Abstract
The high mortality rate of immunocompromised patients with fungal infections and the limited availability of highly efficacious and safe agents demand the development of new antifungal therapeutics. To rapidly discover such agents, we developed a high-throughput synergy screening (HTSS) strategy for novel microbial natural products. Specifically, a microbial natural product library was screened for hits that synergize the effect of a low dosage of ketoconazole (KTC) that alone shows little detectable fungicidal activity. Through screening of ≈20,000 microbial extracts, 12 hits were identified with broad-spectrum antifungal activity. Seven of them showed little cytotoxicity against human hepatoma cells. Fractionation of the active extracts revealed beauvericin (BEA) as the most potent component, because it dramatically synergized KTC activity against diverse fungal pathogens by a checkerboard assay. Significantly, in our immunocompromised mouse model, combinations of BEA (0.5 mg/kg) and KTC (0.5 mg/kg) prolonged survival of the host infected with Candida parapsilosis and reduced fungal colony counts in animal organs including kidneys, lungs, and brains. Such an effect was not achieved even with the high dose of 50 mg/kg KTC. These data support synergism between BEA and KTC and thereby a prospective strategy for antifungal therapy.
Keywords: antifungal, beauvericin, ketoconazole
Serious epidemics of infectious diseases constantly remind us that we live in a universe of microbes, including viruses, bacteria, and fungi, that evolutes faster than the speed at which we develop antiinfective drugs. Fungi have emerged as the fourth most-common pathogens isolated in nosocomial bloodstream infections, nearly 40% of which prove fatal (1, 2). The risk of fungal infections is greatly increased in patients who are severely immunocompromised due to cancer chemotherapy, organ or bone marrow transplantation, or through human immunodeficiency viral infections (3–5). Resistance to existing classes of drugs is also on the rise and, because there are limited classes of antifungal drugs available today and few new drugs in development, there is a need to develop new approaches (6–9). Unfortunately, only one new class of antifungal agents with novel mechanisms of action has been discovered in the past two decades (10).
Systems biology has revealed that fungal pathogens are composed of complex networking systems with redundant, convergent, and divergent signaling pathways (11, 12). Therefore, to cure diseases, multicomponent therapies along the disease pathway may need to be manipulated simultaneously for an effective treatment. When current drugs aimed only at one target are used, the required high dosages for efficacy often produce bioavailability problems and unwanted side effects, and drug-resistance problems may also emerge. Perhaps, if we can focus on multiple targets in a pathway through the use of codrugs, high dosages of single drugs will not be necessary. Given that combinations are being used more frequently in the clinical setting without evidence-based clinical support, and given that the potential for reduced efficacy or increased toxicity exists, it is critical to build upon the available animal data to demonstrate efficacy and safety in the setting of clinical trials. Indeed, the first large randomized trial of antifungal combination therapy to be conducted in two decades has recently been published (13), and new in vitro and in vivo data on antifungal combinations are proliferating (14). For example, combinations of fungistatic and analgesic agents were shown to generate fungicidal activity against drug-resistant Candida albicans but did not significantly affect human cells (15). On the other hand, such limited combinations of commercially available drugs are only the tip of iceberg of combination space and are unlikely to have resulted in the selection of very potent and diverse combinations among the very large number of possibilities. Increasing the quality and quantity of chemical compounds tested in diverse biological systems should increase the chances of finding new leads for therapeutic agents (16–21). Therefore, a broad screen of natural products is urgently needed, especially for those with no detectable activity as single agents but bearing synergistic effects with other compounds.
Microbial and plant metabolites have led to a doubling of the human lifespan during the 20th century, reduced pain and suffering, and revolutionized medicine (10). Over the years, natural products have accounted for the majority of major therapeutic modalities. This success is largely due to their structural complexity and clinical specificity. Previous work in our laboratories has focused on the development of diversified and low-redundancy microbial natural product libraries. We have used integrated approaches for diversifying and dereplicating microbial strains and their extract libraries while decreasing genetic and chemical redundancy.
Ketoconazole (KTC), initially synthesized in the laboratories of Janssen Pharmaceuticals, inhibits lanosterol 14-demethylase, which is critical for sterol synthesis in fungi and mammals. It is commonly used to treat Candida and mold infections. However, at clinical doses, KTC is associated with toxic side effects, including hepatitis. In addition, resistant strains often emerge during long-term or prophylactic treatment as a result of the need to use high concentrations of the drug.
A high-throughput synergy screening (HTSS) method has been developed for identifying synergistic microbial natural products in combination with a low dosage of KTC against fungal pathogens in vitro (22). Pure compounds are isolated and identified after synergy is observed. Many unexpected synergistic interactions of antifungal activities were observed that may be attributable to the interconnected signaling networks existing within and between fungal pathogens.
Results
HTSS for Antifungal Agents from Microbial Natural Product Libraries.
Many drugs, especially natural products, can be more effective at a reduced dosage if low dosages of other synergistic compounds are introduced simultaneously (22). It is important to develop a synergistic drug discovery approach consonant with a systems biology framework and complementary to the target-based approach. A high-throughput screening method was developed for the isolation and identification of synergistic microbial natural products with a low dosage of KTC that perturbs cellular biosynthetic pathways in fungal pathogens (22). The test fungal strain, Candida parapsilosis (CP; ATCC 22019) is an opportunistic human pathogen once used as a quality control strain to compare minimum inhibitory concentrations (MIC) results in different laboratories (23). CP has become the second most commonly isolated fungus in clinical laboratories after C. albicans (24). It can cause high mortality rates within a very short time, particularly in neonates (25–28).
