Abstract
Habituation is a universal form of nonassociative learning that results in the devaluation of sensory inputs that have little information content. Although habituation is found throughout nature and has been studied in many organisms, the underlying molecular mechanisms remain poorly understood. We performed a forward genetic screen in Drosophila to search for mutations that modified habituation of an olfactory-mediated locomotor startle response, and we isolated a mutation in the glycogen synthase kinase-3 (GSK-3) homolog Shaggy. Decreases in Shaggy levels blunted habituation, whereas increases promoted habituation. Additionally, habituation acutely regulated Shaggy by an inhibitory phosphorylation mechanism, suggesting that a signal transduction pathway that regulates Shaggy is engaged during habituation. Although shaggy mutations also affected circadian rhythm period, this requirement was genetically separable from its role in habituation. Thus, shaggy functions in different neuronal circuits to regulate behavioral plasticity to an olfactory startle and circadian rhythmicity.
Keywords: behavior
All animals are constantly confronted with a deluge of sensory information. A major challenge for the nervous system is to cull a meaningful signal from all the noise. Habituation is a form of behavioral adaptation to repeated sensory stimuli that devalues environmental stimuli with low information content, giving an animal greater ability to discriminate salient information, such as food, mates, or danger (1).
Many, if not all, sensory modalities demonstrate habituation. The most thoroughly studied example of habituation is the Aplysia gill withdrawal reflex, in which repeated application of a nonnoxious mechanical stimulus results in reduced gill withdrawal (2). Gill withdrawal is partially mediated by a simple sensory-motorneuron circuit, and a number of molecular and cellular processes required for gill withdrawal habituation have been described, leading to a model whereby release of neurotransmitter is reduced by changes in presynaptic calcium currents and synaptic vesicle availability (3, 4). Recent data have suggested that postsynaptic alterations also contribute to habituation (5). Analyses of other forms of habituation and utilization of different methodologies are likely to give additional perspectives on the underlying molecular mechanisms.
Habituation in Drosophila has been described for a number of sensory behaviors, including a taste-mediated proboscis extension reflex, a touch-mediated cleaning reflex, and a visually mediated escape response (6–8). Genetic studies of preexisting mutants point to an important role for cAMP signal transduction in these habituation paradigms. Flies also exhibit habituation of an olfactory startle response. When flies are first presented with a novel odorant, they react by immediately and transiently increasing their walking speed (9). Repeated discrete exposures to the same odorant reduce the magnitude of this olfactory-mediated startle response (10). Attenuation of the olfactory startle response is an example of habituation, meeting a number of its classical criteria (1). Perhaps most importantly, the habituated olfactory startle response can be dishabituated upon presentation of a strong and unrelated sensory stimulus. As expected, cAMP signal transduction is important for olfactory startle habituation. Olfactory startle habituation is a promising paradigm for identifying the molecular, cellular, and neural circuitry for nonassociative learning. The neural circuit mediating olfaction is well described, leading from the olfactory sensory neurons, via the projection neurons, to the mushroom bodies and the lateral horn. Components of this circuit are required for aspects of associative learning as well as for olfactory startle habituation (10, 11). Furthermore, the olfactory startle habituation paradigm, which has been adapted to high-throughput analysis, is well suited for forward genetic screens to identify new genes involved in this ubiquitous form of neural plasticity.
Glycogen synthase kinase-3β (GSK-3β) functions in cell fate specification, cell polarity, and energy metabolism and is well studied for it's evolutionarily conserved roles in Wnt and insulin signal transduction pathways (12). In the nervous system, GSK-3β is involved in the establishment and maintenance of neuronal polarity (13, 14). Additionally, the Drosophila GSK-3 homolog Shaggy (SGG) controls synapse growth at the neuromuscular junction (15), possibly as a transducer of Wg/Wnt signals (16). There is growing evidence that signaling pathways that use GSK-3β may be involved in mental illness, specifically in bipolar disorder and schizophrenia. Lithium, one of the most effective available treatments for bipolar disorder, targets and inactivates a handful of signal transduction molecules, including GSK-3β (17). Consistent with the notion that lithium acts by inhibiting GSK-3β are the findings that either a 50% reduction of GSK-3β or lithium treatment result in a similar constellation of behavioral alterations in mice (18). Schizophrenics can exhibit defects in prepulse inhibition, a measure of sensorimotor gating, and defects in habituation to startling sensory stimuli (19, 20). GSK-3β activity levels are correlated with the strength of prepulse inhibition in rodents (21). Moreover, treatment with antipsychotics, the only effective treatment for schizophrenia, alters GSK-3β protein levels in the rat brain (22). Therefore, GSK-3β may play an important role in psychiatric pathophysiology by regulating behavioral plasticity.
