Abstract
The neural mechanisms underlying the cellular and behavioral responses to antidepressants are not yet known. Up-regulation of growth factors and adult neurogenesis suggest a role for one or more of these factors in the action of antidepressants. One candidate of interest is vascular endothelial growth factor (VEGF), which was initially characterized for its role in angiogenesis, but also exerts direct mitogenic effects on neural progenitors in vitro. Results of this study demonstrate that VEGF is induced by multiple classes of antidepressants at time points consistent with the induction of cell proliferation and therapeutic action of these treatments. We find that VEGF signaling through the Flk-1 receptor is required for antidepressant-induced cell proliferation. We also show that VEGF-Flk-1 signaling is required and sufficient for behavioral responses in two chronic and two subchronic antidepressant models. Taken together, these studies identify VEGF and VEGF-Flk-1 signaling as mediators of antidepressant actions and potential targets for therapeutic intervention.
Keywords: desipramine, electroconvulsive shock, endothelial cells, fluoxetine, neurogenesis
Depression is a pervasive and debilitating disease affecting as many as one in five Americans (1). The neural mechanisms underlying the pathophysiology of depression and the actions of antidepressants remain unknown. A leading hypothesis of depression suggests that neurotrophic factors and adult neurogenesis play critical roles in mediating the behavioral responses to antidepressants (2, 3). Previous studies have demonstrated that the trophic factor BDNF is regulated by antidepressants (4) and exerts an antidepressant-like effect in short-term behavioral models of depression (5). However, BDNF does not underlie the neurogenic actions of antidepressants (6), and a direct link between growth factor signaling and the behavioral actions of antidepressants has not been identified.
In the current study, we demonstrate that vascular endothelial growth factor (VEGF) is an essential mediator of both the neurogenic and behavioral actions of multiple classes of antidepressants. Originally described as an endothelial cell mitogen and survival factor (7), recent studies demonstrate neurogenic and neuroprotective effects of VEGF (8–10), which influence hippocampus-dependent processes, such as learning and memory (11). VEGF also modulates synaptic transmission (12), suggesting that the effects of this factor are multifaceted. VEGF signals through two high-affinity receptor tyrosine kinases (Flk-1 and Flt-1) (13, 14). Flk-1 signaling is most intriguing because this receptor is expressed in the hippocampus by endothelial cells and neural progenitors (15, 16) and has been shown to mediate the mitogenic effects of VEGF in vitro (8).
Using a combination of approaches, including expression studies, pharmacological inhibitors, and an infusion of recombinant VEGF, in both cellular and behavioral assays of antidepressant activity, we identify VEGF as a critical mediator of antidepressant treatment and a therapeutic target for depression.
Results
Multiple Classes of Antidepressants Increase Hippocampal VEGF Expression.
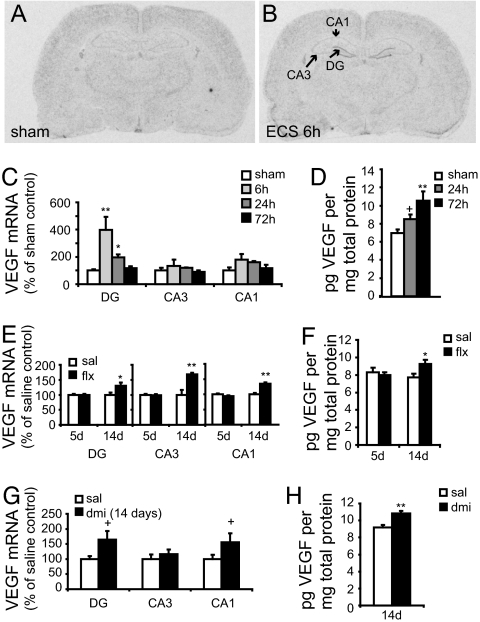
Previous microarray work from our laboratory has shown that VEGF mRNA is up-regulated in the hippocampus 2 h after electroconvulsive seizure (ECS) (17), a potent stimulator of neurogenesis and a highly efficacious antidepressant. Here we demonstrate that ECS increases the expression of VEGF mRNA in the granule cell layer (GCL) of the hippocampus at 6 (Fig. 1 A and B) and 24 h after ECS, returning to baseline levels by 72 h (Fig. 1C). Analysis of VEGF immunoreactivity, determined by ELISA of hippocampus homogenates, demonstrates a strong trend for an increase after 24 h and a significant increase 72 h post-ECS (Fig. 1D).
Fig. 1.