Instead of using the established therapeutic dosage of KTC, we used a much-reduced dosage to sensitize and potentiate fungal pathogens in a screen for potent codrugs in an established microbial natural product library (19). A suboptimal dose of 0.01 μg/ml KTC consistently generated 10–20% of the maximum antifungal activity (22). This procedure takes advantage of KTC at low dosage concentration and screens for those synergistic partners from broad microbial natural products to enhance efficacy. Twenty thousand extracts from our microbial natural product library screened in a high-throughput manner gave a 0.1% hit rate and generated >90% of the maximum activity in combination with 0.01 μg/ml KTC; the microbial extract alone had little to no effect.
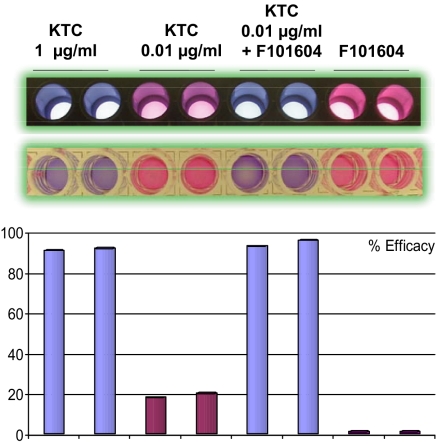
As an example (shown in Fig. 1Top), a marine microbial extract, F101604, was identified and reconfirmed as one of the potent hits (J.B., K. Yan, C. Zheng, H. Sun, Z.C., N. Sun, Y.S., Y. Zhuo, J.Y., J.K., et al., unpublished results). KTC at therapeutic dosage of 1 μg/ml, shown in blue, gave 90% inhibition of fungal growth. A decreased concentration of KTC at 0.01 μg/ml showed a pink color and ≈20% inhibition. The F101604 extract had no antifungal activity by itself, as shown in red. However, the combination of 0.01 μg/ml KTC with F101604 achieved ≈95% inhibition, better than that of the 1 μg/ml KTC treatment (Fig. 1 Bottom) (16). Thus, antifungal activities with KTC and extract F101604 greatly exceeded those seen with either sample alone.
Fig. 1.
Synergistic effect of F101604 with a low dosage of KTC. (Top) The samples were treated as labeled in duplicate. The assay plates after incubation overnight at 35°C in a moistured chamber. Regrowth of samples (Top) in drug-free fresh RPMI media 1640 is shown (Middle). Fluorescence was measured at excitation wavelength (Ex) 544 nm and emission wavelength (Em) 590 nm and converted as percentage of growth inhibition (Bottom).
To determine whether the fungal pathogen was dead or merely inhibited in growth after compound treatment, 2 μl of the above overnight culture was transferred to drug-free RPMI1640 medium in excess with Alamar blue dye. The sample in the well with 0.01 μg/ml KTC turned red, indicating that the pathogen was just inhibited, and that the mode of action was stasis. However, the well with the combined treatment remained blue, indicating that the pathogen was eradicated and the mode of action fungicidal (Fig. 1 Middle). To confirm these results, 0.1 ml of the above samples were spread on Sabouraud agar plates and incubated at 35°C for 48 h. No colonies were formed from the combined treated samples, whereas many colonies were observed for the samples treated with KTC alone (data not shown). Hence, KTC alone is fungistatic, whereas the combination of beauvericin (BEA) and KTC is fungicidal.
Evaluation of Antifungal Spectrum of Isolated Hits.
To confirm the hits, we tested the in vitro activity of those combinations against potentially pathogenic or opportunistic species of yeasts and molds with 12 reference organisms and 10 clinical isolates. The fungal pathogens used in this study included type strains [American Type Culture Collection (ATCC), Rockville, MD] C. albicans, ATCC 90028; C. albicans, ATCC 11651; C. albicans, ATCC 96901 (KTC and fluconazole resistant); CP, ATCC 14054; Candida glabrata, ATCC 90030; Candida krusei, ATCC 6258; Candida tropicalis, ATCC 750; Aspergillus fumigatus, ATCC 46645; Saccharomyces cerevisiae, ATCC 2601; Aspergillus niger, ATCC 10535; Aspergillus terreus, ATCC 1012; Cryptococcus neoformans, ATCC 14116; and Fusarium oxysporum, ATCC 12581. The clinical isolates were received from Theodore C. White's laboratory at the University of Washington (Seattle) and local hospitals in China, including four KTC-resistant C. albicans and two strains each of Aspergillus, Cryptococcus, and Fusarium. Voriconazole and echinocandins were used as references for the treatment of invasive aspergillosis. Twelve hits were ranked based on their potency against the broad spectrum of fungal pathogens.
Human Cell Toxicity of Selected Hit Compounds.
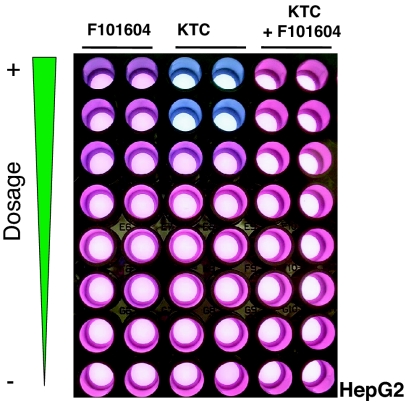
A critical problem in combating fungal infections is that many of the existing antifungal drugs target eukaryotic processes, which are common to fungi and mammals. The potential and reality of drug toxicity are readily apparent, because these drug targets are homologous between fungi and mammals. To eliminate those extracts that may be toxic to human cells, we used human hepatoma (HepG2) cells as a surrogate system to mimic potential therapeutic side effects in the human body. Using the F101604 extract as an example, equal amounts of HepG2 cells, RPMI medium 1640 and Alamar blue dye were placed in each well. The treatment is indicated on the top of the duplicated samples (Fig. 2). From the top to the bottom of Fig. 2, there was a series of 2-fold dilutions of the compounds. The top wells containing F101604 extracts showed a moderate cell-killing effect, and KTC was toxic at 100 and 50 μg/ml, as reported clinically (29), whereas a fixed concentration of 6.25 μg/ml KTC in combination with series dilution of F101604 extracts did not kill HepG2 cells. The top seven leading hits were chosen and ranked by using Spotfire (Somerville, MA) software. In addition, we tried to fractionate and purify the bioactive compounds from those extracts that synergized the activity of KTC.
