Abstract
The gene encoding the conserved bacterial G protein CgtA (Obg) is essential for viability in every organism in which it has been studied. CgtA has been reported to be involved in several diverse bacterial functions, including ribosome assembly, DNA repair, sporulation, and morphological development. However, none of these functions have been identified as essential. Here we show that depletion of CgtA in Vibrio cholerae causes global changes in gene expression that are consistent with induction of a classical low nutrient stress response or “stringent” response. We show that depletion of CgtA leads to increased ppGpp levels that correlate with induction of the global stress response and cessation of growth. The enzyme RelA is responsible for synthesis of the alarmone ppGpp during the stringent response. We show that CgtA is no longer essential in a relA deletion mutant and thus conclude that the essentiality of CgtA is directly linked to its ability to affect ppGpp levels. The enzyme SpoT degrades ppGpp, and here we show that SpoT is essential in a RelA+ CgtA+ background but not in a relA deletion mutant. We also confirmed that CgtA interacts with SpoT in a two-hybrid assay. We suggest that the essential function of CgtA is as a repressor of the stringent response that acts by regulating SpoT activity to maintain low ppGpp levels when bacteria are growing in a nutrient-rich environment.
Keywords: alarmone, cholera, GTPase, starvation, transcription
Bacteria respond to starvation for amino acids by dramatically changing gene expression patterns in what is know as the nutrient stress response or “stringent response” in Escherichia coli (1). RelA and SpoT regulate the nutritional stress response by regulating levels of the small molecule alarmone ppGpp. Both RelA and SpoT synthesize ppGpp by phosphorylating GTP to pppGpp in response to specific low nutrient conditions. The pppGpp is converted to ppGpp, and together the pppGpp and ppGpp are termed (p)ppGpp. RelA activity is induced by uncharged tRNAs entering the A site in the ribosome, whereas SpoT phosphorylation activity is triggered by low concentrations of nitrogen, iron, carbon, phosphate, and fatty acids (2–6). SpoT also has hydrolytic activity, breaking down ppGpp into GTP and PPi (7). The spoT gene is essential in relA+ strains, presumably because ppGpp is not degraded efficiently while basal (p)ppGpp synthetic activity by RelA occurs even in rich media, gradually increasing ppGpp concentration (8). Nutritional stress induces production of ppGpp, and this alarmone is thought to regulate the nutrient stress response by altering the affinity of RNA polymerase σ factor for its target promoters (1). In a nutrient-rich environment, ppGpp levels are low, and RNA polymerase is predominantly associated with the σ70 transcription factor. This situation correlates with high expression of the translation machinery, including ribosomal proteins and initiation and elongation factors (9, 10). Under nutrient poor conditions, levels of ppGpp are high. The change in ppGpp concentration alters σ factor affinity of RNA polymerase, causing an increase in the affinity for alternative σ factors.
The cgtA gene is a member of a well conserved family of bacterial P-loop GTPases found in prokaryotes and eukaryotes (11, 12). It is often annotated as obg/GTP1 or yhbZ and is essential in every organism in which it has been studied, including Escherichia coli, Vibrio cholerae, Vibrio harveyi, Caulobacter crescentus, and Bacillus subtilis (13–17). CgtA/Obg is involved in sporulation in B. subtilis and morphological development in S. coelicolor (18–20). It has been suggested that Obg senses guanine nucleotide levels and that GTP concentration regulates developmental and morphological processes (18, 21). Neither of these developmental functions is essential for viability. It has also been reported to respond to DNA replication fork arrest in E. coli (22). CgtA/Obg proteins interact with components of the stress response pathways in B. subtilis and E. coli. In B. subtilis, Obg interacts with the general stress response pathway and is necessary for activation of the stress resistance transcription factor, σB, which is induced by both nutritional and physical stress (23). In E. coli, CgtA interacts with SpoT (24).
In E. coli, CgtA is involved in late steps of ribosome assembly and maturation (24, 25), and is specifically required for optimal incorporation of specific ribosomal proteins into the large ribosomal subunit (26). This is probably a conserved function because in other organisms, such as V. harveyi, B. subtilis, C. crescentus, and E. coli, CgtA interacts with the 50S ribosomal subunit and has been shown to be involved in assembly or stability of the 50S subunit (17, 23, 27, 28). Interestingly, a yeast mitochondrial CgtA homolog is essential for translation, likely through an involvement in ribosome biogenesis (29). In C. crescentus, a temperature-sensitive mutant was isolated with a defect in 50S ribosome assembly or stability, but this defect occurred at both the permissive and restrictive temperatures, indicating that this is not the essential function of CgtA (28). The mutant was not viable at the restrictive temperature, but there were no additional ribosomal defects. In E. coli, a similar conditional mutant has the same effect (26). This suggests that CgtA has an essential function apart from ribosome biogenesis, and that this unidentified function is the essential function.
To identify the essential function of CgtA in V. cholerae, we compared the transcriptional profiles of CgtA-depleted and CgtA-expressing strains to identify changes in gene expression due to CgtA depletion. We determined that gene expression in CgtA-depleted cultures resembles that of cultures in stringent response, implicating the reported CgtA–SpoT interaction as functionally important (24). We then tested the interactions of CgtA with RelA and SpoT and showed that V. cholerae CgtA and SpoT interact, and that ppGpp concentration increases when CgtA is not expressed. Taken together, our results suggest that CgtA–SpoT interaction is necessary to prevent inappropriate induction of the stress response, and that CgtA is necessary for SpoT hydrolysis activity. This suggests that the essential function of CgtA is to regulate SpoT activity to keep intracellular ppGpp concentrations low and this in turn prevents induction of the stress response and allows cells to maintain active growth.