Here, we demonstrate that Drosophila SGG regulates habituation of an olfactory startle response. We also show that the role of SGG in startle habituation is distinct from its characterized role in circadian rhythms. Our data demonstrate a potentially conserved role for GSK-3β in forms of behavioral plasticity often affected by mental illness and suggest that Drosophila may be an appropriate model to address some of the molecular mechanisms underlying complex behavioral disorders in humans.
Results
tweaker Mutants Are Defective for Olfactory Habituation.
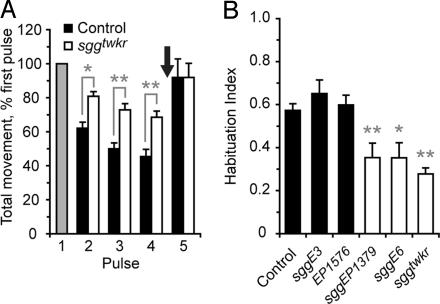
In a screen for mutants with altered behavior, we identified tweaker (twkr), a mutant showing reduced habituation to repeated discrete presentations of the odorant ethanol. Naïve wild-type and twkr flies showed a robust startle when presented with a brief, 30-s exposure to ethanol vapor (9, 10). After exposure to four pulses of ethanol vapor delivered at 6-min intervals, however, wild-type control flies habituated to 43% of naïve response levels, whereas twkr mutants habituated to only 72% of naïve response levels (Fig. 1A). twkr mutants retained the ability to dishabituate upon presentation of a mechanical stimulus (arrow in Fig. 1A). Thus, twkr mutants display a reduced acquisition of habituation but a normal discrimination between divergent stimuli.
Fig. 1.
sgg mutants show reduced olfactory startle habituation. (A) Groups of twenty genetically identical flies were exposed to four 30-sec pulses of ethanol vapor at 6-min intervals, and the total distance traveled per fly was measured and normalized to the first pulse. A mechanical dishabituating stimulus was delivered after the fourth ethanol pulse (arrow). Control flies correspond to the w1118 Berlin genetic background. n = 15 groups of 20 flies, ∗, P < 0.05; ∗∗, P < 0.01; one-way ANOVA, Neuman–Keuls multiple comparison test. (B) Habituation index {ratio of normalized distance traveled between pulse 1 and 4 [1-(P4/P1)]} for sgg alleles. n = 10–20, ∗∗, P < 0.01; one-way ANOVA, Neuman–Keuls multiple comparison test.
tweaker Is an Allele of sgg.
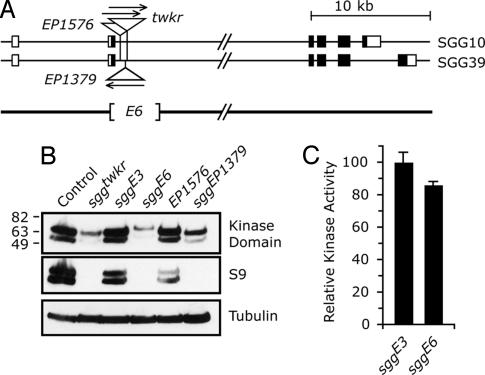
The twkr transposon is inserted in an intron in the sgg locus [also known as Zeste-white 3 (Zw3)] on the X chromosome (Fig. 2A). At least five distinct SGG proteins are produced through the use of different transcription start sites and differential splicing (23). In the adult fly, two SGG protein isoforms predominate, SGG10 and SGG39, whereas the other isoforms primarily show regulated expression during development. SGG10 and SGG39 (here collectively termed SGG) most closely resemble mammalian GSK-3β, containing the signature kinase domain and an amino-terminal serine residue that can be phosphorylated to form an autoinhibitory pseudosubstrate (S9 in both mammals and flies). Antibodies recognizing the kinase domain detected SGG proteins of reduced quantity and molecular weight in twkr mutants (Fig. 2B). S9 is encoded by the exon immediately upstream of the twkr insertion site; antibodies directed against sequences surrounding S9 were unable to detect protein, indicating that this domain is lacking in the mutant and that a novel initiator methionine provides an in-frame translation start site (Fig. 2B).