Fig. 2.
Nontoxicity to human HepG2 cells treated with low dosage of KTC and extract F101604. The same amount of HepG2 cells was seeded in each of the 96 wells. After 24-h incubation at 37°C CO2 incubator with a humidified chamber, colors were developed based on the cell viability (see text).
Identification of Single Compounds as the Active Component in the Hits.
We developed a dereplication procedure for the rapid identification and elimination of known compounds or previously identified chemical isolates present in crude extracts. Analytical HPLC with Photo-Diode Array (PDA) data were acquired for all extracts selected based on biological profiling. These data provided the input descriptors used in pattern-recognition algorithms, such as Hierarchical Cluster Analysis or Principle Component Analysis (16). The resulting clusters were analyzed to assess the chemical diversity among extracts, with only representative nonduplicate (or “dereplicated”) extracts being further evaluated. The HPLC-PDA and mass spectrometric data provided “chemical fingerprints” that were used for dereplication. The ability to rapidly dereplicate biologically active samples significantly shortens the time and decreases the effort required to discover novel molecules (16).
An identified codrug can be one pure compound or mixture of compounds that produce beneficial interactions. Reported here are single compounds and their producing strains identified from our screen (Table 1). For example, the fungal strain producing the F101604 extract was identified genetically and morphologically as Fusarium proliferatum (ref. 22; J.B., K. Yan, C. Zheng, H. Sun, Z.C., N. Sun, Y.S., Y. Zhuo, J.Y., J.K., et al., unpublished results). Upon activity-guided fractionation and purification, BEA, isolated from the F101604 crude extract mixture, reproduced a synergistic phenotype with 0.01 μg/ml KTC in inhibiting the growth of CP. Six pure natural product compounds were identified with their producers deciphered based on rDNA sequencing and morphology (Table 1). BEA showed the most potent activity. This compound is a cyclic hexadepsipeptide that was first studied for its insecticidal properties (30, 31) and later identified as a specific cholesterol acyltransferase inhibitor (32). No antifungal activity had been previously reported for BEA. The activity was confirmed by use of the commercially available BEA obtained from Sigma (St. Louis, MO).
Table 1.
Synergistic antifungal hits isolated from HTSS with 0.01 μg/ml KTC
| Hits identified | Molecular formula | Molecular weight | Producer |
|---|---|---|---|
| Beauvericin | C45H57N3O9 | 784 | F. proliferatum |
| Berberine | C20H18NO4 | 336 | Berberis fremontii |
| Cyclosporin A | C62H111N11O12 | 1,203 | Tolypocladium inflatum |
| Geldanamycin | C29H40N2O9 | 561 | Streptomyces hygroscopicus |
| Lovastatin | C24H36O5 | 405 | A. terreus |
| Radicicol | C18H17C1O6 | 365 | Diheterospora chlamydosporia |
To determine whether the mechanism of combination was synergistic, we performed a checkerboard assay on the diverse fungal pathogens. Although the checkerboard method has not been standardized for testing molds, it has the advantage of simplicity in performance and interpretation compared with Etest and time-kill studies (33). MICs and fractional inhibitory concentration indices (FICIs) were determined, and the result showed a synergistic effect on inhibiting CP with FICI <0.5 (J.B., K. Yan, C. Zheng, H. Sun, Z.C., N. Sun, Y.S., Y. Zhuo, J.Y., J.K., et al., unpublished results). Synergy between BEA and KTC was also observed against 9 of 14 isolates of Saccharomyces, Candida, and Aspergillus spp., including four KTC-resistant C. albicans. For Cryptococcus and Fusarium spp., the combination of BEA and KTC suggested indifference. Antagonism between BEA and KTC was rare on the tested fungal strains. Although using a limited number of fungal pathogens, this in vitro study shows that BEA frequently exhibits synergy with KTC when tested against Saccharomyces, Candida, and Aspergillus spp. To our knowledge, this work presents previously undescribed codrug antifungal activity of BEA with KTC.
Establishment of an Antifungal Mouse Model.
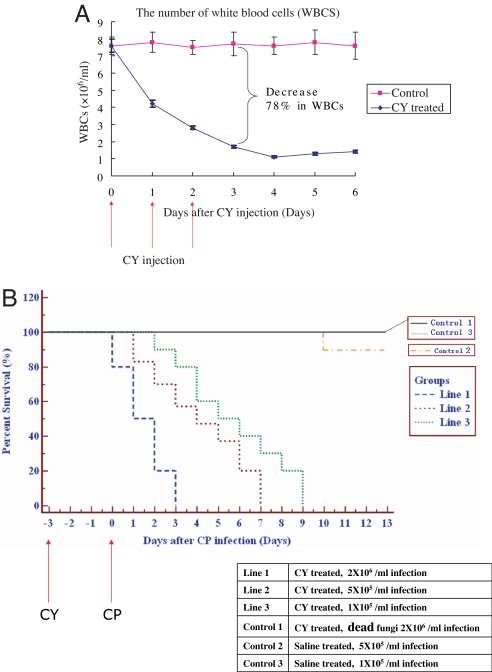
Many synergistic combinations have been reported in vitro (6, 11–15, 34–36), but fungal infections are mainly fatal to immunocompromised patients. It is crucial to test the efficacy of the synergistic pairs in a living immunocompromised animal model. We developed an immunocompromised mouse model by i.p. injection of cyclophosphamide (CY) at a dosage of 100 mg/kg (body weight) once daily for 3 consecutive days to specific pathogen-free female ICR mice. The animals were monitored on various days posttreatment by determining the number of white blood cells (WBCs) and by body weight. A decrease in the number of WBCs (shown in Fig. 3A) and a reduction in body weight (data not shown) compared with saline-treated mice are indicative of an immunocompromised state.