Results
The cgtA Gene Is Essential in V. cholerae.
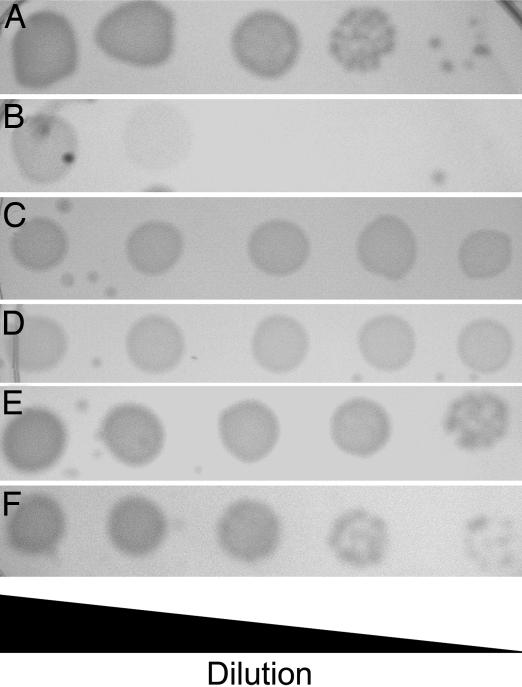
The cgtA gene was identified as an essential gene by TnAraOut transposon mutagenesis (15). The TnAraOut transposon introduced the arabinose-inducible PBAD promoter upstream of cgtA, producing strain NJ267, in which viability depends on the presence of arabinose in the growth medium. When NJ267 is grown in LB containing 0.1% arabinose, its growth rate is similar to wild type (15). However, when NJ267 is grown in medium containing lower concentrations of arabinose, or without arabinose, it grows very slowly and is unable to escape stationary phase until several hours after the wild type. Complementation with cgtA in trans confirmed the role of CgtA in the slow-growth phenotype. A strain containing an in-frame deletion of cgtA on the chromosome was constructed, while maintaining a plasmid with cgtA under control of PBAD, DR209/pDR291 [ΔcgtA/PBAD::cgtA]. This strain also required arabinose for growth, including during the construction of the in-frame deletion (Fig. 1 A and B). We were unable to construct an in-frame deletion of cgtA in a wild-type strain, further supporting the evidence that cgtA is essential.
Fig. 1.
Dependence on arabinose for growth. Indicated strains were grown on LB/0.1% arabinose (A and C) or LB only (B and D–F) and spotted as 10-fold serial dilutions from left to right. (A and B) DR209/pDR291. (C and D) DR210/pDR308. (E) DR214. (F) N16961ΔrelAΔspoT.
Using Depletion Strains to Characterize Function.
We used the depletion strain, DR209/pDR291 [ΔcgtA/PBAD::cgtA], to characterize CgtA function by determining changes that occur during depletion. In E. coli, the CgtA homolog Obg is necessary for chromosome segregation, and an Obg deficiency produces elongated cells with nonpartitioned chromosomes (30). It has also been reported that the E. coli Obg protein is involved in monitoring DNA damage during DNA replication (22). In contrast, CgtA in V. harveyi does not affect does not affect chromosome segregation (17). In V. cholerae, CgtA-depletion does not cause cell elongation, and no chromosomal defects were observed when nucleoids were visualized with DAPI staining (data not shown). This suggests that V. cholerae CgtA is functionally similar to V. harveyi CgtA.
Depletion of CgtA Leads to Changes in Gene Expression Consistent with Stringent Response.
To characterize CgtA function further, we conducted transcriptional profiling studies using the cgtA-depletion strain DR209/pDR291 to identify cgtA-coregulated genes, and to determine how cells respond during early stages of CgtA depletion. The goal was to identify transcriptional changes that occur before pleiotropic effects arise due to general stress.
Transcriptional profiling was performed by using cultures in mid-log growth phase and CgtA-depleted or mock-depleted for 20 min. Among the most down-regulated genes in the CgtA-depleted cultures were ribosomal proteins and other genes involve in protein synthesis, as well as genes involved in cell growth and metabolism, and many hypothetical ORFs [Table 1 and supporting information (SI) Table 3]. Genes with increased expression during CgtA-depletion included a large number of hypothetical ORFs, plus several transporters and transcriptional regulators. Studies of E. coli and B. subtilis have shown that during nutritional stress responses, the genes encoding proteins important for cell growth, specifically including protein synthesis, are down-regulated while transporters are up-regulated (9, 10). Because the B. subtilis CgtA-homolog Obg is involved in the general stress response, we hypothesized that CgtA was likely to be involved in nutritional stress responses or specifically the stringent response that occurs after the accumulation of uncharged tRNAs in V. cholerae.
Table 1.