Hippocampal VEGF expression is increased by fluoxetine, desipramine, and ECS. VEGF mRNA expression was determined by ISH analysis. (A and B) Representative autoradiograms illustrate induction of VEGF mRNA in the dentate gyrus granule cell layer (DG), CA3 and CA1 pyramidal cell layers of the hippocampus 6 h after sham (A) or ECS treatment (B). The graphs show optical density values as a percent of sham-handled controls. (C) The time course of VEGF mRNA induction was quantified in rats receiving a single ECS [DG, F(3, 12) = 7.612, P < 0.01; CA3, F(3, 12) = 0.608, n.s.; CA1, F(3, 12) = 1.970, P > 0.05]. (D) Quantification of VEGF protein levels in whole hippocampus homogenates, determined by ELISA after a single ECS [F(2, 14) = 6.965, P < 0.01]. (E) VEGF mRNA expression was quantified following 5 or 14 days of fluoxetine treatment [FLX-14d: DG, F(1, 6) = 6.211, P < 0.05; CA3, F(1, 6) = 15.272, P < 0.01; CA1, F(1, 6) = 32.514, P < 0.01; FLX-5d: DG, F(1, 8) = 0.001, n.s.; CA3, F(1, 8) = 0.093, n.s.; CA1, F(1, 8) = 2.206, n.s.]. (G) VEGF mRNA induction by 14 days of desipramine [DMI-14d: DG, F(1, 10) = 4.261, P = 0.06; CA3, F(1, 10) = 0.532, n.s.; CA1, F(1, 10) = 2.901, P = 0.1]. VEGF protein levels after fluoxetine (F) or desipramine (H) treatment [FLX-14d, F(1, 8) = 5.247, P ≤ 0.05; FLX-5d, F(1, 8) = 0.231, n.s.; DMI-14d, F(1, 10) = 16.042, P < 0.01; n = 4–7 per group in each experiment]. ∗, P ≤ 0.05; ∗∗, P ≤ 0.01; +, P ≤ 0.1; n.s., P > 0.1.
Regulation of VEGF by other classes of antidepressants, including specific serotonin (5-HT) or norepinephrine reuptake inhibitors, has not been shown. To address this question, rats were treated with fluoxetine or desipramine for 14 days, at which time the neurogenic effects of antidepressants are observed (18). Both antidepressants increased VEGF mRNA in the GCL compared with controls (Fig. 1 E and G) and increased VEGF protein in hippocampal homogenates (Fig. 1 F and H). Five days of fluoxetine treatment, a time point at which no proliferative effects have been seen, had no effect on VEGF mRNA or protein (Fig. 1 E and F).
VEGF Is Required for Antidepressant-Induced Cell Proliferation in the SGZ.
One of the most reproducible findings in antidepressant research is that different classes of antidepressants increase hippocampal cell proliferation and neurogenesis (18–20), and this effect is thought to contribute, in part, to the actions of antidepressants (3, 18). Because VEGF is a mitogen for both endothelial cells and neural progenitors, the induction of VEGF by antidepressants could lead to proliferation of multiple cell types in the hippocampus. To address this issue, animals were injected with the mitotic marker BrdU, and immunohistochemical analysis of BrdU (Fig. 2A) and colabeling with the endothelial cell marker RECA were determined (Fig. 2B). The total number of BrdU-positive cells in the SGZ was counted to confirm that 14 days of fluoxetine (Fig. 2C) or ECS (Fig. 2D) increases cell proliferation, including neuronal precursors (18, 19). The lower baseline in the fluoxetine relative to ECS experiment could be due to the age (1 month older) and/or increased stress of daily injections received by saline animals, both of which reduce cell proliferation (21, 22). Results demonstrate that chronic fluoxetine administration significantly increases endothelial cell proliferation in the subgranular zone (SGZ) (Fig. 2E). In addition, we confirm that ECS significantly increases the number of proliferating endothelial cells in the SGZ (23) (Fig. 2F). Taken together, these results indicate that the time course of antidepressant-induced VEGF expression (Fig. 1) correlates with the induction of proliferation for both endothelial cells and neural progenitors. The time course after a single ECS is notable because there is a correlation with the time lag (72 h) for VEGF protein and cell proliferation.
Fig. 2.
Antidepressant-induced cell proliferation in the SGZ is mediated by VEGF-Flk-1 signaling. Immunohistochemical visualization of BrdU was followed by confocal analysis and z-sectioning to identify newborn endothelial cells. (A) Representative coronal section through the dentate gyrus with immunoperoxidase labeled BrdU (black arrowheads). (Scale bar, 50 μm.) (B) Representative colabeled cell in the SGZ labeled expressing both BrdU (red) and the endothelial cell marker RECA (green) at 63× magnification. (Scale bar, 10 μm.) (C) Quantification of BrdU-positive cells in the SGZ of the hippocampus after chronic fluoxetine injections (14 days) compared with saline controls [F(1, 8) = 34.129, P < 0.01]. (D) BrdU-positive cells 24 or 72 h after a single ECS compared with sham-handled controls [F(2, 9) = 24.575, P < 0.01]. Quantification of BrdU/RECA-positive cells following fluoxetine treatment (E) [F(1, 8) = 18.289, P < 0.01] or ECS (F) [F(2, 9) = 6.358, P < 0.05; n = 4–5 per group in each experiment]. ∗, P ≤ 0.05; ∗∗, P ≤ 0.01; +, P ≤ 0.1; n.s., P > 0.1.
To directly examine whether VEGF signaling through the Flk-1 receptor is required for the induction of cell proliferation by antidepressants, we used two selective and potent inhibitors, SU5416 and SU1498 (24, 25). ECS causes a rapid (3-day; Fig. 2C) and robust induction of proliferation compared with chemical antidepressants, making it a preferable test system for the initial analysis of Flk-1 inhibitors. SU5416 or vehicle was delivered intracerebroventricularly (i.c.v.) 30 min before one ECS, followed by three daily infusions. SU5416 had no effect on basal cell proliferation, but completely blocked the ability of ECS to induce SGZ proliferation (Fig. 3A). SU1498 also attenuated ECS-induced proliferation in the SGZ (Fig. 3A). In contrast, infusion of K252a, a Trk-receptor antagonist (26), had no effect on ECS induction of SGZ proliferation (Fig. 3A). Interestingly, there was a trend for attenuation of basal cell proliferation by K252a.
Fig. 3.