Fig. 3.
Establishment of an immunocompromised mouse model for fungal infection by i.p. injection of CY at a dosage of 100 mg/kg (body weight) once daily for 3 consecutive days. (A) The number of WBCs counted at various days with CY and saline control treatment. (B) Percent survival of mice infected with high, medium, and low concentration of CP blastospores (see text).
To test the model with fungal pathogens, mice were infected with 0.1 ml of CP suspension at different concentrations in warmed saline (35°C) by the lateral tail vein on day 3 after pretreatment with CY. The mortality rate was increased, elicited by CP infection (Fig. 3B). A high inoculum of 2 × 105 blastospores per mouse caused disseminated candidosis that led to death in immunocompromised mice within a 3-day period; four of the mice died immediately after infection. It is unlikely due to the cell mass effect that the same amount of heat-killed blastospores would not cause any mortality after infection (control 1). Disseminated candidosis failed in naive mice treated with only warm saline followed by injection of 5×104 blastospores per mouse with 90% survival rate until day 10 (control 2). Only in CY-immunocompromised animals did we succeed in attempts to induce disseminated candidosis using a 5 × 104 blastospore infection that resulted in 100% mortality within 5–7 days [mean survival time (MST) was 5.9 ± 0.2]. The optimal inoculum for C. albicans was found to be 1 × 104 blastospores per mouse by titration. This concentration caused 100% mortality within 8–10 days (MST was 8.2 ± 0.4) and was also used for subsequent drug-evaluation experiments.
Once the fungi enter the vasculature, they have the capacity to rapidly overwhelm the normally very effective defenses of the bloodstream, similar to invasive human fungal infection. Macroscopic observation of the tissues, including kidneys, lungs, and brains of infected mice with CP, revealed numerous microabscesses, which demonstrated congestion, hemorrhages, tubular degeneration, and heterophilic infiltration by H&E staining and hyphal structure of fungal pathogens by periodic acid Schiff staining (Y. Zhang, K. Yan, F. Min, J.B., W.Z., R.H., L.Z., unpublished data). The same concentration of inoculum was tested in noncompromised mice, but no mortality was observed during the entire observational period (control 3). The results showed that the CY-immunocompromised mice were suitable for fungal infection.
BEA Synergized the KTC Effect for the Treatment of Fungal Infection in CY-Immunocompromised Mice.
To test whether the low dosage of KTC combined with BEA had a synergistic effect in the CY-immunocompromised mouse model, we infected mice i.p. with 1×104 blastospores of CP. Test compound(s) either alone or in combination were administered orally by gavage 6 h postinfection and once daily thereafter for 5 days. A control group received 0.1 ml of saline by the same route as the placebo regimens. Organs of dead and killed mice at the 19th day after infection were collected for H&E/periodic acid Schiff staining, homogenized in sterile saline, diluted, and spread onto sabouraud dextrose agar plates. Colony counts were determined after 48 h at 35°C for calculation of geometric means. Comparative efficacy assessed by changes in the fungal density in kidney, lung, and brain tissue of the infected mice after treatment, percent survival, and MST were undertaken for each test compound(s) either alone or in combination.
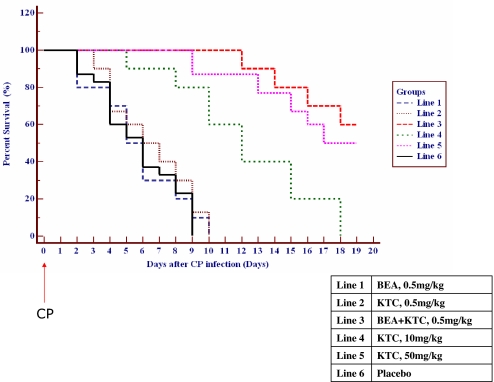
Data obtained from four consecutive experiments showed that preparations of either BEA or KTC did not have any significant therapeutic effect on the survival rate of infected mice compared with the placebo group (Fig. 4). The course of infection indicates that the MST was 8.9 ± 1.4, 9.3 ± 0.3, and 8.4 ± 0.6 days for the placebo control, 0.5 mg/kg BEA, and 0.5 mg/kg KTC-treated groups, respectively (Table 2), and all of the mice in these groups died at day 10 after infection.
Fig. 4.
Percent survival of systemic infected mice treated with test compounds. Blastospores (1 × 104) of CP were injected into the immunocompromised mice at day 0, and the test compounds were administered orally by gavage 6 h postinfection and once daily thereafter for 5 days. For better observation, the number of dead mice was adjusted approximately ±3% in case of overlap when drawing the figure.
Table 2.