Genes down-regulated by category due to CgtA depletion or serine hydroxamate treatment
| Function | CgtA-depleted | % | Serine hydroxamate-treated | % | Genes in common | % | CgtA operons* | % | Serine hydroxamate operons† | % |
|---|---|---|---|---|---|---|---|---|---|---|
| Amino acid biosynthesis | 6 | 2.9 | 6 | 0.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Biosynthesis of cofactors, prosthetic groups, and carriers | 5 | 2.4 | 20 | 2.8 | 0 | 0.0 | 1 | 1.1 | 0 | 0.0 |
| Cell envelope | 8 | 3.9 | 41 | 5.7 | 3 | 3.8 | 4 | 4.3 | 7 | 5.1 |
| Cellular processes | 15 | 7.3 | 30 | 4.1 | 2 | 2.5 | 6 | 6.5 | 5 | 3.7 |
| Central intermediary metabolism | 9 | 4.4 | 16 | 2.2 | 2 | 2.5 | 2 | 2.2 | 2 | 1.5 |
| DNA metabolism | 3 | 1.5 | 20 | 2.8 | 1 | 1.3 | 1 | 1.1 | 2 | 1.5 |
| Energy metabolism | 23 | 11.3 | 85 | 11.8 | 13 | 16.4 | 16 | 17.4 | 24 | 17.6 |
| Fatty acid and phospholipid metabolism | 3 | 1.5 | 17 | 2.3 | 2 | 2.5 | 3 | 3.3 | 5 | 3.7 |
| Mobile and extrachromosomal element functions | 10 | 4.9 | 9 | 1.2 | 1 | 1.3 | 3 | 3.3 | 3 | 2.2 |
| Protein fate | 10 | 4.9 | 31 | 4.3 | 6 | 7.6 | 6 | 6.5 | 10 | 7.3 |
| Protein synthesis | 13 | 6.4 | 58 | 8.0 | 10 | 12.7 | 11 | 12.0 | 27 | 19.8 |
| Purines, pyrimidines, nucleosides, and nucleotides | 6 | 2.9 | 30 | 4.1 | 4 | 5.0 | 4 | 4.3 | 4 | 2.9 |
| Regulatory functions | 10 | 4.9 | 37 | 5.1 | 4 | 5.0 | 4 | 4.3 | 6 | 4.4 |
| Transcription | 2 | 1.0 | 13 | 1.8 | 1 | 1.3 | 2 | 2.2 | 1 | 0.7 |
| Transport and binding proteins | 18 | 8.8 | 65 | 9.0 | 6 | 7.6 | 6 | 6.5 | 13 | 9.6 |
| Unknown function | 63 | 30.9 | 245 | 33.9 | 24 | 30.4 | 23 | 25.0 | 27 | 19.8 |
| Total | 204 | 100 | 723 | 100 | 79 | 100 | 92 | 100 | 136 | 100 |
*Total number of genes that were down-regulated in the CgtA-depleted cultures that were present in operons or functional clusters that were down-regulated in the serine hydroxamate treated cultures.
†Total number of genes that were down-regulated in the serine hydroxamate-treated cultures that were present in operons or functional clusters that were down-regulated in the CgtA-depleted cultures.
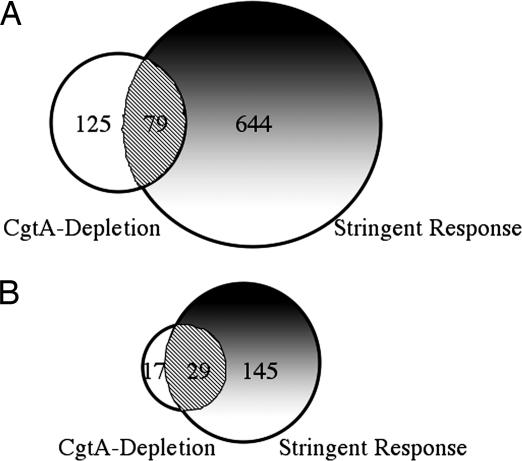
In bacterial cells, two ribosome-associated proteins, RelA and SpoT, regulate entry into stringent response by modifying intracellular ppGpp concentration (8). RelA phosphorylates GTP to produce the alarmone ppGpp when uncharged tRNAs are present in the A site of the ribosome. SpoT hydrolyzes ppGpp to GTP and PPi. Decreasing RelA concentration leads to lowered levels of ppGpp, whereas decreasing SpoT concentration leads to elevated ppGpp levels. Because CgtA depletion produced changes in gene expression similar to that seen in stringent response in other organisms, we decided to compare CgtA depletion with stringent response in V. cholerae. Serine hydroxamate is an amino acid analog that inhibits tRNAser aminoacylation and thus causes accumulation of uncharged tRNAser and induction of the stringent response (31). Thus, stringent response effects were determined by comparing transcriptional profiles of cultures treated with serine hydroxamate for 20 min to that of untreated cultures. The serine hydroxamate-treated cultures produced a transcriptional profile similar to that seen in stringent response-induced cultures of E. coli and B. subtilis, including decreased expression of genes involve in protein synthesis, energy metabolism, DNA replication, and many genes involved in growth (Table 1 and SI Table 4) (9, 10). CgtA depletion decreased expression of genes involved in protein synthesis, as well as genes involved in cell growth and energy metabolism (Table 1 and SI Table 4). The number of genes affected by CgtA depletion was less than the number of genes affected in stringent response (Fig. 2, Table 1, and SI Tables 3 and 4). This was not surprising because CgtA depletion also does not produce as severe a growth defect as addition of serine hydroxamate. CgtA-depleted cultures see a gradual slowing of growth instead of a complete cessation as seen with addition of serine hydroxamate (data not shown), which is expected due to functional CgtA remaining even as no new expression is occurring. Seventy-nine of the 204 genes that were down-regulated during CgtA depletion were also down-regulated during stringent response (Fig. 2A). These were enriched for genes in energy metabolism, protein synthesis, and protein fate (Fig. 2B and Table 1). During CgtA depletion, there were 46 down-regulated genes belonging to these gene classes; of these, 29 were also down-regulated during stringent response (Fig. 2B and Table 1). Many genes that were down-regulated during CgtA depletion that were not found to be down-regulated during stringent response were in operons or functional clusters that had genes down-regulated because of serine hydroxamate treatment, and vice versa. For instance, there is a cluster of genes involved in chemotaxis, including cheW, cheB, cheA, cheZ, and cheY, which is located between VC2059 and VC2065 loci on the large chromosome. CgtA causes down-regulation of VC2059 to VC2062, and stringent response causes down-regulation of VC2063 to VC2065, indicating that they regulate the same function while not including the same genes. If these genes are considered overlapping, the number of genes involved in energy metabolism and protein synthesis/fate is further enriched, to ≈44% of all down-regulated genes (Table 1).