Fig. 2.
The sgg locus is disrupted in nonhabituating mutants. (A) Two transcriptional splice variants that code for SGG proteins detectable in adults (additional SGG forms are primarily expressed during development) (23). The coding region is shaded. twkr is a p[GawB] transposon inserted at position 8858 of AE104140. sggE6 is a 3,330-base deletion initiating 1,016 bases 5′ to the twkr insertion site, deleting the translation initiation codon of the adult proteins. Ser-9 (S9) is coded by the exon deleted in sggE6. (B) Western blot of adult fly heads, probed with antibodies against SGG kinase domain, S9-containing peptide from human sequence, and α-tubulin. SGG39 and SGG10 are the higher and lower molecular weight bands, respectively. (C) Kinase activity of SGG proteins. Equivalent amounts of control (sggE3) and mutant (sggE6) proteins were immunoprecipitated from fly heads. sggE6 has an 8-fold reduction in levels of SGG protein. Kinase activity was measured on a CREB peptide substrate, and values were normalized to the control after background subtraction. Error bars are standard deviation. P > 0.05 by t test.
Excision of the twkr transposon generated two new alleles, a precise excision, sggE3, and an imprecise excision, sggE6. sggE3 completely reverted the behavioral (Fig. 1B) and molecular (Fig. 2B) phenotypes of twkr, indicating that the transposon insertion was responsible for the habituation defect. The sggE6 deletion removed the entire transposon, protein coding sequences just downstream of the translation initiation codon, and the splice donor site of the first exon (Fig. 2A). sggE6 deletion mutants showed reduced habituation similar in extent to that of twkr mutants (Fig. 1B). Additionally, sggE6 mutants had 8-fold reduced SGG protein levels (as measured by quantitative immunoprecipitation), an increased protein molecular weight (suggesting the use of a novel initiator methionine), and undetectable S9 immunoreactivity (Fig. 2B). An independent transposon allele, sggEP1379, had behavioral and molecular phenotypes similar to twkr. Flies containing another transposon, EP1576, that is inserted in the genome near EP1379 (but in the opposite orientation) was wild type for both habituation and protein levels. These data indicate that SGG is abnormal in the habituation-defective twkr, E6, and EP1379 mutants.
To determine whether the residual protein was functional in the sgg behavioral mutants, we assayed their kinase activity in vitro. The kinase activity of SGG protein that was quantitatively immunoprecipitated from adult heads and adjusted for equal SGG protein levels showed no appreciable difference between sggE3 and sggE6 (Fig. 2C). Thus, in the habituation-defective sggE6 mutant, and presumably in sggtwkr and sggEP1379, there exist low levels of SGG protein that retains full kinase activity, yet this kinase is not subject to regulation through phosphorylation of S9. Additionally, these data indicate that the novel N-terminal protein sequences present in sggtwkr and sggE6 are unlikely extend into the kinase domain, which begins at amino acid position 54 in both adult SGG isoforms.
sgg Behavioral Mutants Are Defective for Circadian Period.
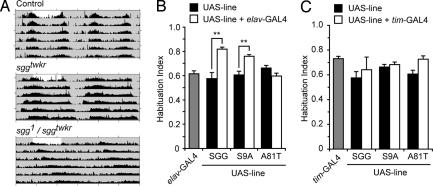
sgg functions in the fly circadian clock pacemaker neurons by regulating the phosphorylation and nuclear import of Timeless (24, 25). Previous experiments demonstrated that increasing SGG levels in the pacemaker neurons shortened circadian period length (25). In addition, sgg null mutants that were rescued for lethality by ubiquitously supplying sgg during development but not during adulthood showed an increased period length. We replicated these findings: overexpressing wild-type (UAS-SGG10) or S9 phosphorylation site mutated (UAS-SGG10.S9A), but not kinase dead (UAS-SGG.A81T), SGG in clock cells resulted in a shortening of circadian period (Table 1). Importantly, sggtwkr and sggE6 both caused a significant lengthening of circadian period (Table 1 and Fig. 3A). Because sgg is located on the X chromosome, we analyzed allelic interactions in females. sggtwkr behaved as a recessive allele because sggtwkr heterozygous flies had a normal period length. sggtwkr acted as a strong loss-of-function mutation when placed in trans to the null allele sgg1. Indeed, with respect to period length, EP1576 = sggtwkr/+ = sgg1/ep1576 < sggtwkr/sggtwkr < sgg1/sggtwkr. These data demonstrate that sggtwkr and sggE6 are recessive loss-of-function alleles of sgg. Unfortunately, we were unable to test flies containing these stronger allele combinations to determine whether sgg was absolutely required for olfactory habituation because these flies displayed a strong increase in baseline locomotor activity both before and between ethanol pulse applications.