Outcome of antifungal treatment in experimental murine infections
| Treatment | Mean ± SD (P value) |
|||
|---|---|---|---|---|
| Survival days | Kidney (log cfu) on day 12 (n = 5) | Lung (log cfu) on day 12 (n = 5) | Brain (log cfu) on day 12 (n = 5) | |
| Placebo | 8.2 ± 0.4 | 8.9 ± 1.4 | 5.1 ± 0.7 | 3.2 ± 0.2 |
| BEA, 0.5 mg/kg | 8.3 ± 1.2 (>0.05) | 9.3 ± 0.3 (>0.05) | 5.2 ± 1.1 (>0.05) | 3.2 ± 0.3 (>0.05) |
| KTC, 0.5 mg/kg | 9.2 ± 1.4 (>0.05) | 8.4 ± 0.6 (>0.05) | 4.8 ± 0.5 (>0.05) | 2.8 ± 0.6 (>0.05) |
| BEA + KTC, 0.5 mg/kg | >21 (<0.01) | 1.3 ± 0.6 (<0.01) | 1.2 ± 0.2 (<0.01) | 0.9 ± 0.2 (<0.01) |
| KTC, 10 mg/kg | 14.2 ± 1.8 (<0.01) | 7.6 ± 0.8 (<0.01) | 3.5 ± 0.7 (<0.01) | 2.1 ± 0.8 (<0.01) |
| KTC, 50 mg/kg | 19.4 ± 2.0 (<0.01) | 5.5 ± 1.5 (<0.01) | 2.6 ± 0.6 (<0.01) | 1.5 ± 0.6 (<0.01) |
Increasing doses of KTC prolonged significantly (P < 0.01) the MST to 14.2 ± 1.8 and 19.4 ± 2.0 days for the 10 mg/kg and 50 mg/kg KTC-treated groups, respectively. The remaining fungi [log10 cfu (cell/g)] in kidney was reduced from 8.9 to 7.6 and 5.5, respectively, as was the case in brain and lungs. However, the dosage of 50 mg/kg administered in mice caused high liver toxicity and other side effects (33). However, the combination of 0.5 mg/kg BEA and 0.5 mg/kg KTC showed 60% survival 19 days after infection. The [log10 cfu (cell/g)] in kidney, lungs, and brain was reduced dramatically (P < 0.01) from 8.4 to 1.3, 5.1 to 1.2, and 3.2 to 0.9, respectively. Importantly, a low dosage of KTC combined with BEA gave better antifungal efficacy than a high single-therapy dosage of 50 mg/kg KTC.
Discussion
Testing combinations of antifungal compounds in patients has been applied as an explicit strategy for drug improvement by physicians (13, 37). However, mixing two or more effective drugs with different mechanisms of action cannot guarantee an improved outcome compared with the results seen with a single agent. BEA was identified before as a potentiator of antifungal miconozole activity in vitro (38, 39). Some nonantifungal compounds were also found to enhance the activity of conventional antifungal agents (40). Recent discoveries indicate that Hsp90 and calcineurin inhibitors potentiate drug-resistant fungal pathogens to fungistatic agents (12), but none of these observations had preclinical backup, whereas fungal infections are mainly fatal to immunocompromised patients.
Using our HTSS technology to screen microbial bioactive metabolites with a low dosage of KTC in cell-based assays, we observed and purified some compounds responsible for synergistic interactions, as what was reported in other systems using pure compounds (15, 41). The antifungal activities of KTC and BEA combinations reported here greatly exceeded those seen with either drug alone in vitro and in an immunocompromised mouse model. BEA not only improved the efficacy of a much-reduced dosage of KTC but also broadened its spectrum on drug-resistant strains and reduced its side effects. BEA had little antifungal activity, but the synergistic activity of KTC and BEA resulted in fungicidal, in contrast to fungistatic, action of KTC. Furthermore, the combination did not significantly affect the proliferation of primary human liver HepG2 cells, indicating that the combination is selective for fungal pathogens relative to mammalian cells.
The synergy mechanism of BEA and KTC is still under investigation. Our preliminary observations suggest that an efflux pump in the fungal cell membrane might be a target (J.B., K. Yan, C. Zheng, H. Sun, Z.C., N. Sun, Y.S., Y. Zhuo, J.Y., J.K., et al., unpublished results; ref. 42). BEA and other synergistic compounds isolated from microbial extracts will enable the existing drug KTC to be more effective and will contribute to a better understanding of multiple pathways to cure fungal infections.
Materials and Methods
Antifungal Agents.
Pure KTC dihydrochloride powder was purchased from the local hospital pharmacy, and BEA, amphotericin B, voriconazole, echinocandins, and other compounds from Sigma, unless specified. These compounds were reconstituted according to the manufacturers' directions.
HTSS Procedure for Antifungal Activities.
Microbes from a variety of ecosystems were collected and grown in different physiological media to generate a highly diversified and low redundant microbial natural product library (19). We grew selected microbes in 40 ml of liquid or solid culture in 250-ml flasks under shaking or static conditions at two different temperatures (ref. 22; J.B., K. Yan, C. Zheng, H. Sun, Z.C., N. Sun, Y.S., Y. Zhuo, J.Y., J.K., et al., unpublished results).