Fig. 2.
Down-regulated genes in common in CgtA depletion and stringent response induced cells. (A) Complete set of down-regulated genes. (B) Genes involved in protein synthesis, protein fate, and energy metabolism. Open, genes down-regulated because of CgtA depletion; shaded, genes down-regulated because of treatment with serine hydroxamate; hatched, overlap between these gene sets.
CgtA Depletion Causes an Increase in Intracellular Concentration of ppGpp.
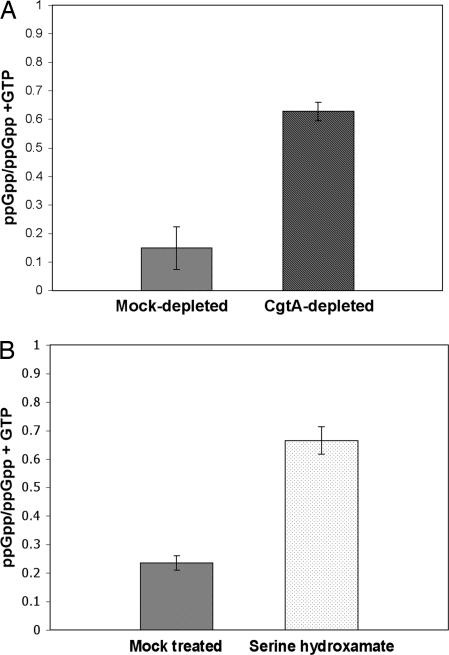
Transcriptional profiling suggested that a stringent response-like effect was induced by CgtA depletion. We monitored production of ppGpp during depletion of CgtA using strain DR209/pDR291 [ΔcgtA/PBAD::cgtA] to determine whether this was the case. CgtA was depleted for 20 min after which cells were harvested and ppGpp identified by thin-layer chromatography. After CgtA depletion, the percentage of ppGpp in the total guanine nucleotide pool increased from 14.7% to 62.7% (Fig. 3A). Addition of serine hydroxamate caused an increase in ppGpp concentration from 23.5% to 66.5% (Fig. 3B). The rise in ppGpp levels due to depletion of CgtA and addition of serine hydroxamate are very similar and occur on a similar time scale. After 40 and 60 min, ppGpp levels remained about the same as at 20 min (data not shown). The increased production of ppGpp is consistent with down-regulation of protein synthesis genes and cessation of growth, suggesting the reason for CgtA depletion and serine hydroxamate addition affecting growth rate.
Fig. 3.
CgtA depletion causes increased ppGpp concentration. (A) Strain DR209/pDR291 [ΔcgtA/ PBAD::cgtA] was arabinose-depleted for 20 min, then cells were harvested and analyzed for nucleotide content by TLC. Mock-depleted cultures were cells washed and reinoculated into LB/0.1% arabinose. The ppGpp concentration is represented as the percentage of ppGpp of the amount of GTP and ppGpp combined. (B) Strain N16961Sm treated with 20 mM serine hydroxamate or an equal volume of dH2O. The ppGpp concentration was determined as in A.
Essentiality of cgtA Depends on relA.
CgtA appears to be necessary to maintain low ppGpp levels in rich media. Because activation of RelA increases ppGpp concentration, we tested whether CgtA expression is necessary in a strain containing an in-frame relA deletion. We were able to construct a clean deletion of cgtA in strain DR200 [ΔrelA], and this strain, DR214 [ΔrelAΔcgtA], grew normally (Fig. 1E). This showed that cgtA is only essential if RelA is functional given that CgtA depletion in relA+ cells produced growth inhibition (see DR209/pDR291 above) (Fig. 1B).
Interactions Between RelA, SpoT, and CgtA Proteins.
Wout et al. (2004) showed that CgtA interacts with SpoT in E. coli, initially by copurifying SpoT with tagged CgtA, and confirmed the interaction using the yeast two-hybrid system. To test whether V. cholerae CgtA and SpoT interact, we used yeast two-hybrid analysis testing CgtA for interactions with SpoT and RelA, as well as CgtA–CgtA interaction (Table 2). Consistent with the data from E. coli, CgtA showed an interaction with SpoT. There was also self-interaction, consistent with the data showing CgtA–SpoT interaction in E. coli and also consistent with structural data showing that CgtA is found as a dimer in B. subtilis and Thermus thermophilus (32, 33). CgtA did not interact with RelA, despite significant sequence similarity between RelA and SpoT in V. cholerae (32% identity/51% similarity). This result suggests that CgtA–SpoT interaction may play a role in SpoT activity. CgtA depletion leads to increased ppGpp concentration, and SpoT is a ppGpp hydrolase, suggesting that the CgtA interaction may be important for SpoT hydrolytic activity. Thus, the likely essential function of CgtA in V. cholerae is to interact with SpoT to regulate ppGpp hydrolysis.
Table 2.
Yeast two-hybrid interactions
| BD | AD | β-Galactosidase units | SE |
|---|---|---|---|
| GAL-BD | RelA | 0 | 0 |
| GAL-BD | CgtA | 1.15 | 0.06 |
| GAL-BD | SpoT | 0.34 | 0.26 |
| CgtA | GAL-AD | 0 | 0 |
| CgtA | RelA | 0.25 | 0.25 |
| CgtA | CgtA | 2.84 | 0.09 |
| CgtA | SpoT | 11.90 | 0.13 |
SE, standard error.