Table 1.
Circadian period (τ) of sgg mutants
| Genotype | Sex | τ, h | SD, h | Rhythmic, % | n |
|---|---|---|---|---|---|
| Control | ♂ | 23.6 | 0.36 | 100 | 16 |
| EP1576 | ♂ | 23.8 | 0.35 | 87 | 26 |
| sggtwkr | ♂ | 24.7** | 0.31 | 100 | 29 |
| sggE6 | ♂ | 25.3** | 0.42 | 61 | 26 |
| tim-Gal4/EP1576 | ♂ | 20.9** | 0.41 | 100 | 16 |
| tim-Gal4/UAS-SGG10 | ♂ | 19.1** | 0.16 | 100 | 10 |
| tim-Gal4/UAS-SGG10.S9A | ♂ | 19.7** | 0.13 | 100 | 16 |
| tim-Gal4/UAS-SGG10.A81T | ♂ | 23.5 | 0.17 | 100 | 15 |
| tim-Gal4 | ♂ | 24.5 | 0.26 | 100 | 15 |
| UAS-SGG10 | ♂ | 23.7 | 0.30 | 100 | 29 |
| UAS-SGG10.A81T | ♂ | 23.3 | 0.22 | 100 | 16 |
| UAS-SGG10.S9A | ♂ | 23.7 | 0.21 | 100 | 25 |
| EP1576/EP1576 | ♀ | 24.1 | 0.18 | 100 | 16 |
| sggtwkr/+ | ♀ | 24.3 | 0.27 | 84 | 24 |
| sggtwkr/sggtwkr | ♀ | 25.3** | 0.33 | 47 | 15 |
| sgg1/EP1576 | ♀ | 24.5 | 0.27 | 95 | 22 |
| sgg1/sggtwkr | ♀ | 26.4** | 0.43 | 50 | 40 |
All strains contain w1118 and are in the Berlin genetic background. Control is w1118 Berlin and tim-GAL4 is tim-(UAS)GAL4 (42). All transgenes are autosomal and heterozygous, and sgg is X-linked and thus hemizygous in males and either heterozygous or homozygous in females. One-way ANOVA,
**, P < 0.01 by Tukey's multiple comparison test: for males, asterisks indicate significance to both control and EP1576; for females, significance is to both EP1576/EP1576 and sggtwkr/+.
Fig. 3.
Habituation and circadian rhythms are separable. (A) Double-plotted actograms showing circadian locomotor activity patterns. The first day (not shaded) is a 12-h light 12-h dark cycle, and the remaining days are in constant darkness (shaded). (B) Overexpression of UAS transgenes encoding S9 mutated (S9A) or wild-type (SGG), but not kinase-dead (A81T) SGG, by the weak panneuronal driver line elav-GAL4 3E1 results in superhabituation (n = 8–15, ∗∗, P < 0.01; one-way ANOVA, Newman–Keuls multiple comparison test). (C) Overexpression of UAS transgenes by the circadian clock pacemaker neuron-specific driver tim-GAL4 (42). One-way ANOVA found no effect of genotype (n = 7–15).
Increased Expression of SGG Increases Olfactory Habituation.
SGG protein appears to be expressed ubiquitously in the Drosophila central nervous system (F.W.W., unpublished results). To determine whether SGG levels determine the extent of habituation, we overexpressed SGG in all postmitotic neurons using the elav-Gal4 driver. In contrast to loss of sgg function, overexpression of either wild-type or nonphosphorylatable SGG (SGG10.S9A) protein resulted in increased habituation (Fig. 3B). Increased expression of kinase-dead SGG (SGG.A81T) had no effect on habituation. The magnitude of the initial olfactory startle response as well as nonstimulated baseline locomotion were unaffected by overexpression of SGG (data not shown).
Circadian and Habituation Functions of sgg Are Separable.
The circadian clock could regulate olfactory habituation, in which case the role of sgg in habitation would be indirect. To test this possibility, we determined the habituation behavior of flies overexpressing SGG in the clock pacemaker cells. Although overexpression of wild-type and S9 mutated SGG in clock cells shortened circadian period length, neither manipulation had an effect on olfactory startle habituation (Fig. 3C). Thus, the roles of sgg in olfactory startle habituation and circadian rhythms are separable.
