Abstract
A key feature of memory processes is to link different input signals by association and to preserve this coupling at the level of synaptic connections. Late-phase long-term potentiation (L-LTP), a form of synaptic plasticity thought to encode long-term memory, requires gene transcription and protein synthesis. In this study, we report that a recently cloned coactivator of cAMP-response element-binding protein (CREB), called transducer of regulated CREB activity 1 (TORC1), contributes to this process by sensing the coincidence of calcium and cAMP signals in neurons and by converting it into a transcriptional response that leads to the synthesis of factors required for enhanced synaptic transmission. We provide evidence that TORC1 is involved in L-LTP maintenance at the Schaffer collateral–CA1 synapses in the hippocampus.
Keywords: BDNF, calcineurin, cAMP-response element-binding protein, long-term potentiation, memory
Transducers of regulated cAMP-response element-binding protein (CREB) activity (TORCs) are newly discovered coactivators that dramatically increase CREB's transcriptional activity independently of CREB Ser-133 phosphorylation (1, 2). Recently, it has been shown that TORC2 functions as a pancreatic coincidence detector. In insulinoma cells, glucose and gut hormones, via respective activation of L-type calcium channels and the cAMP pathway, synergistically promote the dephosphorylation and the concomitant nuclear translocation of TORC2 (3). In the brain, encoding and storing associative memories requires detection of the coincidence of different input signals and translation of these associations into changes in the number, structure, or function of synapses. Therefore, it appears that short-lived coincidences result in the transcriptional activation of genes encoding factors required for enhanced synaptic transmission. TORCs present two features that neurons could use to create an association: they can detect the coincidence of the two most important second messengers, calcium and cAMP, and they are potent coactivators of CREB, a transcription factor known to drive the expression of genes underlying synaptic plasticity, late-phase long-term potentiation (L-LTP), learning, and memory (4–7).
CREB-dependent promoters have been generally thought to respond to various intracellular and extracellular cues by the stimulus-dependent phosphorylation of CREB at Ser-133 and resultant recruitment of the coactivator CREB binding protein (CBP) (5, 6, 8, 9). Modification of CREB at this site often mirrors the activation of neurons, leading to the idea that the expression of plasticity-related genes relies on CREB/CBP interaction. However, some studies revealing a discrepancy between CREB phosphorylation and CREB-mediated gene transcription have challenged this model. For instance, monocular deprivation induces LacZ expression in the visual cortex of cAMP-response element (CRE)-LacZ transgenic mice (10), whereas phosphorylation of CREB at Ser-133 remains static (11). Similarly, the mechanism underlying CREB activation during LTP in the Schaffer collateral–CA1 pathway is still unclear. Tethering a strong transcriptional activation domain (VP16) to CREB facilitates hippocampal LTP (12), whereas transgenic mice expressing a mutant CREB unphosphorylable at Ser-133 show normal hippocampal potentiation (13). Furthermore, CRE-mediated gene expression, but not CREB phosphorylation, correlates with the expression of hippocampal L-LTP in CRE-LacZ reporter mice (14). Although CREB phosphorylation could still be required for L-LTP induction, these contradictory results raise the possibility that neuronal activity could regulate CREB through additional mechanisms.
Here, we provide evidence that the recruitment of TORC1 is an important step in the CRE-regulated transcription underlying the modulation of synaptic strength. We show that TORC1 is expressed in adult mouse brain and cultured neurons, and that it translocates to the nucleus upon the concomitant activation of calcium and cAMP signaling pathways. Functionally, nuclear translocation of TORC1 results in the synergistic activation of CREB-mediated transcription. Our data also indicate that TORC1 is playing a role in the activation of brain-derived neurotrophic factor (BDNF) by calcium and cAMP. Finally, we show that TORC1 is involved in L-LTP maintenance at the Schaffer collateral–CA1 synapses in the hippocampus. Our results unveil a pivotal role of TORC1 in the molecular mechanism of synaptic plasticity and long-term memory.
Results
Expression and Calcium and cAMP-Dependent Nuclear Translocation of TORC1 and TORC2 in Neurons.
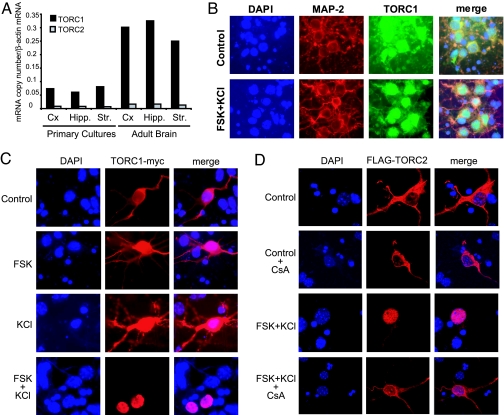
To assess whether TORCs could function as calcium- and cAMP-sensitive coincidence detectors in the central nervous system, we first analyzed by real-time RT-PCR the expression of TORC1 and TORC2 mRNAs in adult mouse brain and in primary neuronal cultures. We found that both TORCs are present in the cortex, hippocampus, and striatum, with TORC1 mRNA being ≈20 times more abundant than that for TORC2 (Fig. 1A). We next investigated whether TORCs could serve as coincidence detectors in neurons in a similar way as has been reported for insulinoma cells. In these cells, forskolin (FSK) decreases TORC2 phosphorylation via cAMP-mediated inhibition of salt-inducible kinases, whereas KCl triggers TORC2 dephosphorylation via calcium entry and resultant activation of calcineurin/protein phosphatase 2B. Subsequently, unphosphorylated TORC2 molecules are released from 14-3-3 proteins, and migrate to the nucleus to activate CRE-regulated genes (3, 15). By using a pan-TORC antibody to visualize endogenous TORCs (Fig. 1B), or following overexpressed TORC1 (Fig. 1C) and TORC2 (Fig. 1D), we observed nuclear translocation of the coactivators on combined application of FSK and KCl. In contrast, FSK or KCl alone had little effect (Fig. 1C), and the calcineurin inhibitor cyclosporine A (CsA) abolished the nuclear entry of TORCs (Fig. 1 D and data not shown). Inhibition of nuclear export by leptomycin B (LMB) was necessary to detect the nuclear accumulation of transfected TORC1, whereas TORC2 readily translocated in the absence of LMB. Translocation of endogenous proteins recognized by the pan-TORC antibody also required LMB pretreatment, further indicating that cortical neurons mainly express TORC1.