Essentiality of CgtA and SpoT Protein Expression.
If the essential CgtA function is its interaction with SpoT, this suggests that SpoT-depletion phenotypes and transcriptional profiles should be similar to what is seen during CgtA depletion. We constructed a SpoT-depletion strain in the same manner as the CgtA-depletion strain, an in-frame deletion on the chromosome with a plasmid expressing SpoT, DR210/pDR308 [ΔspoT/PBAD::spoT]. We were unable to construct an in-frame deletion of spoT in a wild-type N16961 strain, suggesting that expression of SpoT is essential. Interestingly, the SpoT-depletion strain, DR210/pDR308, did not show a growth defect from removing arabinose from the growth media (Fig. 1 C and D), despite the fact that SpoT is necessary for ppGpp hydrolysis and is required for growth in a relA+ background in E. coli (8). Viability of strain DR210/pDR308 without induction could be due to leaky expression from the PBAD promoter producing enough SpoT to hydrolyze ppGpp.
To address the question of whether spoT is essential in V. cholerae, we attempted to cure the ΔspoT strain DR210/pDR308 of the SpoT expression plasmid through plasmid incompatibility. The plasmid pRK2013 is incompatible with ColE1-derived plasmids and carries a kanamycin-resistance cassette (34). The SpoT expression plasmid pDR308 is a ColE1-derived plasmid that provides ampicillin resistance and was shown to be incompatible with pRK2013 in SpoT+ cells (data not shown). Plasmid pRK2013 was introduced into ΔspoT strain DR210/pDR308 by conjugation, and transconjugates were isolated on LB with streptomycin and kanamycin in the absence of ampicillin. We monitored the continued presence of pDR308 in pRK2013 transconjugates in the absence of ampicillin by scoring ampicillin resistance. However, we were unable to obtain any ampicillin-sensitive colonies, indicating that pDR308 could not be lost and that spoT is essential in V. cholerae. A V. cholerae ΔrelAΔspoT strain was viable (Fig. 1F), indicating that spoT is not essential in a ΔrelA background and only essential if relA is expressed.
Discussion
There are now hundreds of bacterial genome sequences available, and one constant in these genomes is the large number of genes of unknown function; typically, one-third to one-half identified of all predicted ORFs have no known function. Determining the role of these genes is an important emerging area of research. In some cases sequence information can aid in predicting function accurately, but in many cases sequence information is not enough. The sequence of a predicted gene product might predict general aspects of the product's biochemistry (e.g., nucleotide hydrolysis, protease activity, and kinase activity), but this provides no direct information on its function inside intact cells. Postgenomic methods such as transcriptional profiling of cells depleted in the expression of an essential gene product are potentially powerful ways of identifying other biochemical pathways that might be involved in the essential gene product's cellular function (35). Changes in gene expression identified by transcriptional profiling that occur after depletion of an essential gene product indicate how bacteria respond to loss of a specific function. Transcriptional profiles may identify genes that are coregulated with the gene of interest and thus may contribute to the same functional pathway. Alternatively, stress responses that are induced by the depletion of a specific gene product might provide evidence for a block in a particular aspect of biogenesis (e.g., a DNA synthesis block would induce the “SOS” response). Thus, expression profiling of cells depleted for an essential gene product may provide a useful method for deducing the function of a gene product that is not easily uncovered by traditional bioinformatic, genetic or biochemical methods.
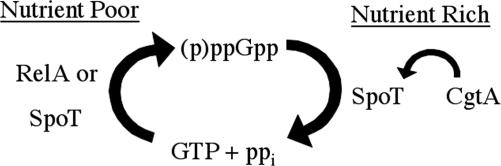
Previous studies had identified a role for CgtA in ribosome biogenesis, but the essential function of this protein remained unknown (25, 26, 28). Based on the transcriptional profile of a V. cholerae CgtA depletion strain, we hypothesized that CgtA functions as a regulator of the nutritional stress response pathway. Expression of CgtA is necessary for V. cholerae growth, and shutting off its expression caused accumulation of the alarmone ppGpp coincident with growth arrest. This suggests a role for CgtA in keeping bacteria out of the nutrient stress response state when sufficient nutrients are present, presumably by keeping ppGpp concentration low. CgtA interacts with SpoT, and presumably this interaction affects the ppGpp hydrolase activity of SpoT (Fig. 4). We do not know the mechanism by which CgtA promotes ppGpp hydrolysis by SpoT, but blocking CgtA expression leads to ppGpp accumulation and cessation of growth. The role of CgtA as a negative regulator of the nutrient stress response is likely to be conserved at least among Gram-negative bacteria, considering that a SpoT–CgtA interaction has also been identified in E. coli, and both genes are very well conserved (24).
Fig. 4.
Simplified model for the essential CgtA activity. Nutritional stress induces RelA or SpoT to generate ppGpp by phosphorylation of GTP. SpoT is required to hydrolyze ppGpp to prevent growth inhibition. CgtA is required to maintain normal SpoT ppGpp hydrolysis activity.
