Abstract
The TRPC (C-type transient receptor potential) class of ion channels has been hypothesized to participate in store-operated Ca2+ entry (SOCE). Recently, however, STIM1 and Orai1 proteins have been proposed to form SOCE channels. Whether TRPCs participate in SOCE that is dependent on or regulated by Orai has not been explored. Here we show that Orai1 physically interacts with the N and C termini of TRPC3 and TRPC6, and that in cells overexpressing either TRPC3 or TRPC6 in a store-depletion insensitive manner, these TRPCs become sensitive to store depletion upon expression of an exogenous Orai. Thus, Orai-1, -2, and -3 enhanced thapsigargin-induced calcium entry by 50–150% in cells stably overexpressing either TRPC3 or TRPC6. Orai1 expression had no significant effect on endogenous, thapsigargin-induced calcium entry in wild-type cells (HEK-293, COS1), in HEK cells expressing a thapsigargin-sensitive variant of TRPC3 (TRPC3a), or in HEK cells overexpressing another membrane protein, V1aR. Single-channel cation currents present in membrane patches of TRPC3-overexpressing cells were suppressed by expression of Orai1. We propose that Orai proteins by interacting with TRPCs act as regulatory subunits that confer STIM1-mediated store depletion sensitivity to these channels.
Keywords: capacitative calcium entry, ion channels, thapsigargin, transmembrane signaling
It is now widely accepted that activation of phospholipase C stimulates calcium entry across the plasma membrane, but the molecular mechanisms are still being elucidated. One mechanism involves receptor-operated Ca2+ entry (ROCE) through calcium-permeable but nonselective cation channels with conductances in the 10- to 100-pS range, which are activated by lipid metabolites of phosphatidylinositol-4,5-bisphosphate. The other mechanism involves calcium-selective channels of unmeasurable conductance that are activated by store depletion independently of phosphatidylinositol-4,5-bisphosphate metabolism, and is referred to as store-operated Ca2+ entry (SOCE) or capacitative Ca2+ entry (1–7).
The members of the C (for canonical) family of TRP (transient receptor potential)-related ion channels (TRPCs) have been postulated as candidates for forming both ROCE and capacitative Ca2+ entry channels (8–10). There are seven TRPC family members (C1–C7; reviewed in ref. 11). When expressed in model cells they form calcium-permeable, largely nonselective cation channels (12, 13). Most of the TRPCs have been shown either to be activated by store depletion or to be essential components of SOCE, including TRPC1 (14, 15), TRPC2 (16, 17), TRPC3 (18, 19), TRPC4 (20), TRPC5 (21), and TRPC7 (22, 23). Gene inactivation (24) and RNAi (25) studies also support a role for TRPCs in SOCE. However, the dependence of TRPCs on store depletion requires special conditions. For TRPCs of the 3-6-7 subfamily, the overriding condition appears to be that they be expressed at low densities (18, 19, 26, 27). The reason for this has not been clarified. One possibility is that at high expression levels the transfected TRPCs titrate one or more regulatory subunits that confer SOCE characteristics to TRPCs. The recent discoveries of STIM and Orai as essential components of SOCE and Icrac may have uncovered in Orai such a missing subunit.
The STIM (stromal interaction molecule) gene, of which there is one in Drosophila and two in mammals, was discovered as a component of the SOCE machinery in two independent RNA suppression screens performed in Drosophila Schneider 2 cells, (S2 cells) (28, 29). STIM proteins reside in the endoplasmic reticulum, span the membrane once, and appear to have a Ca2+-sensing helix–loop–helix motif (EF hand) near their luminal C terminus. Human STIM1 and STIM2 are 685 and 833 aa long, respectively. When expressed by itself in normal cells, human STIM1 has either no or only a slight enhancing effect on thapsigargin (Tg)-stimulated Ca2+ influx. Overexpression of STIM2 inhibits STIM1-dependent SOCE (30).
The Orai gene, of which there are three in the mammalian genome, was identified in three laboratories by using two independent approaches. One was RNAi-mediated suppression of Tg-stimulated Ca2+ entry into S2 cells (31, 32). The second approach tracked both the effect of RNAi in Drosophila cells and the molecular basis of the loss of T cell receptor function in a familial case of severe combined immunodeficiency (SCID), which had been shown to lack SOCE in T cells (33). Positional cloning of the mutant locus identified encompassed the Orai1 gene, whose functional suppression by RNAi in S2 cells caused loss of both Tg-stimulated Ca2+ entry and activation of transfected nuclear factor of activated T cells (34). This factor is known to require SOCE for its activation. Orai molecules are predicted to span the plasma membrane four times and range in size from 250 aa (mouse Orai2) to 301 aa (human Orai1). One of the extracellular loops of Orai1 has a consensus glycosylation site.