VEGF-Flk-1 signaling is required for the induction of cell proliferation by antidepressants. (A) The graph represents the number of BrdU-positive cells in the SGZ of rats receiving sham or ECS and i.c.v. infusions of vehicle or SU5416 [main effect ECS: F(1, 22) = 13.376; P < 0.01; main effect SU5416: F(1, 22) = 12.629; P < 0.01; ECS × SU5416: F(1, 22) = 6.644; P < 0.05; n = 6–8 per group]. The same experiment was performed by using SU1498 [main effect ECS: F (1, 23) = 21.249; P < 0.01; SU1498 F (1, 23) = 0.316, n.s.; ECS × SU1498: F(1, 23) = 3.123; P = 0.09; n = 5–8 per group] and with a kinase inhibitor that does not block Flk-1 signaling, K252a [main effect ECS: F(1, 10) = 28.038, P < 0.01; K252a: F(1, 10) = 1.998; n.s.; K252a × ECS: F(1, 10) = 0.196; n.s.; n = 3–4 per group]. (B) Representative image of a cell in the SGZ coexpressing BrdU (red) and early neuronal marker TUJ-1 (green) (white arrow) and BrdU-positive, TUJ-1-negative cells (arrowhead). (Scale bar, 10 μm.) (C) Percentage of 40 BrdU-positive cells that coexpress TUJ-1 (n.s.; n = 5 per group). (D) Graph illustrating the number of BrdU-positive cells counted per SGZ in animals treated for 14 days with fluoxetine or desipramine and SU5416 or vehicle infusions on days 8, 10, 12, and 14 [main effect AD: F(2, 30) = 2.94, P = 0.06; main effect SU5416: F(1, 30) = 16.277, P < 0.01; AD × SU5416: F(2, 30) = 1.521, n.s.; n = 5–11 per group]. ∗, P ≤ 0.05; ∗∗, P ≤ 0.01; +, P ≤ 0.1; n.s., P > 0.1.
To be certain that i.c.v. cannulations and infusions did not disrupt neurogenesis in the hippocampus, we quantified the percentage of BrdU-labeled cells that coexpressed the early neuronal marker TUJ-1 (Fig. 3B) in animals receiving SU5416 and ECS treatments compared with controls; ≈7–10% of BrdU-positive cells were TUJ-1 positive (Fig. 3C) 2 h after BrdU injection, consistent with previous reports in naïve animals (16). No differences were observed across treatment groups, demonstrating that ECS increases the number of newborn neurons in the GCL, and SU5416 infusions block proliferation of neuronal progenitors in addition to other cell types.
To test whether VEGF-Flk-1 signaling is required for chemical antidepressant-induced proliferation, animals received 14 days of antidepressant or vehicle injections, and SU5416 or vehicle was infused i.c.v. on alternate days for the last week of treatment (days 8, 10, 12, and 14). This paradigm was chosen to minimize the number of SU5416 infusions and still provide sufficient blockade of Flk-1 signaling during the time period when induction of cell proliferation occurs [i.e., between 7 and 14 days of fluoxetine treatment (18)]. Both fluoxetine and desipramine induced a significant increase in BrdU-positive cells in vehicle-infused controls, and SU5416 administration completely blocked the induction of cell proliferation by both antidepressants. There was no effect on basal proliferation in saline-injected animals (Fig. 3D).
To confirm that VEGF infusions stimulate SGZ cell proliferation (8) and assess the magnitude of this effect quantitatively, recombinant rat VEGF164 or vehicle was administered i.c.v. continuously for 3 days via a s.c. osmotic minipump. Rats were injected with BrdU on day 3 and killed 2 h after injection. There were significantly more BrdU-positive cells in the SGZ of rats receiving VEGF infusions compared with controls [control: 2,133 ± 111, VEGF: 3,642 ± 528 BrdU-positive cells ± SEM; F(1, 6) = 7.815, P < 0.05, t test]. Previous work has shown that this infusion procedure increases BrdU-doublecortin-positive neurons in the GCL (8). We also confirm that the infusion paradigm increases VEGF protein in the hippocampus [control: 11.1 ± 1.2, VEGF: 20.6 ± 2.7 pilogram (pg) VEGF per mg total protein, mean ± SEM; F(1, 6) = 10.329, P < 0.05, t test].
VEGF-Flk-1 Signaling Mediates Behavioral Responses to Chronic Antidepressant Treatment.
Novelty suppressed feeding (NSF) and chronic unpredictable stress (CUS) are two models that require chronic antidepressant treatment to illicit a behavioral response, consistent with the time course for the therapeutic actions of antidepressants. Moreover, induction of hippocampal cytogenesis is required for antidepressant responses in these behavioral models (3, 27). In the current study, we examine whether VEGF-Flk-1 signaling is required for these antidepressant-induced behaviors. For all studies, desipramine was chosen because it produces the most efficacious and reproducible behavioral responses (28–31).