SGG Is Phosphorylated During Habituation.
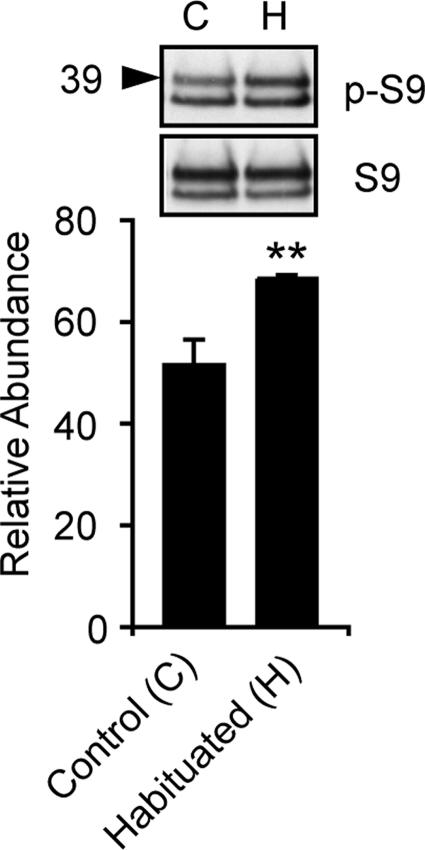
GSK-3/SGG kinase activity is regulated by association with other proteins. In Wnt/Wg signal transduction, a complex that includes APC and axin regulates GSK-3 phosphorylation of β-catenin. Phosphorylation of the pseudosubstrate domain at S9 by upstream kinases, including Akt1, down-regulates GSK-3, promoting activation of glycogen synthase and other substrates (26, 27). To determine whether SGG signaling pathways are engaged during the habituation process, we assessed the status of SGG S9 phosphorylation in habituated and sham-habituated flies. Wild-type control flies were given five 30-s pulses of ethanol vapor separated by 6-min intervals and were then frozen 1.5 min after the fifth pulse. The levels of protein detectable by antibodies against the S9 epitope versus phosphorylated S9 epitope were then quantified by Western blot. After habituation, the relative abundance of S9 phosphorylated SGG39 increased significantly, whereas the abundance of S9-phosphorylated SGG10 did not change (Fig. 4). Overall SGG abundance was unchanged. These data are consistent with an acute down-regulation of SGG activity during habituation by phosphorylation of S9.
Fig. 4.
SGG S9 phosphorylation is increased after habituation. Western blot of heads from control and habituated flies probed with anti-phospho-S9 (p-S9) and S9 peptide antibodies. Below, quantification of p-S9 SGG39 (39) band intensity normalized to SGG39 S9 intensity (n = 8, ∗∗, P < 0.01; t test).
Localizing SGG Function.
SGG is expressed throughout the adult CNS, and increased expression in the CNS increases olfactory startle habituation. Where does SGG function to regulate habituation? We began to address this question by expressing SGG in the known components of the olfactory neural circuit using several GAL4 drivers: Or83b-Gal4 drives expression in a majority of olfactory receptor neurons, GH146 in the second order projection neurons that connect the antennal lobe to the mushroom bodies (MBs) and the lateral horn, and 17D, c747, and 201Y drive expression in neurons of the MBs that are relevant for olfactory habituation (10). Overexpression of SGG in neither Or83b-expressing neurons nor the MBs resulted in changes in olfactory startle habituation [see supporting information (SI) Fig. 5). Expression of SGG in the projection neurons (with GH146) caused lethality, presumably because of glial expression (28). These data suggest that SGG functions downstream of the known olfactory neural circuit (although function in the projection neurons cannot be excluded at this time) or that the role for SGG in habituation is distributed in the nervous system.
Discussion
Here, we show that multiple strong loss-of-function mutant alleles of the Drosophila GSK-3 homolog SGG have defects in two adult behaviors, olfactory startle habituation (reduced habituation) and circadian rhythm period length (longer period). Importantly, SGG overexpression results in an opposite phenotype for both behaviors [this work and Martinek et al. (25)]. In its role in circadian rhythms, SGG phosphorylates Timeless, promoting its movement from the cytoplasm to the nucleus, thus allowing the clock pacemaker to progress (25). As the roles of SGG in habituation and circadian rhythms are separable, the target of SGG activity in habituation remains to be determined.