Coexpression of STIM1 and Orai1 dramatically increases Tg-induced Ca2+ entry as well as Icrac from 8 to 100 times (35–38). The fact that Icrac currents generated by coexpression of Orai and STIM were larger than ever seen before and developed without requiring cotransfection of a TRPC has been taken to mean that Orai1 is a Ca2+-selective pore activated by STIM1 and de-emphasized thinking of TRPCs as components of SOCE channels. In support, mutations in Orai were found to change the ion permeation characteristics of Icrac channels induced by the coexpression of STIM1 and Orai1 (31, 39, 40).
To follow up on these developments, and to address the question whether Orai and TRPCs interact, we expressed Orai in HEK cells stably expressing TRPC3 or TRPC6, and tested the effect that this maneuver would have on Tg-induced Ca2+ entry. As controls we expressed Orai1 in control HEK-293 cells that had not been transfected with a TRPC. The result was puzzling. Orai had no effect in control cells but enhanced SOCE in cells overexpressing TRPC3 or TRPC6. Below we report results that are consistent with the idea that Orai is a regulatory subunit of SOCE channels composed of pore-forming TRPC subunits.
Results
Studying TRPC Regulation in HEK Cells.
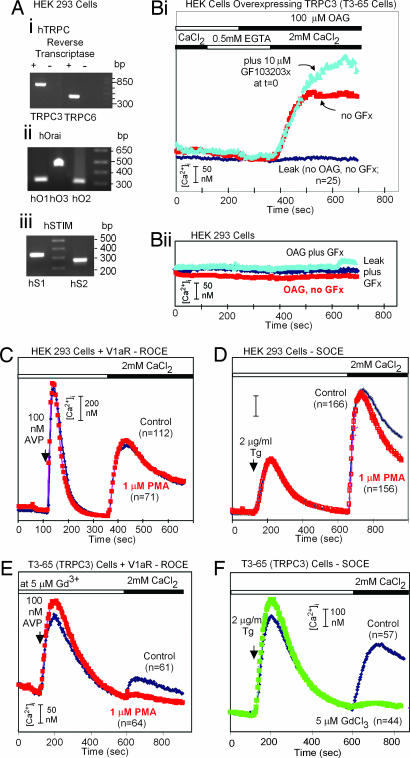
Human embryonic kidney (HEK) cell lines have been used widely to investigate the mechanism of SOCE. As Fig. 1A shows, the HEK-293 cells used in our experiments express not only TRPC3 and TRPC6, confirming a previous report (41), but also a full complement of three Orai and two STIM mRNAs. Fig. 1 C and D also shows that emptying intracellular calcium stores in zero external calcium with either vasopressin, acting through a Gq-coupled hormone receptor, or the SERCA pump inhibitor, Tg, produces robust calcium entry when extracellular calcium is restored. Stimulation of protein kinase C with 2 μM phorbol-myristoyl-acetate (PMA) did not alter these responses, and addition of 100 μM oleyl-acetyl-glycerol (OAG) did not mimic the effect of store depletion (Fig. 1Bii). In each case the data are representative of two to four independent experiments, and only cells that maintained stable baseline calcium signals when extracellular calcium was removed were included in the analysis.
Fig. 1.
Sites responsible for stimulatory and inhibitory effects of diacylglycerol and protein kinase C are occluded in untransfected HEK cells that express TRPC3, TRPC6, three Orai, and two STIMs. (A) Reverse-transcriptase PCR analysis shows that HEK cells transcribe mRNAs coding for TRPCs, STIMs, and Orai. (B) OAG activates TRPC3-mediated Ca2+ entry in T3 cells (Bi) but not in wild-type HEK-293 cells (Bii). (C–E) Activation of protein kinase C with PMA inhibits TRPC3-mediated (Gd3+-resistant) ROCE in T3 cells but does not affect either SOCE or ROCE in HEK-293 cells. (F) Overexpressed TRPC3 is not activated by store depletion. Cells were transfected, plated onto polylysine-coated coverslips 1 day before the test, loaded with Fura-2 acetoxymethyl ester, rinsed, and placed onto the stage of a microscope fitted with an InCyt Im2 ratiometric imaging system, at room temperature in Hepes buffered salt solution (HBSS) with 2 mM CaCl2. HBSS consisted of 140 mM NaCl, 4.7 mM KCl, 1.13 mM MgCl2, 10 mM Hepes (pH 7.5), and 10 mM glucose. For studies with OAG, cells were superfused in succession with HBSS without CaCl2, HBSS without CaCl2 plus 0.05 mM EGTA and 100 μM OAG, and HBSS plus CaCl2 and 100 μM OAG. For tests of ROCE, the indicted type of cells transfected with the indicated expression plasmids were exposed in succession to HBBS without Ca2+ plus 0.05 mM EGTA, HBSS without Ca2+ plus 0.05 mM EGTA and 100 nM arginine vasopressin, and HBSS plus 100 nM arginine vasopressin plus 2 mM Ca2+. When present, the protein kinase C inhibitor GF103203x (GFx) (10 μM), PMA (1 μM), and GdCl3 (5 μM) were added to all solutions from time 0 onwards. n, number of cells analyzed.