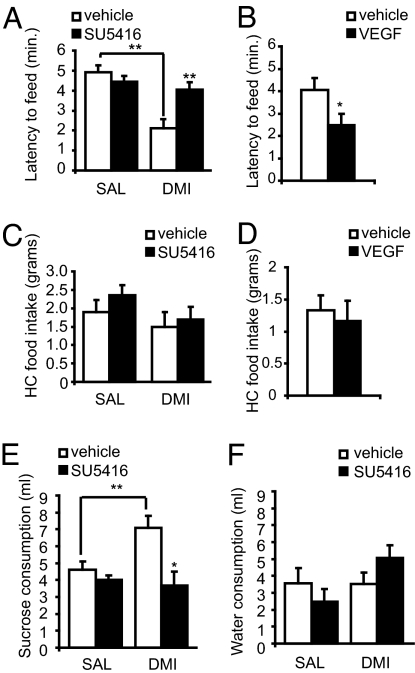
For NSF, rats received 3 weeks of desipramine or saline injections and i.c.v. infusions of SU5416 or vehicle on days 12, 15, 18, and 21. Consistent with earlier studies, desipramine reduced the latency to feed in the center of an open field in vehicle-infused rats (32, 33). Infusions of SU5416 completely blocked the antidepressant effect of desipramine (Fig. 4A). In contrast, continuous i.c.v. infusions of recombinant VEGF (3 weeks) reduced the latency to feed, an antidepressant-like effect (Fig. 4B). There were no effects of treatment on home-cage feeding (Fig. 4 C and D), confirming that there were no general effects on appetite or feeding.
Fig. 4.
VEGF mediates behavioral responses to chronic antidepressant treatment. For NSF, the latency to feed (maximum of 6 min) is quantified in (A) for animals receiving SU5416 or vehicle infusions and injections of saline or desipramine [main effect DMI: F(1, 27) = 20.033, P < 0.01; main effect SU5416: F(1, 27) = 3.9725, P = 0.05), and in (B) for animals infused with recombinant VEGF [F(1, 10) = 4.639, P = 0.05]. Treatments had no effect on home-cage feeding for a 6-min period (C and D) (n = 5–6 per group). For the chronic stress paradigm, a sucrose preference test was performed after 4 weeks of CUS exposure and antidepressant treatment. (E) Graph represents the average sucrose consumption over three 1-h tests [main effect DMI: F(1, 28) = 2.243, P = 0.1; SU5416: F(1, 28) = 7.871, P < 0.01; DMI × SU5416: F(1, 28) = 3.849, P = 0.05]. Water consumption was not significantly affected by treatments (F) (n = 5–11 per group). ∗, P ≤ 0.05; ∗∗, P ≤ 0.01; +, P ≤ 0.1; n.s., P > 0.1.
Another cohort of rats was cannulated and exposed to 4 weeks of CUS [see supporting information (SI) Table 1] and received daily injections of saline or desipramine plus infusions of SU5416 or vehicle as described for NSF. We confirm that desipramine increases sucrose preference, a well established model of anhedonia (31), and demonstrate that SU5416 infusions completely block this effect (Fig. 4E). There was no effect of SU5416 in saline-injected controls (Fig. 4E) and no effects on water consumption during the test (Fig. 4F).
VEGF Also Mediates Subchronic Antidepressant Behavioral Responses.
The learned helplessness (LH) and forced swim test (FST) are well established models that are responsive to multiple classes of antidepressants and thereby serve as valuable predictors of antidepressant activity (29, 30, 32, 33). To test the involvement of VEGF-Flk-1 signaling in these behaviors, a cohort of rats was cannulated and underwent LH training (see Materials and Methods), followed by 6 days of desipramine injections and i.c.v. infusions of SU5416 on the last 3 days. Desipramine decreased the number of escape failures and latency to escape in the active avoidance test, and antidepressants failed to produce a significant effect in SU5416-infused animals (Fig. 5A).
Fig. 5.
VEGF-Flk-1 receptor signaling is required and sufficient for subchronic behavioral responses to desipramine. (A–B) Following LH training, rats received desipramine/saline and infusions of SU5416/vehicle and were tested in LH and FST. Quantification of escape failures and escape latency in (A). [Failures: main effect DMI: F(1, 28) = 13.790, P < 0.01; main effect SU5416: F(1, 28) = 5.062, P < 0.05; DMI × SU5416: F(1, 28) = 1.623, n.s.; Latency: main effect DMI: F(1, 28) = 9.725, P < 0.01; main effect SU5416: F(1, 28) = 3.681, P = 0.06; DMI × SU5416: F(1, 28) = 0.702, n.s.] The FST consisted of one 15-min session, and behaviors were scored by using a sampling technique described in Materials and Methods. (B) Quantification of immobility and climbing behaviors (see Results for swimming) [Immobility: main effect DMI: F(1, 18) = 7.355, P < 0.05; main effect SU5416: F(1, 18) = 0.926, n.s.; DMI × SU5416: F(1, 18) = 11.791, P < 0.01; Climbing: main effect DMI: F(1, 18) = 29.443, P < 0.01; main effect SU5416: F(1, 18) = 4.860, P < 0.05; DMI × SU5416: F(1, 18) = 4.1974, P = 0.05]. Learned helplessness training was followed by continuous i.c.v. infusions of recombinant VEGF164 (10 ng/h). (C) Quantification of escape failures and escape latency from LH test [Failures: F(1, 17) = 4.021, P ≤ 0.05; Latency: F(1, 17) = 3.202, P = 0.09]. (D) Quantification of FST data [Immobility: F(1, 10) = 10.737, P < 0.01; Swim: F(1, 10) = 7.173, P < 0.05; Climb: F(1, 10) = 6.047, P < 0.05; n = 5–6 per group]. ∗, P ≤ 0.05; ∗∗, P ≤ 0.01; +, P ≤ 0.1; n.s., P > 0.1.