Transcriptional profiling of the CgtA-depleted cells was used to identify changes in gene expression while cells were still viable. These changes included down-regulation of components of the translation apparatus and other changes typical of stringent response profiles seen in other bacteria (9, 10). CgtA-depleted cells display a very high intracellular ppGpp concentration, indicative of stringent response. The enzyme RelA is predominantly responsible for synthesis of ppGpp during normal growth and especially during nutrient starvation. Neither cgtA nor spoT is essential in a ΔrelA background, suggesting that CgtA is necessary to keep cells out of a stringent response state through regulation of ppGpp levels. CgtA and SpoT interact, suggesting that the essential function of CgtA is to regulate the SpoT ppGpp hydrolase function to keep ppGpp levels low when in rich nutrient environments. Without CgtA, SpoT does not hydrolyze ppGpp efficiently, causing cells to enter a nutrient-starvation stress response and preventing growth.
RelA and SpoT are regulators of nutritional stress response by their control of intracellular ppGpp concentration. Both RelA and SpoT synthesize (p)ppGpp by phosphorylating GTP when activated by stress caused by low nutrient levels, but they are activated by different low-nutrient signals. In addition, SpoT also has hydrolytic activity, converting ppGpp to GTP and inorganic phosphate during logarithmic growth, keeping ppGpp concentration low and keeping cells from entering the stress response. In V. cholerae we found that both CgtA and SpoT are essential for normal logarithmic growth in a relA+ background, but both are dispensable in a ΔrelA background, suggesting that CgtA plays a role in regulating SpoT activity in a RelA-dependent fashion. It is possible that CgtA directs which SpoT function, (p)ppGpp synthesis or ppGpp hydrolysis, is active. Functional RelA will produce a basal level of ppGpp that will accumulate over time if not hydrolyzed, explaining the essentiality of both cgtA and spoT in this background. However, in a ΔrelA background, the cgtA gene can be knocked out and the strain grows normally, suggesting that the levels of SpoT (p)ppGpp synthetic activity is not that high in a nutrient-rich environment. It is not known what signal activates SpoT (p)ppGpp synthetic and hydrolytic activities, but it seems likely that CgtA is involved, based on the data presented here. If CgtA activity is not present, SpoT might be nonfunctional, leading to an accumulation of (p)ppGpp due to RelA action and the loss of SpoT hydrolase activity. In Gram-positive bacteria, RelA and SpoT functions are generally combined in one protein. The B. subtilis Obg homolog has not been shown to interact with the RelA/SpoT protein, but it does interact with several regulators of the general stress sigma factor, σB, and is required for the general stress response, suggesting that this may be a common role in addition to its role in ribosome biogenesis (36).
It has been shown that purified CgtA/Obg protein from several organisms has in vitro GTPase activity, and, interestingly, can also bind ppGpp in addition to GTP and GDP (21, 32, 33, 37, 38). The role of nucleotide-binding and hydrolysis in CgtA/Obg function is unknown, but it has been shown that CgtA interaction with the ribosome depends on its interaction with GTP (25, 26). Presumably, the ability to bind ppGpp is important as well, due to the interaction with SpoT. In vitro assays of the CgtA/Obg GTPase activity suggest that ppGpp may amplify GTP hydrolytic activity, suggesting a possible mechanism for ppGpp to regulate CgtA/Obg function (32). CgtA activity may be regulated by its interaction with nucleotides, with different activities determined by interactions with GTP or ppGpp. It is possible that CgtA amplifies ppGpp signaling through its interaction with SpoT. CgtA–ppGpp may prevent SpoT hydrolysis or switch SpoT function toward ppGpp synthesis, further increasing intracellular ppGpp concentration. In vitro biochemical studies may provide a direct approach to understanding how CgtA affects SpoT synthetic and hydrolase activities.
Postgenomic methods such as comprehensive two-hybrid analysis have been previously used to determine a significant fraction of the interactome in S. cerevisiae (39, 40). These studies provided data useful for suggesting functions of previously uncharacterized essential genes. Our analysis of a V. cholerae cgtA deletion strain provides a proof of principle that transcriptional profiling is another powerful postgenomic method for systematically characterizing essential genes of unknown function. Unlike two-hybrid analysis, transcriptional profiling of mutants depleted in an essential gene product does not provide direct evidence for an interaction that may be important to the essential gene product's function. However, as demonstrated here, it can provide a simply way to understanding the function of a gene product in intact cells, particularly if the loss of that function produces a “signature” change in the pattern of global gene expression.
Materials and Methods
Bacterial Strains and Growth Conditions.
All V. cholerae strains used in this study were derivatives of the O1 El tor strain N16961Sm (41). Strain NJ267 [N16961Sm, PBAD::cgtA] is described in ref. 15. Plasmid pDR291 was constructed by introducing a PCR fragment of the complete ORF of cgtA and the native Shine–Dalgarno box into the EcoRI and PstI sites on plasmid pBAD18-kan (42). Plasmid pDR299 was constructed by introducing a PCR fragment of the complete ORF of spoT and the native Shine–Dalgarno box into the NheI and PstI sites on plasmid pBAD18-kan (42). Plasmid pDR308 was constructed by introducing a PCR fragment of the complete ORF of spoT into the NheI and PstI sites on plasmid pBAD24 (42). The method for constructing deletion mutants was that of Skorupski and Taylor (43). Strain DR209 was constructed by making an in-frame deletion of cgtA using strain N16961Sm/pDR291 while maintained on 0.1% arabinose. Strain DR210 was constructed by making an in-frame deletion of spoT in strain N16961Sm/pDR299 while maintained on 0.1% arabinose. Strain N16961ΔrelAΔspoT was constructed by first making an in-frame deletion in strain N16961Sm to produce strain DR200, then making an in-frame deletion of spoT in DR200. V. cholerae cultures were grown at 37°C in Luria broth (LB) supplemented with streptomycin (100 μg/ml), kanamycin (50 μg/ml), ampicillin (50 μg/ml), or arabinose (0.1%) as indicated.