In contrast, HEK cells that were stably transfected with a plasmid encoding the human TRPC3 respond to addition of OAG with an increase in calcium influx from the external milieu (Fig. 1Bi), as reported in 1999 by Schultz and collaborators (42) for TRPC3, TRPC6, and TRPC7. Furthermore, in the presence of 5 μM gadolinium chloride, which completely blocks SOCE in TRPC3-expressing cells (Fig. 1F), PMA blocked the remaining ROCE that was stimulated by phospholipase C activation with vasopressin (Fig. 1E). Thus, when TRPC3 channels are expressed in excess, they respond differently to receptor activation of phospholipase C and to store depletion. These results have also been interpreted as evidence for the idea that homomeric (over expressed) TRPC3 channels are insensitive to store depletion (18, 19, 23, 43). Alternatively, we hypothesized that the regulatory sites with which diacylglycerol and protein kinase C need to interact to affect Ca2+ entry are not accessible under physiologic conditions because binding of Orai occludes them. We tested the hypothesis by examining Orai's effect on SOCE in HEK cells overexpressing TRPC3 or TRPC6.
Orai1 Confers to TRPC3 and TRPC6 the Ability to Respond to Store Depletion.
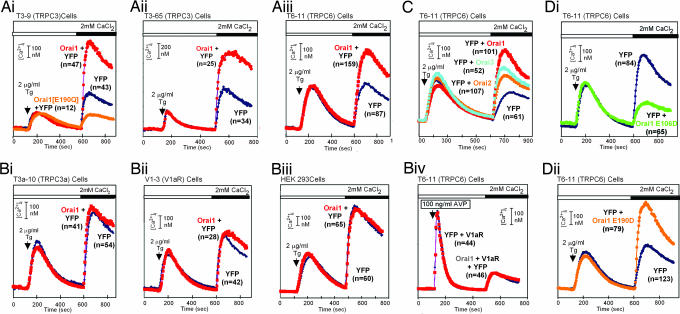
Huang and collaborators (44) showed that the Ca2+ sensor STIM1 binds and activates TRPC1. Whether Orai would affect TRPC channels has not been reported. We therefore tested the effect of Orai1 in cells stably overexpressing TRPCs by comparing SOCE in mock-transfected cells (YFP), which, in addition to the store-insensitive TRPC, express an endogenous complement of SOCE channels of essentially unknown molecular makeup. If Orai1 conferred responsiveness to store depletion to the overexpressed TRPCs, we expected to see an increase in Tg-induced Ca2+ entry. Initial experiments testing for an effect of Orai1 on TRPC3 in this way only showed inhibition of Tg-activated Ca2+ entry with no indication of an effect on TRPC3. This inhibitory effect was in agreement with similar data by two other laboratories (36, 38). Because there was no obvious reason for Orai1 being inhibitory, we tested whether the inhibitory effect might be a reflection of toxic overexpression. To this end we lowered the amount of the Orai-carrying plasmid in the transfection procedure below the standard 1 μg per transfection. Indeed, excess Orai turned out to be deleterious to cells. Lowering the Orai plasmid between 10 and 20 times, to below 100 ng per transfection, brought out a stimulating effect (Fig. 2). This stimulating effect has so far only been seen in cells overexpressing TRPC3 or TRPC6, the only two TRPCs for which we have cell lines overexpressing TRPCs in stable form (Fig. 2A). In untransfected HEK-293 cells, or HEK cells overexpressing another membrane protein (the V1a vasopressin receptor), or in cells overexpressing TRPC3a, a store depletion-sensitive variant of TRPC3 (19), Orai1 did not affect SOCE (Fig. 2 Bi–Biii). Likewise, expression of Orai in COS1 cells did not affect their SOCE (data not shown). Orai also did not affect ROCE in control HEK-293 cells (data not shown) or in HEK cells overexpressing TRPC6 (Fig. 2Biv). The stimulatory effect of Orai on SOCE is not restricted to Orai1. Orai2 and Orai3 also conferred responsiveness to store depletion (Fig. 2C).
Fig. 2.
Expression of Orai potentiates Tg-induced SOCE in cells overexpressing TRPC3 or TRPC6, but not in cells not overexpressing these channels. Shown are (1) the SOCE-enhancing effects of human Orai1 in T3–65 cells (Ai), T3–9 cells (Aii), and T6–11 cells (Aiii), which overexpress TRPC3 or TRPC6; (2) the lack of such an effect in T3a-10 cells expressing the Tg-sensitive TRPC3a splice variant (Bi), in V1–3 cells, which stably overexpress the rat V1a vasopressin receptor (Bii) and in HEK-293 cells (Biii); (3) the lack of an effect on V1a receptor-triggered ROCE in T6–11 cells (Biv); (4) the potentiating effect of human Orai2 and Orai3 on SOCE in T6–11 cells (C); and (5) the effects of human Orai1[E106]D (Di), Orai1[E1900D] (Dii), and Orai1[E190Q] (Ai) on SOCE in T6-11 cells.