In FST, infusions of SU5416 (using the same regimen as described for LH) significantly blocked the antidepressant effect of desipramine on immobility (Fig. 5B). Desipramine also caused a corresponding increase in climbing behavior, consistent with the actions of SNRI antidepressants (34), and infusions of SU5416 reversed this effect (Fig. 5B). There were no significant effects of desipramine on swimming behavior (VEH + SAL: 42 ± 1.9; VEH + DMI: 38.2; n = 5–6 per group), also consistent with previous reports (34).
To determine whether VEGF164 infusions mimic the action of antidepressants in these behavioral paradigms, rats received continuous infusions of VEGF164 or vehicle (i.c.v.) for 3 days between LH training and testing. VEGF decreased the number of escape failures in LH (Fig. 5C) and decreased immobility time in the FST (Fig. 5D), similar to the actions of antidepressant administration (Fig. 5B). There were no effects of infusions of either the Flk-1 inhibitor or VEGF on locomotor activity measured at 5-min intervals over a 30-min period, demonstrating that the actions of these agents in the LH and FST are not due to general effects on ambulatory activity [repeated measures ANOVA: Treatment, F(1, 10) = 0.555, P = 0.47].
Discussion
The results of this study identify VEGF as a key mediator of both the cytogenic and behavioral actions of multiple classes of antidepressants. VEGF is induced by specific serotonin and norepinephrine reuptake inhibitor drugs and ECS in a time-dependent fashion, consistent with the induction of cell proliferation and the therapeutic action of antidepressants (35). Antidepressant induction of VEGF could also block the down-regulation of VEGF in response to stress (40), which could contribute to blockade of stress-inhibition of adult neurogenesis (41). The signaling pathways by which antidepressants induce VEGF expression could involve the cAMP response element binding protein (CREB). Antidepressant treatment increases the function and expression of CREB in the GCL (36), and the VEGF promoter contains a cAMP response element that is induced by receptor-coupled pathways that activate CREB (37).
One of the most interesting findings of this study is that chemical antidepressants increase the number of endothelial cells in the adult hippocampus. The time course for the induction of endothelial cell proliferation is similar to that for the induction of neurogenesis, and the corresponding up-regulation of VEGF could mediate the effects of antidepressant administration on the proliferation of both cell types. 5-HT has been reported to induce proliferation of vascular endothelial cells through activation of 5-HT2 receptors (38), and it is possible that this receptor subtype underlies the actions of fluoxetine. We also confirm that ECS induces endothelial cell proliferation (23), demonstrating that multiple classes of antidepressant produce this effect.
The up-regulation of VEGF expression suggests that VEGF signaling mediates the proliferative actions of antidepressants, and studies were conducted with VEGF-Flk-1 inhibitors to directly test this hypothesis. The inhibitors and doses were chosen based on their specificity in published cell culture studies (8, 24, 25, 39–41). We cannot exclude the possibility that these inhibitors influence targets in addition to Flk-1 [e.g., SU5416 inhibits c-Kit and Ret in other systems (42, 43)]. However, our use of two structurally dissimilar Flk-1 inhibitors strongly supports the involvement of Flk-1. Results demonstrate that Flk-1 inhibitors produce either a complete (SU5416) or near complete (SU1498) blockade of ECS-induced cell proliferation. The difference between these two compounds could be due to minor differences in the properties of these agents (e.g., effective concentration or availability in vivo). Because of the superior efficacy of SU5416, this inhibitor was used to determine whether VEGF-Flk-1 signaling is necessary for the proliferative action of chemical antidepressants. SU5416 completely blocked the effects of both fluoxetine and desipramine, demonstrating that signaling through Flk-1 is required for the actions of these two major classes of antidepressants. This complete blockade of the antidepressant response also indicates that Flk-1 is required for the antidepressant induction of multiple cell types, including neural progenitors and endothelial cells. At the doses chosen, VEGF-Flk-1 inhibitors did not influence basal rates of cell proliferation, demonstrating that blockade of antidepressant responses is not due to a general reduction of cell proliferation. We also confirm that VEGF infusions produce a robust induction of cell proliferation in the hippocampus (8, 9). Infusions of K252a, a potent inhibitor of neurotrophic factor receptors (Trk) (44), did not influence ECS induction of cell proliferation, demonstrating the pharmacological specificity of the VEGF-Flk-1 inhibitors.
VEGF Mediates the Behavioral Response to Antidepressant Treatment.
Although it is difficult to model complex human behaviors in rodents, there are a number of behavioral paradigms that measure antidepressant activity, and in the current study we use four well established models. NSF and CUS tests are responsive to chronic antidepressant treatment (3–4 weeks) and have been linked to alterations in hippocampal cytogenesis (3, 27). We find that VEGF signaling through Flk-1 plays a critical role in mediating responsiveness to desipramine. The results of the current study provide correlative data that VEGF-Flk-1 signaling underlies the neurogenic and behavioral actions of antidepressants, although future studies will be required to demonstrate a causal link.
The escape-directed behaviors in LH and FST are valuable predictors of short-term antidepressant activity (34, 45), and although these actions cannot be explained by induction of neurogenesis, these tests were used to further investigate the effects of VEGF-Flk-1 signaling. Infusion of SU5416 blocks the behavioral action of antidepressants in both of these models, and VEGF infusions mimic the action of an antidepressant. It is interesting that both swimming and climbing, specific serotonin and norepinephrine reuptake inhibitor-responsive behaviors (46), respectively, are increased by VEGF infusions, suggesting that VEGF is a common mediator for these two classes of antidepressants. This possibility is also consistent with the requirement of VEGF signaling in the neurogenic action of both specific serotonin and norepinephrine reuptake inhibitor antidepressants. The mechanisms underlying the actions of VEGF-Flk-1 signaling could include regulation of learning (11) and synaptic activity in the hippocampus (12), effects that could be important for behavioral responses in LH and FST. In this case, we view the neurogenic and behavioral responses to chronic vs. subchronic treatments as two, possibly parallel indices that we have used to identify VEGF signaling as a novel target of antidepressants.