Transcriptional Profiling Experiments.
A whole-genome spotted microarray containing full-length ORFs derived from strain N16961 was hybridized with labeled cDNA as described in refs. 44 and 45. Fifteen microliters of overnight cultures grown in LB supplemented with streptomycin, kanamycin, and arabinose were inoculated into 2 ml of LB/0.1% arabinose. Cultures were grown at 37°C with shaking to an OD600 of 0.3. Cells were harvested, washed twice in LB, and split into two 1-ml cultures. One culture was grown in LB/0.1% arabinose, whereas the other culture was grown in LB only. Both cultures were grown for an additional 20 min. For stringent response induction, serine hydroxamate was added to 20 μM final concentration, and cultures grown 20 min after splitting. Cells were then subjected to centrifugation and resuspended in TRIzol reagent (GIBCO-BRL). Preparation of fluorescently labeled cDNA, hybridization, microarray slide scanning, and data analysis are described in ref. 45. Genepix Pro 4 (Molecular Devices) software was used to analyze microarrays, and Resolver 6.0 (Rosetta Biosoftware) was used to analyze gene expression data. Expression data that produced a P value of <0.05 were considered significant.
ppGpp Assays.
Cultures were grown to mid-log phase at 37°C with shaking in LB supplemented with 0.1% arabinose and 100 μCi/ml H332PO4 (1 Ci = 37 GBq). Depletion studies were performed as described for the transcriptional profiling experiments. Cultures were harvested at the indicated time points and extracted with formic acid on ice. After three freeze/thaw cycles, cell debris was removed by centrifugation, and samples were spotted on polyethyleneimine cellulose F TLC plates. The plates were developed in 1.5 M KH2PO4 (pH 3.5) buffer, and nucleotide was visualized with a phosphorimager. ImageJ software (http://rsb.info.nih.gov/ij) was used to quantify nucleotide spots. The concentration of ppGpp was determined by the percentage of ppGpp versus total guanine nucleotide.
Yeast Two-Hybrid Analysis.
The Matchmaker (Clontech) yeast two-hybrid system was used. Full-length cgtA, spoT, and relA were cloned into the expression vectors pGBKT7 (PADH::GAL4-BD) or pGADT7 (PADH::GAL4-AD) as indicated in Table 2. Combinations of plasmids were introduced into reporter strain AH109. β-Galactosidase assays were performed by using a yeast β-galactosidase assay kit according to the suggested protocol (Pierce). Briefly, yeast strains were grown to mid-log phase (OD600 = 0.5–1.0), and 350 ml of cell culture was added to 350 ml of 1:1 mixture of Y-PER yeast protein extraction reagent and β-galactosidase assay buffer and incubated at 37°C. Reactions were stopped by addition of 300 ml of stop solution, and the A420 of reactions were then determined. β-Galactosidase units were determined by the formula (1,000 × A420)/(t × 0.35 × OD600), where t is the time in minutes.
Supplementary Material
Acknowledgments
We thank Reddy Gali and Paul Grosu (Bauer Center for Genomics Research, Harvard University) for help in the microarray analyses, Su Chiang for comments on the manuscript, and Renee Bina for strain construction. D.M.R. was supported by an Ellison Medical Foundation Fellowship of the Life Sciences Research Foundation. This work was supported by National Institutes of Health Grants AI026289 and AI40053.
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus repository (accession no. GSE6616).
This article contains supporting information online at www.pnas.org/cgi/content/full/0611650104/DC1.
References
- 1.Cashel M, Gentry D, Hernandez VJ, Vinella D. The Stringent Response. Washington, DC: Am Soc Microbiol; 1996. [Google Scholar]
- 2.Spira B, Silberstein N, Yagil E. J Bacteriol. 1995;177:4053–4058. doi: 10.1128/jb.177.14.4053-4058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villadsen IS, Michelsen O. J Bacteriol. 1977;130:136–143. doi: 10.1128/jb.130.1.136-143.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray KD, Bremer H. J Mol Biol. 1996;259:41–57. doi: 10.1006/jmbi.1996.0300. [DOI] [PubMed] [Google Scholar]
- 5.Battesti A, Bouveret E. Mol Microbiol. 2006;62:1048–1063. doi: 10.1111/j.1365-2958.2006.05442.x. [DOI] [PubMed] [Google Scholar]
- 6.Seyfzadeh M, Keener J, Nomura M. Proc Natl Acad Sci USA. 1993;90:11004–11008. doi: 10.1073/pnas.90.23.11004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sy J. J Biol Chem. 1980;255:10056–10059. [PubMed] [Google Scholar]
- 8.Xiao H, Kalman M, Ikehara K, Zemel S, Glaser G, Cashel M. J Biol Chem. 1991;266:5980–5990. [PubMed] [Google Scholar]
- 9.Eymann C, Homuth G, Scharf C, Hecker M. J Bacteriol. 2002;184:2500–2520. doi: 10.1128/JB.184.9.2500-2520.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang DE, Smalley DJ, Conway T. Mol Microbiol. 2002;45:289–306. doi: 10.1046/j.1365-2958.2002.03001.x. [DOI] [PubMed] [Google Scholar]
- 11.Brown ED. Biochem Cell Biol. 2005;83:738–746. doi: 10.1139/o05-162. [DOI] [PubMed] [Google Scholar]