A strong case has been made for Orai1 being itself a pore-forming ion channel (31, 39, 40). A common denominator in the studies that led to this conclusion was that mutations of conserved acidic amino acids, hypothesized to participate in forming a selectivity filter, changed the permeation characteristics of channels formed by coexpression of Orai1 and STIM1 (31, 39, 40). We also tested for effects of mutating Orai1 in cells stably expressing either TRPC3 or TRPC6. When expressed in SCID patient cells lacking SOCE (33) Orai1[E106D] restored SOCE partially (31). When expressed in HEK-293 cells carrying wild-type Orai1, -2, and -3, this mutant reduced endogenous SOCE by ≈50–60% (Fig. 2Di), behaving as a dominant negative. Likewise, Orai1[E190Q], which in SCID cells had lost the ability to reconstitute SOCE, inhibited SOCE (Fig. 2Ai). In contrast, and in agreement with findings by Prakriya and collaborators (31), the Orai1[E190D] mutant, which in SCID patient cells restored SOCE to normal levels (31), was also indistinguishable in our hands from wild-type Orai1 in its ability to confer store depletion sensitivity to TRPC6.
Electrophysiologic Effects of Orai.
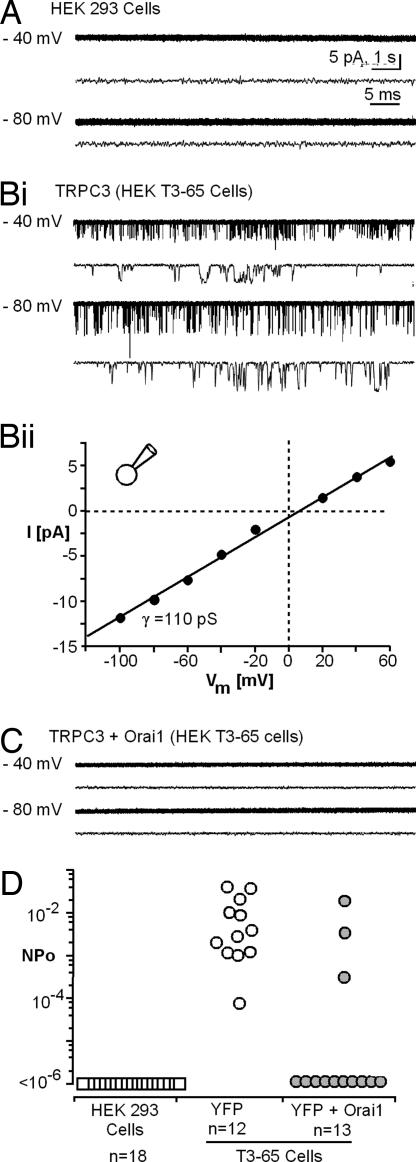
HEK cells stably overexpressing TRPC3 present in cell-attached patches with spontaneously gating nonselective cation channels having a unitary channel conductance of 110 pS (Fig. 3). Channel activity of this type is not observed in parental HEK-293 cells and is suppressed in cells transfected with Orai1 expression plasmid (Fig. 3 C and D), under conditions that elicit store depletion activate Ca2+ entry (Fig. 2Ai). Similar results were obtained with TRPC6-expressing cells (data not shown). As seen by Western blotting with anti-HA, Orai expression did not affect the expression of the HA-tagged TRPC3 or TRPC6 (data not shown).
Fig. 3.
Orai1 suppresses the spontaneous activity of TRPC3 channels. (A) Representative single-channel activity records obtained from cell-attached membrane patches of control HEK-293 cells at holding potentials. (Bi) Representative records T3-65 cells overexpressing TRPC3. (Bii) Single-channel I–V relationship for TRPC3 channels of T3-65 cells. γ, single-channel conductance. (C) Representative records from Orai1-expressing T3–65 cells. (D) Summary of channel activity, expressed as average NPo per patch, recorded from HEK 293 cells, YFP-transfected T3–65 cells, and T3-65 cells transfected with plasmids encoding YFP and Orai1 (60 ng). NPo of Orai1-expressing cells is significantly smaller (P < 0.001 based on Wilcoxon–Mann–Whitney rank sum test).
Physical Interaction of Orai with TRPC.