Together, the results of this study establish that VEGF receptor signaling is necessary and sufficient for the neurogenic and behavioral actions of antidepressants, and identify induction of endothelial cell proliferation as an action of chemical antidepressants. It is interesting to speculate that induction of endothelial cell proliferation and even angiogenesis could be important factors for the treatment of some forms of depression that are characterized by vascular abnormalities, particularly in the elderly (47). The results identify VEGF-Flk-1 signaling as a mediator of antidepressants' responses and suggest new targets for the development of therapeutic interventions.
Materials and Methods
Animals.
Male Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 175–200 g were pair-housed and maintained in standard conditions with a 12-h light/dark cycle and ad libitum access to food and water. Animal use and procedures were in accordance with the National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committees.
Antidepressant Treatments.
ECS was administered via ear-clip electrodes by using a pulse generator (Ugo Basile, Comerio, Italy) (60 mA, 0.5-sec duration, 100-Hz frequency) to induce a generalized grand mal seizure lasting for <15 sec. Sham groups were handled identically, but received no shock. Fluoxetine (5 mg/kg; Eli Lilly & Co., Indianapolis, IN), desipramine [7.5 mg/kg, twice a day (b.i.d.)], or saline was injected i.p. once or twice daily. Doses were chosen based on previous studies of the actions of antidepressants (18, 36). For LH and FST studies, desipramine (10 mg/kg, b.i.d., i.p.) was administered for 6 days based on previous work (48).
Surgical Procedures.
Stereotaxic surgeries were performed under ketamine (80 mg/kg, i.m.) and xylazine (6 mg/kg, i.m.) anesthesia. Guide cannula was implanted into lateral ventricle [coordinates from bregma: −0.9 anterior/posterior (AP), −1.5 ml, −3.3 dorsal/ventral (DV) from dura]. Inhibitors were infused in a 1-μl volume i.c.v. through infusion cannula (0.25 μl/min): SU5416 (4 mM; Sigma Chemical, St. Louis, MO), SU1498 (4 mM; Calbiochem, San Diego, CA), and K252a (1 mM; Calbiochem). Recombinant VEGF164 (10 μg/μl; Sigma) was delivered i.c.v. via s.c. microosmotic pump (Durect Corp., Cupertino, CA) at a rate of 1 μl/h for 3 days, resulting in a dose of 240 ng/day based on previous work (8).
VEGF mRNA and Protein Detection.
For ISH, 14-μm-thick coronal brain sections were cut on a cryostat, thaw mounted onto slides, fixed in 4% paraformaldehyde, acetylated, and dehydrated before hybridization. VEGF riboprobe was generated by PCR with gene-specific primers as described (17) and verified by sequencing. One microgram of the PCR product was used to make a radiolabeled riboprobe using a T7-based in vitro translation kit (Megashortscript Kit; Ambion, Austin, TX). Sections were hybridized with the riboprobe (2 × 106 cpm per section) for 18 h at 55°C. Slides were washed, dried, and exposed to Kodak Biomax film (Kodak IBI, New Haven, CT). National Institutes of Health Image Software was used for analysis. VEGF protein was assessed by ELISA kit according to manufacturer's instructions (R&D Systems, Minneapolis, MN). Total protein concentration was determined by BCA (Pierce Chemical, Rockford, IL).
Bromodeoxyuridine (BrdU) Immunohistochemistry.
BrdU (200 mg/kg, i.p.) and rats were perfused with PBS and 10% formalin. Every sixth 40-μm-thick section was processed for immunoperoxidase labeling or fluorescent immunohistochemistry exactly as described (49). For quantification, a modified unbiased stereological procedure was used as described (18). Sections were coded to ensure that analysis was performed by a blind observer. BrdU-positive cells were counted in the SGZ of the hippocampus on a light microscope under ×400 and ×1,000 magnification. Cells were included in SGZ counts if the cell was in or touching the SGZ (the layer of cells on the border of the GCL and hilus). If a cell was more than two cell diameters from the GCL, that cell was excluded. Every sixth section was counted throughout the extent of the hippocampus, and the sum was multiplied by 6 to provide an estimate of the total number of BrdU-positive cells in the entire region. In double-labeling experiments, at least 30 BrdU-positive cells were examined by confocal microscopy and z-sectioning (0.5-μm steps) to determine whether cells were colabeled with RECA, an antigen that is expressed on the luminal surface of endothelial cells (50), or TUJ-1, a marker of immature neurons.
Behavioral Assays.
NSF procedure was performed as described (3, 51, 52). Rats were food-deprived for 24 h and placed in an open field (76.5 cm × 76.5 cm × 40 cm, Plexiglas) with a small amount of food in the center. Animals explored the open field for 6 min, sessions were videotaped from above, and the latency to feed (specifically, the time it took for the animal to approach and take its first bite of food) was recorded in minutes.
CUS Procedure.