The master plates were prepared for screening by diluting the natural extract stocks 100-fold, 2 μl of which was transferred to a dilution daughter plate with RPMI medium 1640 (2% glucose without bicarbonate or phenol red, buffered with 0.165 M Mops to pH 7.0). Fungal pathogens (including ATCC type strains and clinical isolates) were added to the assay plate postcompound addition by using a Thermo Labsystems (Ventra, Finland) Multidrop in a biosafety cabinet. The antifungal activity and MICs were determined in flat bottom, 96-well microtiter plates (VWR, West Chester, PA) with a total volume of 0.08 ml/well by using a broth microdilution protocol modified from the Clinical and Laboratory Standards Institute (formerly National Committee for Clinical Laboratory Standards) M-38A and M-27A2 methods (43, 44). Cell densities of overnight cultures were determined, and dilutions were prepared so that ≈1 × 104 cells were inoculated into each well in RPMI medium 1640 with 2 μl of samples and 8% Alamar blue (BioSource International, Camarillo, CA) at a 35°C incubator overnight with 80% humidity and 5% CO2. The fluorescence reading is measured at excitation wavelength (Ex) 544 nm and emission wavelength (Em) 590 nm by using a Fusion plate reader (Packard, Meriden, CT) to determine the percentage of remaining viable cells. Amphotericin B and KTC were used as positive controls for antifungal activity. MICs were determined as the concentration of KTC and other compounds that inhibits fungal growth by 50% relative to the corresponding drug-free control. The MICs of each strain were tested at least three times in duplicate. To test whether the fungal pathogens were killed or just growth-inhibited, 2 μl of the overnight culture was transferred to drug-free RPMI medium 1640 with 8% Alamar blue.
Algorithm to Calculate Synergism.
To determine whether the readout of the combination therapy is additive, synergistic, or indifferent, we calculated the FICI by the use of the checkerboard assay (45). The FICI represents the sum of the FICs of each drug tested, where the FIC is determined for each drug by dividing the MIC of each drug when used in combination by the MIC of each drug when used alone. FICI = (MICdrug A in combination/MICdrug A alone) + (MICdrug B in combination/MICdrug B alone) (46). The preassumptions are that (i) testing uses concentrations separated by a factor of 2, and (ii) one-dilution-step MIC changes are within the range of experimental error. Other sophisticated methods have also been used; for example, a contour surface-plot methodology was used in characterizing the nature of three antifungal agents in combinations using various concentrations of each agent (47). Use of response-surface plots illustrated the Loess fit of the association for a particular outcome (such as weight change, fungal tissue burden, or survival) in animal tests (48).
Dereplication and Identification of Active Natural Products.
Extracts that had been prioritized by the dereplication process were separated into multiple fractions by using Tecan's Freedom EVO (Maennedorf, Switzerland) that concentrates and dispenses each fraction into individual wells in plates that were robotically prepared for bioassays. Each fraction was characterized by UV spectrum (to aid in chemical identification) and evaporative light-scattering device (to determine the amount of material present). Bioassay and chemical profiles for each fraction were correlated through proprietary software (10). Test wells containing biologically active molecules were further analyzed by liquid chromatography MS. When sufficient material was present, NMR spectroscopy was performed. If the biological activity and chemical novelty warranted further analysis, the chemical structures of the active compounds were elucidated by using 500 MHz NMR spectrometer (Bruker, Billerica, MA) and quantitative TOF mass spectrometer.
Mouse Model.
Specific-pathogen-free female ICR [Crl: CD-1] mice (female, white, ≈20–22 g) were used throughout the experiment. The animals, housed in cages of five mice per group and fed with standard rodent chow ad libitum, were allowed to acclimate for 1 week before active experimentation carried out in negative-pressure stainless steel isolators. Animals were observed thrice daily for signs of drug-related morbidity or morality. Mice that became immobile or otherwise showed signs of severe illness were humanely terminated and recorded as dying on the following day. Analgesics were not administered to these animals, because the possibility of drug–drug interactions and their influence on the outcome of the study were unknown. Instead, these animals were killed by CO2 exposure followed by cervical dislocation in an area separate from the housing place.
Murine Model of Systemic Infection by CP.
Before infection, mice were rendered neutropenic by i.p. injection of CY (Sigma) daily for 3 consecutive days at a dosage of 100 mg/kg body weight before inoculation. Animals were monitored on various days after the first CY injection by quantitating their total WBCs by using a hemocytometer. Mice were then infected with 0.1 ml of various concentrations (2 × 106, 5 × 105, and 1 × 105 cfu/ml) of inoculum of CP in warmed saline (35°C) by the lateral tail vein on day 3 after pretreatment with CY. A group of mice were injected with 0.1 ml of 2 × 106 cfu/ml dead fungal suspension per mouse as negative control. In addition, two groups of mice were inoculated with 0.1 ml of 5 × 105 and 1 × 105 cfu/ml suspensions per mouse without advance CY treatment as negative controls. Confirmation of infection was determined in duplicate for final isolate candidates. Data were averaged from three experiments.
All groups of mice were observed 19 days after infection. At the end point, all surviving mice were killed by CO2 exposure. Different tissues of mice that died during the observation period and killed survivors were removed under aseptic conditions, which were subjected to detailed necropsy examinations. Left kidneys, lungs, and brains of mice were homogenized in sterile 0.9% saline. Serial dilutions of the homogenates were plated onto sabouraud dextrose agar to calculate the cfus after 48 h of incubation at 35°C. Right-side organs were collected for detailed histopathology. The tissues were processed and 5-μm-thick paraffin sections were stained with H&E. The periodic acid Schiff method was used following standard procedure (Y. Zhang, K. Yan, F. Min, J.B., W.Z., R.H., L.Z., unpublished data) to demonstrate fungal elements in the tissues.
Statistical Analyses.
The LD50 for infection of mice was determined by challenging mice over a range of 104-106 cfu of the fungal pathogen. For each determination, four separate experiments were performed in which more than six mice were infected with each concentration of CP. The change in fungal density in tissues, expressed as change in log10 cfu for both treated and untreated animals, was reported by using descriptive statistics. Data were fitted by using the maximum concentration effect (Emax) model to determine the ED50. Effectiveness (change in fungal density) of the agents alone vs. the combination was undertaken with appropriate statistical tests. The MST data of each group of treated mice were compared with those from untreated controls by using one-way ANOVA. Student's test was used, and the significance level was defined as P < 0.01. Statistical significance of differences in survival times was calculated by using the Kaplan–Meier method (New Statistic for Windows; SPSS, Chicago, IL).