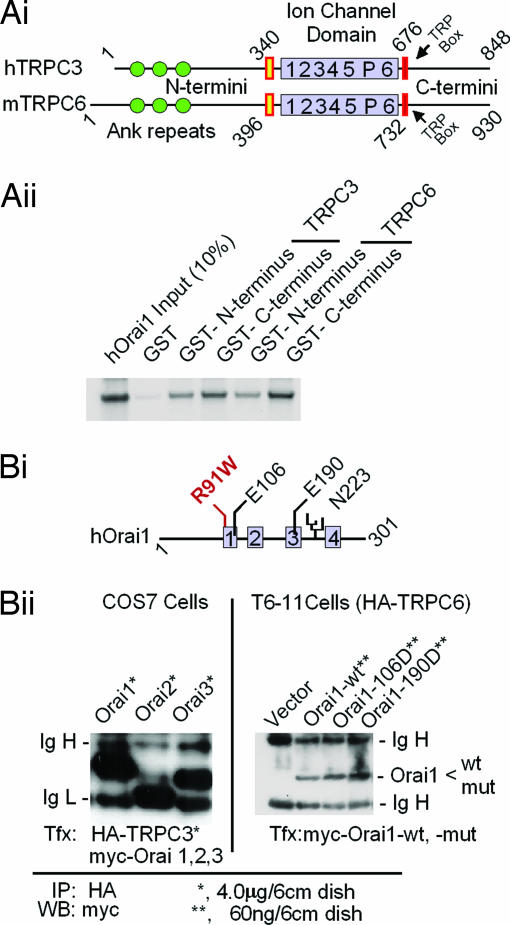
Fig. 4 shows that TRPC and Orai proteins interact directly both in vitro as seen in GST pull-down assays (Fig. 4A) and in the context of intact cells as seen in coimmunoprecipitation experiments (Fig. 4B). For coimmunoprecipitation the cells were lysed, the HA-tagged TRPC protein was immunoprecipitated with anti-HA-coated beads, and the beads were analyzed for associated myc-tagged Orai proteins. Clearly, all three Orai (Fig. 4Bii Left), as well as two Orai1 mutants (Fig. 4Bii Right), bound to TRPCs. Importantly, Orai proteins associated with TRPC6 under conditions known to lead to the changes in SOCE shown in Fig. 2. These experiments show that Orai proteins are able to form relatively stable complexes with a TRPC, and that although the N and C termini both interact with Orai in vitro, there seems to be a preference for interaction with the C termini over the N termini. The effect of the Orai proteins to enhance Ca2+ entry is therefore likely to be due to direct interaction with the TRPCs, as opposed to being indirect and mediated by another cellular component.
Fig. 4.
TRPC3 and TRPC6 interact with Orai in vitro (A) and in vivo (B). (Ai) Domain structures of TRPC3 and TRPC6 as deduced from their respective cDNAs. (Aii) In vitro interaction of in vitro-translated 35S-labeled N an C termini of TRPC3 and TRPC6 with Orai1 fused to GST using as binding/washing buffer 20 mM Tris·HCl (pH 7.4)/150 mM NaCl/1 mM EDTA/5 mM MgCl2/0.5% Nonidet P-40/complete protease inhibitor mixture tablets (one tablet per 50 ml). The retention of Orai1 on the fusion proteins is shown. Orai did not bind to GST without fused TRPC fragments (lane 2). (Bi) Domain structure of Orai1, location of a glycosylation site (N223), and location of mutations tested for activity. Gray boxes, likely transmembrane domains; R91W, a mutation that causes loss of SOCE in human T cells (34). (Bii Left) myc-tagged Orai proteins coimmunoprecipitate with HA-tagged TRPC3 when coexpressed in COS cells. (Bii Right) myc-tagged wild-type or mutated Orai1 coimmunoprecipitates with HA-tagged TRPC6 from T6-11 cells in which they are expressed at levels leading to functional changes. Cells were plated in 6-cm dishes and transfected with the indicated amounts of expression plasmids as for Fura-2 Ca2+ monitoring experiments (Fig. 2) (12). After 36 h at 37°C, the cells were rinsed and solubilized with RIPA buffer (50 mM Tris·HCl, pH 7.4/150 mM NaCl/1% Nonidet P-40/0.25% deoxycholate) supplemented with complete mixture of protease inhibitor tablets (one tablet per 50 ml). The lysates were cleared by centrifugation and incubated with anti-HA antibody coupled to agarose beads for 10 h in the cold. The beads were washed two times with RIPA buffer and analyzed for retention of myc-positive Orai1 by Western blotting.
Discussion
Although the recent series of reports regarding STIM1 seem to be conclusive in as much that it is the molecular entity that senses store depletion and transmits it to the plasma membrane Ca2+ entry channel (28, 44, 45), the evidence that Orai is an ion channel is less compelling. The conclusion that Orai1 embodies an ion channel stems from studies in which mutations in Orai1 were found to change the permeation properties of the Tg-activated Ca2+ entry channel generated by coexpression of Orai1 and STIM1 (31, 39, 40). This type of result is reminiscent of data reported in the early 1990s on the function of a single-pass transmembrane protein of 130 aa. When expressed in Xenopus oocytes it caused the appearance of a slowly activating voltage-gated K+ current resembling cardiac IsK (46, 47). Unlikely as it seemed, the molecule was proposed to be a “minimal voltage gated K+ channel” (minK) when it was found that point mutations in its transmembrane domain caused the induced currents to have altered voltage dependence and altered ion selectivity (48). Yet the molecular structure underlying IsK turned out to be more complex. In 1996 the gene responsible for a familial type-1 long QT syndrome with abnormal IsK was discovered by positional cloning. It encodes a K+ channel, KVLQT1 (later renamed to KCNQ1) (49), a member of the, then, new subfamily of the classical six-transmembrane voltage-gated K+ channels. These K+ channels are now known to be complexes with an α4β2 subunit structure (50) of which KCNQ1 (KVLQT1) is the pore-forming subunit and KCNE1 (minK) is the regulatory β-subunit. minK does not seem either to line the pore of the channel or to form part of its voltage-regulated gate (reviewed in ref. 51). In Xenopus oocytes minK complemented a “dormant” oocyte KCNQ type channel, generating a regulated channel with IsK properties.