Mice were exposed to a sequence of mild and unpredictable stressors b.i.d. for 28 days, and desipramine or saline was injected for the duration of the stress to block the effects of the stressors (31). The stressors used were: cage rotation, light on, light off, isolation, swim stress, cold stress, food or water deprivation, wet bedding, stroboscope, cage tilt, odor exposure, and group housing (see SI Table 1). This sequence of stressors has been shown previously to reduce sucrose drinking compared with nonstressed controls (53), adapted from earlier studies (31, 54). Beginning on day 28, rats were habituated to a 1% (wt/vol) sucrose solution (for 3 days) to prevent neophobia during testing. On day 31, following 4 h of water deprivation, rats were presented with two bottles containing 1% sucrose or water and allowed to drink for 1 h. Sucrose and water intake were recorded, and this test was performed for 3 consecutive days (days 31–33). Sucrose intake was averaged across experiments.
LH was performed as described (48) in custom boxes (MED Associates, St. Albans, VT), in which two compartments with electrified foot-shock floor bars are separated by a retractable door. LH training consisted of 60 inescapable foot shocks (0.8 mA, 15-sec duration, average inner trial interval 45 sec). LH testing, performed on a subsequent day, was a two-way active-avoidance test, consisting of 30 trials (0.65 mA, 15-sec duration, at random intervals averaging 30 sec). The first five trials were FR1 (the rat crosses once to terminate shock), and the subsequent 25 trials were on an FR2 schedule (rat crosses twice to terminate the shock).
For FST, a single testing day was used as has been used in testing chronic antidepressant effects (46). Rats were placed individually in a clear cylinder (25-cm diameter, 65-cm height) with water at a depth of 45 cm (23–25°C) for 15 min. Sessions were videotaped from the side and scored by using a behavioral sampling technique (55) to assess the primary behavior for each 5-sec interval, resulting in a total of 180 samples per 15-min test. Three behaviors were scored as described (56): (i) climbing, rat is thrashing in an upward motion along the sides of the tank; (ii) swimming, movement across the tank into another quadrant; and (iii) immobility, when the animal made the minimal amount of movement required to stay afloat.
Ambulatory locomotor activity was assessed in clear plastic boxes fitted to automated activity meters (Omnitech Electronics, Columbus, OH) consisting of two parallel rows of photosensors (16 sensors per row, 2.5 cm apart). Locomotor activity was recorded in 5-min bins for a total of 30 min by using Micropro software.
Statistical Analyses.
All data are presented as means ± SE. Experiments with two groups were compared with unpaired Student's t test. Experiments with three or more groups were subjected to a two-way ANOVA, followed by the post hoc Bonferroni–Dunn test. Locomotor activity data were analyzed by using the repeated-measures ANOVA, with time being the repeated measure. Markings in figures are based on results from t test or Bonferonni–Dunn post hoc tests. Statistical significance was set at P ≤ 0.05.
Supplementary Material
Acknowledgments
We thank Drs. M. Picciotto, J. Taylor, M. Banasr, and E. F. Schmidt for helpful comments during the preparation of this manuscript; Derek Ng and Joshua Greene for outstanding technical assistance; and Dr. M. Banasr for design/establishment of CUS paradigm. This work was supported by U.S. Public Health Service Grants MH45481 and MH25642, Veterans Administration National Center grant for Posttraumatic Stress Disorder, and the Connecticut Mental Health Center.
Abbreviations
- 5-HT
serotonin
- CREB
cAMP response element binding protein
- CUS
chronic unpredictable stress
- ECS
electroconvulsive seizure
- FST
forced swim test
- GCL
granule cell layer
- LH
learned helplessness
- NE
norepineprine
- NSF
novelty suppressed feeding
- SGZ
subgranular zone
- VEGF
vascular endothelial growth factor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610282104/DC1.
References
- 1.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 2.Duman RS. Neuromol Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- 3.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 4.Nibuya M, Morinobu S, Duman RS. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara N, Gerber HP, LeCouter J. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 8.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG. Brain Pathol. 2004;14:237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storkebaum E, Lambrechts D, Carmeliet P. BioEssays. 2004;26:943–954. doi: 10.1002/bies.20092. [DOI] [PubMed] [Google Scholar]
- 11.Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 12.McCloskey DP, Croll SD, Scharfman HE. J Neurosci. 2005;25:8889–8897. doi: 10.1523/JNEUROSCI.2577-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 14.Quinn TP, Peters KG, De Vries C, Ferrara N, Williams LT. Proc Natl Acad Sci USA. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang SZ, Zhang LM, Huang YL, Sun FY. Anat Rec A Discov Mol Cell Evol Biol. 2003;274:851–856. doi: 10.1002/ar.a.10103. [DOI] [PubMed] [Google Scholar]