- 12.Caldon CE, March PE. Curr Opin Microbiol. 2003;6:135–139. doi: 10.1016/s1369-5274(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 13.Arigoni F, Talabot F, Peitsch M, Edgerton MD, Meldrum E, Allet E, Fish R, Jamotte T, Curchod ML, Loferer H. Nat Biotechnol. 1998;16:851–856. doi: 10.1038/nbt0998-851. [DOI] [PubMed] [Google Scholar]
- 14.Trach K, Hoch JA. J Bacteriol. 1989;171:1362–1371. doi: 10.1128/jb.171.3.1362-1371.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Judson N, Mekalanos JJ. Nat Biotechnol. 2000;18:740–745. doi: 10.1038/77305. [DOI] [PubMed] [Google Scholar]
- 16.Maddock J, Bhatt A, Koch M, Skidmore J. J Bacteriol. 1997;179:6426–6431. doi: 10.1128/jb.179.20.6426-6431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sikora AE, Zielke R, Datta K, Maddock JR. J Bacteriol. 2006;188:1205–1210. doi: 10.1128/JB.188.3.1205-1210.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okamoto S, Ochi K. Mol Microbiol. 1998;30:107–119. doi: 10.1046/j.1365-2958.1998.01042.x. [DOI] [PubMed] [Google Scholar]
- 19.Kok J, Trach KA, Hoch JA. J Bacteriol. 1994;176:7155–7160. doi: 10.1128/jb.176.23.7155-7160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidwans SJ, Ireton K, Grossman AD. J Bacteriol. 1995;177:3308–3311. doi: 10.1128/jb.177.11.3308-3311.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin B, Covalle KL, Maddock JR. J Bacteriol. 1999;181:5825–5832. doi: 10.1128/jb.181.18.5825-5832.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foti JJ, Schienda J, Sutera VA, Jr, Lovett ST. Mol Cell. 2005;17:549–560. doi: 10.1016/j.molcel.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Scott JM, Ju J, Mitchell T, Haldenwang WG. J Bacteriol. 2000;182:2771–2777. doi: 10.1128/jb.182.10.2771-2777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wout P, Pu K, Sullivan SM, Reese V, Zhou S, Lin B, Maddock JR. J Bacteriol. 2004;186:5249–5257. doi: 10.1128/JB.186.16.5249-5257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato A, Kobayashi G, Hayashi H, Yoshida H, Wada A, Maeda M, Hiraga S, Takeyasu K, Wada C. Genes Cells. 2005;10:393–408. doi: 10.1111/j.1365-2443.2005.00851.x. [DOI] [PubMed] [Google Scholar]
- 26.Jiang M, Datta K, Walker A, Strahler J, Bagamasbad P, Andrews PC, Maddock JR. J Bacteriol. 2006;188:6757–6770. doi: 10.1128/JB.00444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin B, Thayer DA, Maddock JR. J Bacteriol. 2004;186:481–489. doi: 10.1128/JB.186.2.481-489.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Datta K, Skidmore JM, Pu K, Maddock JR. Mol Microbiol. 2004;54:1379–1392. doi: 10.1111/j.1365-2958.2004.04354.x. [DOI] [PubMed] [Google Scholar]
- 29.Datta K, Fuentes JL, Maddock JR. Mol Biol Cell. 2005;16:954–963. doi: 10.1091/mbc.E04-07-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi G, Moriya S, Wada C. Mol Microbiol. 2001;41:1037–1051. doi: 10.1046/j.1365-2958.2001.02574.x. [DOI] [PubMed] [Google Scholar]
- 31.Tosa T, Pizer LI. J Bacteriol. 1971;106:972–982. doi: 10.1128/jb.106.3.972-982.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buglino J, Shen V, Hakimian P, Lima CD. Structure (London) 2002;10:1581–1592. doi: 10.1016/s0969-2126(02)00882-1. [DOI] [PubMed] [Google Scholar]
- 33.Kukimoto-Niino M, Murayama K, Inoue M, Terada T, Tame JR, Kuramitsu S, Shirouzu M, Yokoyama S. J Mol Biol. 2004;337:761–770. doi: 10.1016/j.jmb.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 34.Figurski DH, Helinski DR. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mnaimneh S, Davierwala AP, Haynes J, Moffat J, Peng WT, Zhang W, Yang X, Pootoolal J, Chua G, Lopez A, et al. Cell. 2004;118:31–44. doi: 10.1016/j.cell.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Scott JM, Haldenwang WG. J Bacteriol. 1999;181:4653–4660. doi: 10.1128/jb.181.15.4653-4660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welsh KM, Trach KA, Folger C, Hoch JA. J Bacteriol. 1994;176:7161–7168. doi: 10.1128/jb.176.23.7161-7168.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sikora AE, Datta K, Maddock JR. Biochem Biophys Res Commun. 2006;339:1165–1170. doi: 10.1016/j.bbrc.2005.11.129. [DOI] [PubMed] [Google Scholar]
- 39.Uetz P, Giot L, Cagney G, Mansfield TA, Judson RS, Knight JR, Lockshon D, Narayan V, Srinivasan M, Pochart P, et al. Nature. 2000;403:623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- 40.Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O'Shea EK. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 41.Fullner KJ, Mekalanos JJ. Infect Immun. 1999;67:1393–1404. doi: 10.1128/iai.67.3.1393-1404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guzman LM, Belin D, Carson MJ, Beckwith J. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skorupski K, Taylor RK. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- 44.Dziejman M, Balon E, Boyd D, Fraser CM, Heidelberg JF, Mekalanos JJ. Proc Natl Acad Sci USA. 2002;99:1556–1561. doi: 10.1073/pnas.042667999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. Proc Natl Acad Sci USA. 2002;99:3129–3134. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.