Our data also demonstrate an acute phosphorylation and, thus presumably, down-regulation of activity of SGG protein during habituation. A signaling pathway that utilizes SGG is therefore engaged by olfactory startle habituation. The Akt1 signaling pathway, which can be activated by insulin receptors and can result in SGG/GSK-3 S9 phosphorylation, is conserved in flies and regulates growth and metabolism (29). Although Drosophila Akt1 has not yet been directly implicated in behavior, the insulin signal transduction pathway has been shown to function in flies in feeding, ethanol sensitivity, lifespan, and stress resistance (30–32). Recent findings with mice carrying deletion of PTEN, a negative regulator of the insulin pathway, in specific brain regions suggest that signaling via Akt1 and GSK-3β regulate neuronal structure and social interactions in mice (33).
Reduced levels of SGG resulted in a strong reduction in habituation and increased levels of SGG resulted in an increase in habituation, suggesting that SGG activity normally promotes the development of habituation. Perhaps counterintuitively, then, we also found that SGG is likely to be inactivated by S9 phosphorylation during habituation. These observations are not necessarily conflicting. Although the presence of SGG that can be regulated by phosphorylation may be required for habituation to proceed in wild-type flies, our mutant alleles already have strongly reduced levels of SGG that cannot be phosphorylated at S9 and thus further inhibited: our sgg alleles may mimic the habituated state, precluding further habituation by presentation of olfactory stimuli. Alternatively, SGG could have two roles in habituation, one that is engaged directly during habituation, and a second that is perturbed when SGG levels are altered chronically. Indeed, there exists evidence that SGG/GSK-3 inactivation by the pseudosubstrate mechanism and by altered protein associations can proceed independently (37). It remains to be determined, however, whether the acute change in SGG phosphorylation is relevant to the development of habituation.
Genetic and pharmacological evidence supports GSK-3β function in adult behavior in mammals. Mice that overexpress nonphosphorylatable (S9A), and thus presumably over-active GSK-3β, in the nervous system are generally hyperactive, have an enhanced acoustic startle response (ASR), and show less habituation to the ASR (34). Gsk-3β+/− mice show reduced exploratory behavior, similar to what is observed upon treatment with lithium (18). Although the heterozygous mice, which have a 50% reduction in GSK-3β protein levels, are normal for ASR and ASR habituation, they show decreased locomotor activation induced by amphetamine (35). Interestingly, we also isolated sggEP1379 in a genetic screen for mutants with increased sensitivity to cocaine (L. Tsai and U.H., unpublished observation). Thus, although the data are still incomplete in both mice and flies, our data support a potentially opposite function for SGG in flies and mice. Although our mutant alleles have strongly reduced levels of SGG protein (≈10% of normal levels), they also lack S9 regulation of the remaining kinase-competent protein. We believe that the habituation defects in our mutants are due to reduced protein levels because these same alleles behave as loss-of-function for circadian period length and because overexpression of wild-type or S9A-mutated SGG cause increases in habituation.
In summary, we show that SGG is involved in regulating the ability of Drosophila to habituate to olfactory stimuli. Whether SGG function is specific to olfactory habituation, or is generally required for behavioral plasticity will be a topic of future studies. It will also be of interest to determine how upstream signaling pathways regulate SGG function, and to determine the cellular substrates of SGG in habituation.
Methods
Fly Strains.
All Drosophila strains were out-crossed for at least five generations to the Berlin wild-type strain carrying the marker mutation w1118 or were out-crossed against noncomplementing sgg alleles that were in the Berlin genetic background. sggtwkr was identified as a P[GawB] transposon insertion containing transgenic line that responded abnormally in tests for ethanol vapor-induced locomotor activation (data not shown). sggE3 and sggE6 were produced by excision of sggtwkr by standard methods. All other strains were obtained from the Bloomington Stock Center (Bloomington, IN) or Szeged Stock Center (Szeged, Hungary).