- 16.Palmer TD, Willhoite AR, Gage FH. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, Duman RS. J Neurosci. 2003;23:10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 20.Kodama M, Fujioka T, Duman RS. Biol Psychiatry. 2004;56:570–580. doi: 10.1016/j.biopsych.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Mirescu C, Gould E. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn HG, Dickinson-Anson H, Gage FH. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellsten J, Wennstrom M, Bengzon J, Mohapel P, Tingstrom A. Biol Psychiatry. 2004;55:420–427. doi: 10.1016/j.biopsych.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Sun L, Tran N, Tang F, App H, Hirth P, McMahon G, Tang C. J Med Chem. 1998;41:2588–2603. doi: 10.1021/jm980123i. [DOI] [PubMed] [Google Scholar]
- 25.Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, et al. Cancer Res. 1999;59:99–106. [PubMed] [Google Scholar]
- 26.Knusel B, Hefti F. J Neurochem. 1992;59:1987–1996. doi: 10.1111/j.1471-4159.1992.tb10085.x. [DOI] [PubMed] [Google Scholar]
- 27.Jayatissa MN, Bisgaard C, Tingstrom A, Papp M, Wiborg O. Neuropsychopharmacology. 2006;31:2395–2404. doi: 10.1038/sj.npp.1301041. [DOI] [PubMed] [Google Scholar]
- 28.Malberg JE, Duman RS. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 29.Martin P, Soubrie P, Simon P. Pharmacol Biochem Behav. 1986;24:177–181. doi: 10.1016/0091-3057(86)90334-5. [DOI] [PubMed] [Google Scholar]
- 30.Martin P, Soubrie P, Simon P. Prog Neuropsychopharmacol Biol Psychiatry. 1987;11:1–7. doi: 10.1016/0278-5846(87)90026-1. [DOI] [PubMed] [Google Scholar]
- 31.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 32.Chen AC, Shirayama Y, Shin KH, Neve RL, Duman RS. Biol Psychiatry. 2001;49:753–762. doi: 10.1016/s0006-3223(00)01114-8. [DOI] [PubMed] [Google Scholar]
- 33.Sherman AD, Sacquitne JL, Petty F. Pharmacol Biochem Behav. 1982;16:449–454. doi: 10.1016/0091-3057(82)90451-8. [DOI] [PubMed] [Google Scholar]
- 34.Cryan JF, Markou A, Lucki I. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 35.Blier P. Eur Neuropsychopharmacol. 2003;13:57–66. doi: 10.1016/s0924-977x(02)00173-6. [DOI] [PubMed] [Google Scholar]
- 36.Nibuya M, Nestler EJ, Duman RS. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Impey S, McCorkle SR, Cha-Molstad H, Dwyer JM, Yochum GS, Boss JM, McWeeney S, Dunn JJ, Mandel G, Goodman RH. Cell. 2004;119:1041–1054. doi: 10.1016/j.cell.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 38.Pakala R, Willerson JT, Benedict CR. Circulation. 1994;90:1919–1926. doi: 10.1161/01.cir.90.4.1919. [DOI] [PubMed] [Google Scholar]
- 39.Mendel DB, Laird AD, Smolich BD, Blake RA, Liang C, Hannah AL, Shaheen RM, Ellis LM, Weitman S, Shawver LK, Cherrington JM. Anticancer Drug Des. 2000;15:29–41. [PubMed] [Google Scholar]
- 40.Sondell M, Lundborg G, Kanje M. J Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strawn LM, McMahon G, App H, Schreck R, Kuchler WR, Longhi MP, Hui TH, Tang C, Levitzki A, Gazit A, et al. Cancer Res. 1996;56:3540–3545. [PubMed] [Google Scholar]
- 42.Smolich BD, Yuen HA, West KA, Giles FJ, Albitar M, Cherrington JM. Blood. 2001;97:1413–1421. doi: 10.1182/blood.v97.5.1413. [DOI] [PubMed] [Google Scholar]
- 43.Mologni L, Sala E, Cazzaniga S, Rostagno R, Kuoni T, Puttini M, Bain J, Cleris L, Redaelli S, Riva B, et al. J Mol Endocrinol. 2006;37:199–212. doi: 10.1677/jme.1.01999. [DOI] [PubMed] [Google Scholar]
- 44.Tapley P, Lamballe F, Barbacid M. Oncogene. 1992;7:371–381. [PubMed] [Google Scholar]
- 45.Thiebot MH, Martin P, Puech AJ. Br J Psychiatry Suppl. 1992:44–50. [PubMed] [Google Scholar]
- 46.Cryan JF, Page ME, Lucki I. Psychopharmacology (Berl) 2005;182:335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- 47.Kales HC, Maixner DF, Mellow AM. Am J Geriatr Psychiatry. 2005;13:88–98. doi: 10.1176/appi.ajgp.13.2.88. [DOI] [PubMed] [Google Scholar]
- 48.Dow AL, Russell DS, Duman RS. J Neurosci. 2005;25:4908–4916. doi: 10.1523/JNEUROSCI.5155-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madsen TM, Yeh DD, Valentine GW, Duman RS. Neuropsychopharmacology. 2005;30:27–34. doi: 10.1038/sj.npp.1300565. [DOI] [PubMed] [Google Scholar]
- 50.Duijvestijn AM, van Goor H, Klatter F, Majoor GD, van Bussel E, van Breda Vriesman PJ. Lab Invest. 1992;66:459–466. [PubMed] [Google Scholar]
- 51.Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. Psychopharmacology (Berl) 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- 52.Bodnoff SR, Suranyi-Cadotte B, Quirion R, Meaney MJ. Psychopharmacology (Berl) 1989;97:277–279. doi: 10.1007/BF00442264. [DOI] [PubMed] [Google Scholar]
- 53.Banasr M, Valentine GW, Li XY, Gourley S, Taylor J, Duman RS. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2007.02.006. in press. [DOI] [PubMed] [Google Scholar]
- 54.Ortiz J, Fitzgerald LW, Lane S, Terwilliger R, Nestler EJ. Neuropsychopharmacology. 1996;14:443–452. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- 55.Detke MJ, Wieland S, Lucki I. Psychopharmacology (Berl) 1995;119:47–54. doi: 10.1007/BF02246053. [DOI] [PubMed] [Google Scholar]
- 56.Lucki I. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.