With this scenario in mind, the data so far available on Orai proteins are equally well explained by either a model in which Orai are self-contained ion channels activated by STIM1, as proposed (31, 39, 40), or a model in which the SOCE/Icrac channels are made up of TRPC pore-forming α-subunits and Orai regulatory β subunits (Fig. 5). In this model the β-subunit's role would be to transduce the store depletion signal from STIM to TRPC. By receiving signals from either PLC and inositol trisphosphate receptor (52, 53) or STIM1-Orai (54, 55), TRPCs could be the underlying ion channels responsible for both ROCE and SOCE. Definitive proof in support of Orai being regulatory subunits of TRPCs will have to come from identification of such complexes as the normal complement of nontransfected cells. Such experiments would also allow the testing of the hypothesis that the lack of effects of diacylglycerols and protein kinase activation in wild-type nontransfected cells (Fig. 1) is due to physical occlusion of their sites of action by Orai, the hypothetical regulatory subunit. It also remains to be established whether all TRPCs can interact with all Orai and/or whether their interactions follow selectivity rules.
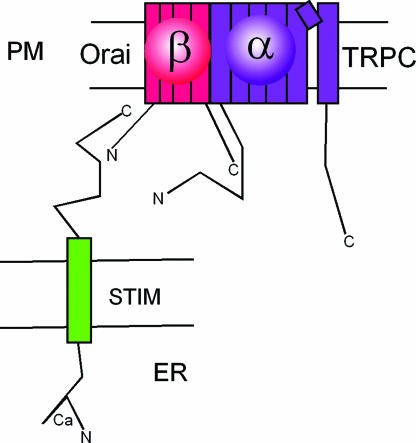
Fig. 5.
A plausible model of the molecular makeup of Icrac channels activated by STIM1. STIM1 senses the depletion of Ca2+ from the endoplasmic reticulum stores. Orai, a regulatory β-subunit of the TRPC ion channel α-subunit, is shown as the transducer of the STIM1 signal into activation of TRPC. PM, plasma membrane; ER, endoplasmic reticulum.
Experimental Procedures
Cell Lines.
HEK-293 cells were from the American Type Culture Collection (cat no. CRL-1573). COS7 cells were a gift from Savio Woo (Baylor College of Medicine, Houston, TX). T3–9, T3–65, and T3a-10 are HEK-293 cell-derived cell clones that stably overexpress TRPC3 or TRPC3a tagged at their N termini with the HA epitope (MYPYDVPDYA) (12, 19). T6–11 is a HEK-293 cell-derived clone that overexpresses the murine HA-tagged (N terminus) TRPC6 in stable form (56).
The protocols to measure changes in intracellular concentrations of cytosolic Ca2+ in single cells by videomicroscopy using the fluorescent indicator-dye Fura-2 have been described (12, 19). The concentrations shown are the averages of the indicated number of cells on two to three coverslips that resulted from single transfections. Each transfection was repeated two to four times with similar results. Cells in which Ca2+ levels dropped >10% upon switching into Ca2+-free medium were omitted from the analyses. The GST pull-down conditions used to assess interaction between GST-fusion proteins synthesized in BL-21 E. coli cells and attached to glutathione-beads (57) and proteins synthesized in vitro by a reticulocyte lysate using 35S-labeled methionine and cysteine were as described in refs. 19, 53, and 57. Solubilization and coimmunoprecipitation of HA-tagged TRPCs and myc-tagged Orai1 were as in ref. 57.
Reagents.
Monoclonal anti-myc antibody 9E10 for Western blotting was from Clontech. Agarose-coupled monoclonal anti-HA HA-7 antibody used for immunoprecipitation was from Sigma. Monoclonal anti-HA antibody 5CA12, purified from mouse ascites, used for immunoprecipitation, was a gift from Arnold Berk (Molecular Biology Institute, University of California, Los Angeles).
cDNAs encoding human Orai1, -2, and -3, cloned into an expression vector under the control of a CMV promoter were from OriGene Technologies. The nucleotide sequence encoding the myc epitope (MASMQKLISEEDL) was attached to the 5′ end of the ORF by standard molecular biology techniques. Site-directed mutagenesis was performed by using QuikChange reagents and protocols from Stratagene. Oligonucleotides were from Invitrogen or Sigma–Genosys. Plasmid pGST-GEX-4T-3 (Amersham Biosciences) served as backbone for the construction of GST-Orai fusion proteins (GST at N terminus). Fusion proteins attached to glutathione-agarose beads were prepared according to protocols supplied in kit form by Amersham Biosciences. pEYFP was from Clontech. The PKC inhibitor GF103203x was from Calbiochem. Oleyl-sn-acetyl-glycerol (OAG) was from Sigma. Complete protease inhibitor mixture tablets were from Roche.