Molecular Biology and Biochemistry.
sggtwkr was found to be inserted at position 8858 of AE104140 by inverse PCR. Immunoprecipitation and SGG kinase activity determination were essentially as in Lin (36) and Papadopoulou (37). Briefly, SGG was quantitatively immunoprecipitated from extracts of 25 (sggE3) and 200 (sggE6) 2- to 5-day-old male heads (equalized for SGG protein content) with antibodies 2G2C5 and 2G1A3 (23) bound to Protein A/G PLUS-Agarose (Santa Cruz Biotechnology, Santa Cruz, CA) in 100 mM KCl, 20 mM Hepes (pH 7.6), 10 mM EDTA, 5% glycerol, 0.1% Triton X-100, 1 mM DTT with protease (Roche Diagnostics, Indianapolis, IN), and phosphatase inhibitors (Sigma, St. Louis, MO). Bound proteins were assayed for activity in 25 μl of 50 mM Tris (pH 7.5), 100 mM NaCl, and 10 mM MgCl2 with 75 μM CREB peptide (KRREILSRRP[pSer]YR, [pSer] indicating phosphorylated serine, biotinylated C terminus), 6 μCi of [γ-32P]ATP (1 Ci = 37 GBq), and 100 μM ATP for 10 min. Reactions were processed according to Lin (38). The experiment was done in triplicate on two different days, each replicate from independent head extracts. Significance was determined by an unpaired two tailed t test. Other antibodies used were 4G-1E (Upstate Biotechnology, Lake Placid, NY), Phospho-GSK-3α/β (Ser-21/9), and GSK-3α/β (Ser-21/9) (Cell Signaling Technology, Danvers, MA). Western blot densitometry was done with ImageJ (NIH). Band intensity was determined by area under the curve and calibration with standards on the same blot.
Behavioral Analyses.
Habituation of olfactory-mediated locomotor startle response was measured as described (10). In this study, groups of 20 genetically identical adult male flies that were 2–5 days old were used. After a brief acclimation period in the booz-o-mat test apparatus, flies were given 30-sec pulses of ethanol vapor (ethanol/humidified air ratio of 70/80, where 70 units of flux is equivalent to 2.4 liter/min) at 6-min intervals. Flies were dishabituated with a physical bang stimulus 2 min after the fourth pulse. The habituation index (HI) is 1-(P4/P1), where P4 and P1 are the areas under the locomotor activity curve for pulse 4 and pulse 1 of ethanol vapor exposure, such that an HI of 1 indicates complete habituation, and an HI of 0 indicates no habituation. For circadian rhythms, locomotor activity of 2- to 5-day-old adult flies was measured at 10-sec intervals in a Drosophila Activity Monitor (Trikinetics, Waltham, MA) for 3–4 days in a 12-h light 12-h dark cycle, followed by 8–10 days in constant darkness at 25°C. The circadian period of the flies was calculated by using the “χ2 periodogram” analysis (α = 0.01) on the ClockLab software package (Actimetrics, Wilmette, IL). Flies were judged to be rhythmic and used for period calculations when the χ2 statistic exceeded the significance line by 5. Statistical significance was determined by the Kruskal–Wallis statistic with a Dunn's multiple comparison post hoc test.
Supplementary Material
Acknowledgments
We thank Marc Bourouis (Université de Nice, France) for a generous gift of SGG monoclonal antibodies, Melissa Sniffen for technical assistance, Dan Wang for advice on the kinase assay, Quasar Padaith for guidance with circadian behavior assays, Eric Kong for statistical advice, and David Kapfhamer and other members of the Heberlein laboratory for helpful suggestions. This work was supported by the Damon Runyon Cancer Research Foundation (F.W.W.), the American Psychological Association Program for Minority Research Training in Psychiatry (W.C.), and National Institute of Health Grants AA014594 (to F.W.W.), AA015470 (to S.L.), and AA13105 (to U.H.).
Abbreviation
- GSK-3
glycogen synthase kinase.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700493104/DC1.