Acknowledgments
We thank Theodore C. White for providing drug resistant clinical fungal isolates and David P. Nicolau for designing preclinical experiments. We thank Xiaorong Liu, Baoliang Cui, Yucai Peng, Patrick Bonner, Victoria Knight, Lauren LeBrun, and Dennis DiTullio for technical help. We thank Haian Fu and Richard Roberts for critical reading of the manuscript and helpful discussions. This work was supported in part by National 863 Project 2006AA09Z402, Chinese Academy of Sciences Innovation Projects 062A131BB4, 973 Project 2007CB707802, by grants from the South China Sea Institute of Oceanology, the Chinese Academy of Sciences, and Guangzhou Consun Pharmaceuticals, Inc.
Abbreviations
- HTSS
high-throughput synergy screening
- BEA
beauvericin
- KTC
ketoconazole
- CY
cyclophosphamide
- WBCs
white blood cells
- CP
Candida parapsilosis
- MST
mean survival time
- MIC
minimum inhibitory concentration
- FICI
fractional inhibitory concentration indices.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Eckmanns T, Ruden H, Gastmeier P. J Infect Dis. 2006;193:1408–1418. doi: 10.1086/503435. [DOI] [PubMed] [Google Scholar]
- 2.Morrell M, Fraser VJ, Kollef MH. Antimicrob Agents Chemother. 2005;49:3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H, Shadduck RK, Shea TC, Stiff P, Friedman DJ, et al. N Engl J Med. 1992;326:845–851. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- 4.Larocco MT, Burgert SJ. Clin Microbiol Rev. 1997;10:277–297. doi: 10.1128/cmr.10.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez V, Vasquez JA, Barth-Jones D, Dembry L, Sobel JD, Zervus MJ. Am J Med. 1993;94:577–582. doi: 10.1016/0002-9343(93)90207-6. [DOI] [PubMed] [Google Scholar]
- 6.Graybill JR, Revankar SG, Patterson TF. In: Topley & Wilson's Microbiology and Microbial Infections. 9th Ed. Ajello L, Hay RJ, editors. Vol 4. London: Arnold; 1998. pp. 163–165. [Google Scholar]
- 7.Rex JH, Rinaldi MG, Pfaller MA. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghannoum MA, Rice LB. Clin Microbiol Rev. 1999;12:501–517. doi: 10.1128/cmr.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugar AM, Alsip SG, Galgiani JN. Antimicrob Agents Chemother. 1997;31:1874–1878. doi: 10.1128/aac.31.12.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demain AL, Zhang L. In: Natural Products: Drug Discovery and Therapeutics Medicines. Zhang L, Demain AL, editors. Totowa, NJ: Humana; 2005. pp. 3–32. [Google Scholar]
- 11.Cowen LE, Lindquist S. Science. 2005;309:2185–2189. doi: 10.1126/science.1118370. [DOI] [PubMed] [Google Scholar]
- 12.Heitman J. Science. 2005;309:2175–2176. doi: 10.1126/science.1119321. [DOI] [PubMed] [Google Scholar]
- 13.Johnson MD, MacDougall C, Ostrosky-Zeichner L, Perfect JR, Rex JH. Antimicrob Agents Chemother. 2004;48:693–715. doi: 10.1128/AAC.48.3.693-715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rex JH, Pappas PG, Karchmer AW, Sobel J, Edwards J, Hadley S, Brass C, Vazquez JA, Chapman S, Horowitz H, et al. Clin Infect Dis. 2003;36:1221–1228. doi: 10.1086/374850. [DOI] [PubMed] [Google Scholar]
- 15.Borisy AA, Elliott PJ, Hurst NW, Lee MS, Lehar J, Price ER, Serbedzija G, Zimmermann GR, Foley MA, et al. Proc Natl Acad Sci USA. 2003;100:7977–7982. doi: 10.1073/pnas.1337088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L. In: Natural Products: Drug Discovery and Therapeutics Medicines. Zhang L, Demain AL, editors. Totowa, NJ: Humana; 2005. pp. 33–56. [Google Scholar]
- 17.Newman DJ, Cragg GM. In: Natural Products: Drug Discovery and Therapeutics Medicines. Zhang L, Demain AL, editors. Totowa, NJ: Humana; 2005. pp. 275–294. [Google Scholar]
- 18.Donia M, Hamann MT. Lancet Infect Dis. 2003;3:338–348. doi: 10.1016/S1473-3099(03)00655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight V, Sanglier JJ, DiTullio D, Braccili S, Bonner P, Waters J, Hughes D, Zhang L. Appl Microbiol Biotechnol. 2003;62:446–458. doi: 10.1007/s00253-003-1381-9. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, An R, Wang J, Sun N, Zhang S, Hu J, Kuai J. Curr Opin Microbiol. 2005;11:655–662. doi: 10.1016/j.mib.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Jensen PR, Fenical W. In: Natural Products: Drug Discovery and Therapeutics Medicines. Zhang L, Demain AL, editors. Totowa, NJ: Humana; 2005. pp. 315–328. [Google Scholar]