PCR.
Reverse-transcript PCR (RT-PCR) was performed as described in ref. 58 (primer sequences are available upon request). Negative controls were obtained by analyzing a mock reverse transcript prepared in parallel by omitting the reverse transcriptase during the reverse-transcription step.
Cell Culture and Transfections.
For SOCE measurements, cells were plated at 1.5 × 106 cells per 60-mm dish and transfected with a mixture of 5.5 μg expression plasmids containing 0.5 μg of pEYFP, 60–90 ng of pOrai, and pcDNA3 with or without other inserts (e.g., via receptor cDNA) using Lipofectamine 2000 as the transfecting agent and protocols supplied by the manufacturer (Invitrogen). The cells were incubated at 37°C in a CO2 incubator for 24 h and then replated onto 40 mm × 40 mm microscope cover glasses for Fura-2 imaging. HEK and COS7 cells were maintained in DMEM and 10% heat-inactivated FBS supplemented with 100 units/ml penicillin G and 100 μg/ml streptomycin.
Electrophysiology.
Unitary TRPC currents were recorded under voltage-clamp in the cell-attached patch configuration with an EPC-10 amplifier (HEKA Electronik). The patch pipettes (1.5–2.0 MÙ) were coated with Sylgaard 184 elastomer (Dow Corning) and filled with 140 mM NaCl, 0.1 mM EGTA, and 10 mM Hepes with the pH adjusted to 7.4. All experiments were performed at room temperature (20–24°C) on cells bathed in high potassium to zero the membrane potential. The extracellular bath solution consisted of 145 mM KCl, 2 mM MgCl2, 0.1 mM CaCl2, and 10 mM Hepes with the pH adjusted to 7.4 with KOH. Thirty-second segments of single-channel activity were recorded at different holding potentials. Records were acquired at 20 kHz and filtered at 4 kHz. Single-channel records were analyzed with TAC and TACFit software (Bruxton, Seattle, WA) to determine channel amplitude and activity (NPo).
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Sciences.
Abbreviations
- OAG
oleyl-acetyl-glycerol
- PMA
phorbol-myristoyl-acetate
- ROCE
receptor-operated Ca2+ entry
- SOCE
store-operated Ca2+ entry
- Tg
thapsigargin
- YFP
yellow fluorescent protein.
Footnotes
The authors declare no conflict of interest.
References
- 1.Takemura H, Hughes AR, Thastrup O, Putney JW. J Biol Chem. 1989;246:12266–12271. [PubMed] [Google Scholar]
- 2.Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, Drobak BK, Bjerrum BJ, Christensen SB, Hanley MR. Agents Actions. 1989;27:17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- 3.Putney JW., Jr Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 4.Putney JW., Jr Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 5.Hoth M, Penner R. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 6.Franzius D, Hoth M, Penner R. Pflügers Arch. 1994;428:433–438. doi: 10.1007/BF00374562. [DOI] [PubMed] [Google Scholar]
- 7.Fasolato C, Hoth M, Matthews G, Penner R. Proc Natl Acad Sci USA. 1993;90:3068–3072. doi: 10.1073/pnas.90.7.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardie RC, Minke B. Trends Neurosci. 1993;91:371–376. doi: 10.1016/0166-2236(93)90095-4. [DOI] [PubMed] [Google Scholar]
- 9.Selinger Z, Doza YN, Minke B. Biochim Biophys Acta. 1993;1179:283–299. doi: 10.1016/0167-4889(93)90084-3. [DOI] [PubMed] [Google Scholar]
- 10.Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. Proc Natl Acad Sci USA. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abramowitz J, Yildirim E, Birnbaumer L. In: The TRP Family of Ion Channels. Liedke W, editor. Boca Raton, FL: CRC; 2006. pp. 1–30. [Google Scholar]
- 12.Zhu X, Jiang M, Peyton MJ, Boulay G, Hurst R, Stefani E, Birnbaumer L. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]
- 13.Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, Mori Y. J Biol Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- 14.Zitt C, Zobel A, Obukhov Y, Harteneck C, Kalkbrenner F, Lückhoff A, Schultz G. Neuron. 1996;16:1189–1196. doi: 10.1016/s0896-6273(00)80145-2. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Wang W, Singh BB, Lockwich T, Jadlowiec J, O'Connell B, Wellner R, Zhu MX, Ambudkar IS. J Biol Chem. 2000;275:3403–3411. doi: 10.1074/jbc.275.5.3403. [DOI] [PubMed] [Google Scholar]
- 16.Vannier B, Peyton M, Boulay G, Brown D, Qin N, Jiang M, Zhu X, Birnbaumer L. Proc Natl Acad Sci USA. 1999;96:2060–2064. doi: 10.1073/pnas.96.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jungnickel MK, Marrero H, Birnbaumer L, Lémos JR, Florman HM. Nat Cell Biol. 2001;3:499–502. doi: 10.1038/35074570. [DOI] [PubMed] [Google Scholar]