References
- 1.Christoffersen GR. Prog Neurobiol. 1997;53:45–66. doi: 10.1016/s0301-0082(97)00031-2. [DOI] [PubMed] [Google Scholar]
- 2.Pinsker H, Kupfermann I, Castellucci V, Kandel E. Science. 1970;167:1740–1742. doi: 10.1126/science.167.3926.1740. [DOI] [PubMed] [Google Scholar]
- 3.Bailey CH, Chen M. J Neurosci. 1988;8:2452–2459. doi: 10.1523/JNEUROSCI.08-07-02452.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edmonds B, Klein M, Dale N, Kandel ER. Science. 1990;250:1142–1147. doi: 10.1126/science.2174573. [DOI] [PubMed] [Google Scholar]
- 5.Glanzman DL. Biol Bull. 2006;210:271–279. doi: 10.2307/4134563. [DOI] [PubMed] [Google Scholar]
- 6.Corfas G, Dudai Y. J Neurosci. 1989;9:56–62. doi: 10.1523/JNEUROSCI.09-01-00056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duerr JS, Quinn WG. Proc Natl Acad Sci USA. 1982;79:3646–3650. doi: 10.1073/pnas.79.11.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engel JE, Wu CF. J Neurosci. 1996;16:3486–3499. doi: 10.1523/JNEUROSCI.16-10-03486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf FW, Rodan AR, Tsai LT, Heberlein U. J Neurosci. 2002;22:11035–11044. doi: 10.1523/JNEUROSCI.22-24-11035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho W, Heberlein U, Wolf FW. Genes Brain Behav. 2004;3:127–137. doi: 10.1111/j.1601-183x.2004.00061.x. [DOI] [PubMed] [Google Scholar]
- 11.Davis RL. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- 12.Cohen P, Frame S. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 13.Jiang H, Guo W, Liang X, Rao Y. Cell. 2005;120:123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Shi SH, Cheng T, Jan LY, Jan YN. Curr Biol. 2004;14:2025–2032. doi: 10.1016/j.cub.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Franco B, Bogdanik L, Bobinnec Y, Debec A, Bockaert J, Parmentier ML, Grau Y. J Neurosci. 2004;24:6573–6577. doi: 10.1523/JNEUROSCI.1580-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciani L, Salinas PC. Nat Rev Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 17.Klein PS, Melton DA. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, Klein PS. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braff DL, Geyer MA, Swerdlow NR. Psychopharmacology (Berlin) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 20.Geyer MA, Braff DL. Psychophysiology. 1982;19:1–6. doi: 10.1111/j.1469-8986.1982.tb02589.x. [DOI] [PubMed] [Google Scholar]
- 21.Amar S, Jones BC, Nadri C, Kozlovsky N, Belmaker RH, Agam G. Genes Brain Behav. 2004;3:178–180. doi: 10.1111/j.1601-183X.2004.00065.x. [DOI] [PubMed] [Google Scholar]
- 22.Alimohamad H, Rajakumar N, Seah YH, Rushlow W. Biol Psychiatry. 2005;57:533–542. doi: 10.1016/j.biopsych.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 23.Ruel L, Pantesco V, Lutz Y, Simpson P, Bourouis M. EMBO J. 1993;12:1657–1669. doi: 10.1002/j.1460-2075.1993.tb05811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cyran SA, Yiannoulos G, Buchsbaum AM, Saez L, Young MW, Blau J. J Neurosci. 2005;25:5430–5437. doi: 10.1523/JNEUROSCI.0263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinek S, Inonog S, Manoukian AS, Young MW. Cell. 2001;105:769–779. doi: 10.1016/s0092-8674(01)00383-x. [DOI] [PubMed] [Google Scholar]
- 26.Jope RS, Johnson GV. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 27.McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stocker RF, Heimbeck G, Gendre N, de Belle JS. J Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Stocker H, Hafen E. Curr Opin Genet Dev. 2000;10:529–535. doi: 10.1016/s0959-437x(00)00123-4. [DOI] [PubMed] [Google Scholar]
- 30.Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. Proc Natl Acad Sci USA. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corl AB, Rodan AR, Heberlein U. Nat Neurosci. 2005;8:18–19. doi: 10.1038/nn1363. [DOI] [PubMed] [Google Scholar]
- 32.Wu Q, Zhao Z, Shen P. Nat Neurosci. 2005;8:1350–1355. doi: 10.1038/nn1540. [DOI] [PubMed] [Google Scholar]
- 33.Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, Daneels G, Bouwknecht JA, Steckler T. J Neurosci. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Proc Natl Acad Sci USA. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin JM, Schroeder A, Allada R. J Neurosci. 2005;25:11175–11183. doi: 10.1523/JNEUROSCI.2159-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papadopoulou D, Bianchi MW, Bourouis M. Mol Cell Biol. 2004;24:4909–4919. doi: 10.1128/MCB.24.11.4909-4919.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin JM, Kilman VL, Keegan K, Paddock B, Emery-Le M, Rosbash M, Allada R. Nature. 2002;420:816–820. doi: 10.1038/nature01235. [DOI] [PubMed] [Google Scholar]
- 39.Zars T, Fischer M, Schulz R, Heisenberg M. Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]
- 40.Blau J, Young MW. Cell. 1999;99:661–671. doi: 10.1016/s0092-8674(00)81554-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.