- 22.Zhang L. WO 2005/051303. PCT Patent. 2005
- 23.Rex JH, Pfaller MA, Lancaster M, Odds FC, Bolmstrom A, Rinaldi MG. J Clin Microbiol. 1996;34:816–817. doi: 10.1128/jcm.34.4.816-817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagano L, Antinori A, Ammassari A, Mele L, Nosari A, Melillo L, Martino B, Sanguinetti M, Equitani F, Nobile F, et al. Eur J Haematol. 1999;63:77–85. doi: 10.1111/j.1600-0609.1999.tb01120.x. [DOI] [PubMed] [Google Scholar]
- 25.Weems JJ, Jr, Chamberland ME, Ward J, Willy M, Padhye AA, Solomon SL. J Clin Microbiol. 1987;25:1029–1032. doi: 10.1128/jcm.25.6.1029-1032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saiman L, Ludington E, Pfaller M, Rangel-Frausto S, Wiblin RT, Dawson J, Blumberg HM, Patterson JE, Rinaldi M, Edwards JE, et al. Pediatr Infect Dis J. 2000;19:319–324. doi: 10.1097/00006454-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Tbienpont D, Van Cutsem J., Van Gerven F., Heeres J., Jansen PAJ. Experientia. 1979;35:606–607. doi: 10.1007/BF01960348. [DOI] [PubMed] [Google Scholar]
- 28.Rex JH, Rinaldi MG, Pfaller MA. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perfect JR, Lindsay MH, Drew RH. Drug Saf. 1992;7:323–363. doi: 10.2165/00002018-199207050-00003. [DOI] [PubMed] [Google Scholar]
- 30.Hamill RL, Higgens CE, Boaz HE, Gorman M. Tetrahedron Lett. 1969;49:4255–4258. [Google Scholar]
- 31.Wilson JP, Jurjevic Z, Hanna WW, Wilson DM, Potter TL, Coy AE. Mycopathologia. 2006;161:101–107. doi: 10.1007/s11046-005-0170-7. [DOI] [PubMed] [Google Scholar]
- 32.Tomoda H, Huang XH, Nishida H, Nagao R, Okuda S, Tanaka H, Omura S, Arai H, Inoue K. J Antibiot. 1992;45:1626–1632. doi: 10.7164/antibiotics.45.1626. [DOI] [PubMed] [Google Scholar]
- 33.Lewis RE, Diekema DJ, Messer SA, Pfaller MA, Klepser ME. J Antimicrob Chemother. 2002;49:345–351. doi: 10.1093/jac/49.2.345. [DOI] [PubMed] [Google Scholar]
- 34.Chung E, Nafziger AN, Kazierad DJ, Bertino JS., Jr Clin Pharmacol Ther. 2006;79:350–361. doi: 10.1016/j.clpt.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 35.El-Dahshan KF, Bakr MA, Donia AF, Badr AE, Sobh MA. Am J Nephrol. 2006;226:293–298. doi: 10.1159/000094133. [DOI] [PubMed] [Google Scholar]
- 36.Ficker CE, Arnason JT, Vindas PS, Alvarez LP, Akpagana K, Gbeassor M, De Souza C, Smith ML. Mycoses. 2003;46:29–37. doi: 10.1046/j.1439-0507.2003.00838.x. [DOI] [PubMed] [Google Scholar]
- 37.Lewis RE, Kontoyiannis DP. Pharmacotherapy. 2001;21:149S–164S. doi: 10.1592/phco.21.12.149s.34505. [DOI] [PubMed] [Google Scholar]
- 38.Fukuda T, Arai M, Yamaguchi Y, Masuma R, Tomoda H, Omura S. J Antibiot. 2004;57:110–116. doi: 10.7164/antibiotics.57.110. [DOI] [PubMed] [Google Scholar]
- 39.Fukuda T, Hasegawa Y, Hagimori K, Yamaguchi Y, Masuma R, Tomoda H, Omura S. J Antibiot. 2006;59:480–485. doi: 10.1038/ja.2006.67. [DOI] [PubMed] [Google Scholar]
- 40.Afeltra J, Verweij PE. Eur J Clin Microbiol Infect Dis. 2003;22:397–407. doi: 10.1007/s10096-003-0947-x. [DOI] [PubMed] [Google Scholar]
- 41.Hung DT, Shakhnovich EA, Pierson E, Mekalanos JJ. Science. 2005;310:670–674. doi: 10.1126/science.1116739. [DOI] [PubMed] [Google Scholar]
- 42.Xu Z, Zhang LX, Zhang JD, Cao YB, Yu YY, Wang DJ, Ying K, Chen WS, Jiang YY. Int J Med Microbiol. 2006;296:421–434. doi: 10.1016/j.ijmm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 43.National Committee for Clinical Laboratory Standards. Approved Standard. Wayne, PA: Natl Comm Clin Lab Stand; 2002. [Google Scholar]
- 44.National Committee for Clinical Laboratory Standards. Approved Standard. 2nd Ed. Wayne, PA: Natl Comm Clin Lab Stand; 2002. [Google Scholar]
- 45.Barchiesi F, Falconi DF, Scalise G. Antimicrob Agents Chemother. 1997;41:1812–1814. doi: 10.1128/aac.41.8.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eliopoulos GM, Moellering RC. In: Antibiotics in Laboratory Medicine. Lorian V, editor. Baltimore, MD: Williams & Wilkins; 1991. pp. 432–492. [Google Scholar]
- 47.Ghannoum MA, Fu Y, Ibrahim AS, Mortara LA, Shafiq MC, Edwards JE, Jr, Criddle RS. Antimicrob Agents Chemother. 1995;39:2459–2465. doi: 10.1128/aac.39.11.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsen RA, Bauer M., Weiner JM, Diamond DM, Leal ME, Ding JC, Rinaldi MG, Graybill JR. Antimicrob Agents Chemother. 1996;40:2178–2182. doi: 10.1128/aac.40.9.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]