- 18.Vazquez G, Wedel BJ, Trebak M, Bird GS, Putney JW., Jr J Biol Chem. 2003;278:21649–21654. doi: 10.1074/jbc.M302162200. [DOI] [PubMed] [Google Scholar]
- 19.Yildirim E, Kawasaki BT, Birnbaumer L. Proc Natl Acad Sci USA. 2005;102:3307–3311. doi: 10.1073/pnas.0409908102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philipp S, Cavalie A, Freichel M, Wissenbach U, Zimmer S, Trost C, Marquart A, Murakami M, Flockerzi V. EMBO J. 1996;15:6166–6171. [PMC free article] [PubMed] [Google Scholar]
- 21.Philipp S, Hambrecht J, Braslavski L, Schroth G, Freichel, Murakami M, Cavalie A, Flockerzi V. EMBO J. 1998;77:4274–4282. doi: 10.1093/emboj/17.15.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riccio A, Medhurst AD, Mattei C, Kelsell RE, Calver AR, Randall AD, Benham CD, Pangalos MN. Brain Res. 2002;109:9510–9514. doi: 10.1016/s0169-328x(02)00527-2. [DOI] [PubMed] [Google Scholar]
- 23.Lievremont JP, Bird GS, Putney JW., Jr Am J Physiol. 2004;287:C1709–C1716. doi: 10.1152/ajpcell.00350.2004. [DOI] [PubMed] [Google Scholar]
- 24.Freichel M, Suh SH, Pfeifer A, Schweig U, Trost CP, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, et al. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- 25.Ohba T, Watanabe H, Murakami M, Takahashi Y, Iino K, Kuromitsu S, Mori Y, Ono K, Iijima T, Ito H. J Mol Cell Cardiol. 2006 doi: 10.1016/j.yjmcc.2006.10.020. in press. [DOI] [PubMed] [Google Scholar]
- 26.Yue L, Peng JB, Hediger MA, Clapham DE. Nature. 2001;410:705–709. doi: 10.1038/35070596. [DOI] [PubMed] [Google Scholar]
- 27.Schindl R, Kahr H, Graz I, Groschner K, Romanin C. J Biol Chem. 2002;277:26950–26958. doi: 10.1074/jbc.M203700200. [DOI] [PubMed] [Google Scholar]
- 28.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL. Curr Biol. 2006;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 31.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 32.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feske S, Prakriya M, Rao A, Lewis RS. J Exp Med. 2005;202:651–662. doi: 10.1084/jem.20050687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 35.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 37.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, et al. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riccio A, Mattei C, Kelsell RE, Medhurst AD, Calver AR, Randall AD, Davis JB, Benham CD, Pangalos MN. J Biol Chem. 2002;277:12302–12309. doi: 10.1074/jbc.M112313200. [DOI] [PubMed] [Google Scholar]
- 42.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 43.Zhu X, Jiang M, Birnbaumer L. J Biol Chem. 1998;273:133–142. doi: 10.1074/jbc.273.1.133. [DOI] [PubMed] [Google Scholar]
- 44.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 45.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Proc Natl Acad Sci USA. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takumi T, Ohkubo H, Nakanishi S. Science. 1988;242:1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- 47.Murai T, Kakizuka A, Takumi T, Ohkubo H, Nakanishi S. Biochem Biophys Res Commun. 1989;161:176–181. doi: 10.1016/0006-291x(89)91577-5. [DOI] [PubMed] [Google Scholar]
- 48.Goldstein SA, Miller C. Neuron. 1991;7:403–408. doi: 10.1016/0896-6273(91)90292-8. [DOI] [PubMed] [Google Scholar]
- 49.Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, et al. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 50.Chen H, Kim LA, Rajan S, Xu S, Goldstein SA. Neuron. 2003;40:15–23. doi: 10.1016/s0896-6273(03)00570-1. [DOI] [PubMed] [Google Scholar]
- 51.Melman YF, Krummerman A, McDonald TV. Trends Cardiovasc Med. 2002;12:182–187. doi: 10.1016/s1050-1738(02)00158-5. [DOI] [PubMed] [Google Scholar]
- 52.Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 53.Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Proc Natl Acad Sci USA. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luik RM, Wu MM, Buchanan J, Lewis RS. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu MM, Buchanan J, Luik RM, Lewis RS. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, Birnbaumer L. J Biol Chem. 1997;272:29672–29680. doi: 10.1074/jbc.272.47.29672. [DOI] [PubMed] [Google Scholar]
- 57.Kawasaki BT, Liao Y, Birnbaumer L. Proc Natl Acad Sci USA. 2006;103:335–340. doi: 10.1073/pnas.0508030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yildirim E, Dietrich A, Birnbaumer L. Proc Natl Acad Sci USA. 2003;100:2220–2225. doi: 10.1073/pnas.0438036100. [DOI] [PMC free article] [PubMed] [Google Scholar]