Fig. 1.
TORC1 and TORC2 are expressed in adult mouse brain and translocate to the nucleus of cultured neurons upon activation of calcium and cAMP pathways. (A) TORC1 and TORC2 mRNA levels in mouse neuronal cultures and adult brain as measured by real-time RT-PCR. Cx, cortex; Hipp., hippocampus; Str., striatum. (B) Nuclear translocation of endogenous TORCs in mouse cortical neurons exposed to FSK and KCl (preincubation with LMB). Microtubule-associated protein 2 (MAP-2) and DAPI stain neuronal processes and nuclei, respectively, are shown. (C) Immunofluorescence of neurons transfected with mouse TORC1-myc showing its nuclear translocation only in the presence of FSK and KCl (preincubation with LMB). (D) Immunofluorescence of neurons transfected with mouse Flag-TORC2 showing its nuclear translocation in the presence of FSK and KCl (no preincubation with LMB). Inhibition of calcineurin by CsA blocks the nuclear accumulation of TORC2 triggered by FSK and KCl. DAPI staining is shown to localize nuclei.
TORC1 Mediates the Synergistic Activation of CREB-Mediated Transcription by Calcium and cAMP in Neurons.
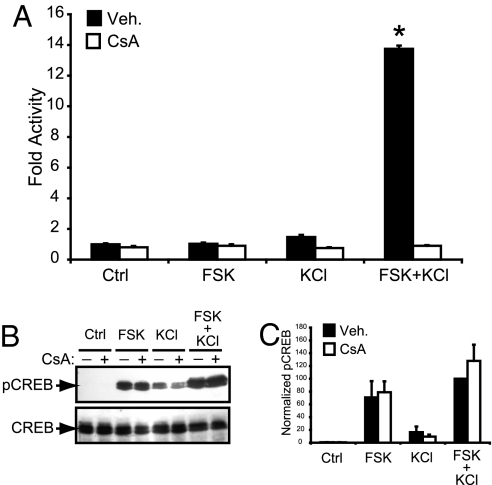
Having shown the predominant presence of TORC1 in neurons, and its nuclear translocation, we next assessed its role in CREB-mediated transcription. We measured CRE-dependent transcriptional activation by using a reporter gene transfected into mouse cortical neurons after exposure to FSK and KCl, either separately or in combination. Interestingly, only the pairing of both stimuli efficiently activated CREB-dependent transcription (Fig. 2A). Consistent with the involvement of TORCs, this synergistic activation was abolished by the immunophillin-binding calcineurin inhibitors CsA and FK506, but not by rapamycin, an immunophillin-binding compound that does not inhibit calcineurin [Fig. 2A and supporting information (SI) Fig. 6]. In a parallel experiment, we quantified the phosphorylation status of CREB at 30 min, 1 h, or 2 h after FSK and KCl exposure (Fig. 2 B and C and SI Fig. 7). At each time point, CsA treatment never reduced Ser-133 phosphorylation, demonstrating that the inhibition of CREB-mediated transcription by CsA is not because of altered levels of phosphorylated CREB. Moreover, these data clearly show that CREB phosphorylation per se is not sufficient to sustain an efficient transcriptional activation, further suggesting that the calcineurin-dependent nuclear translocation of TORCs is required for the activation of CREB-mediated transcription in neurons.
Fig. 2.
Calcium and cAMP pathways activate CREB synergistically in a calcineurin-dependent manner. (A) Effect of FSK and KCl on mouse cortical neurons transfected with a CRE-luciferase reporter in the absence (Veh.) or in the presence of CsA. Results are displayed as the mean (±SEM) of fold luciferase activity (n = 3) relative to control. ∗, Significantly different from all of the other conditions (two-way ANOVA followed by Bonferroni's post hoc test; P < 0.05). (B) Western blot of phospho-(Ser-133) CREB (pCREB) and total CREB levels in cortical neurons exposed for 30 min to FSK and/or KCl. (C) Mean densitometric band analysis of the Western blots shown in B. Data are representative of triplicate determinations. Intensity values were normalized to the FSK plus KCl condition, which was set equal to 100%.
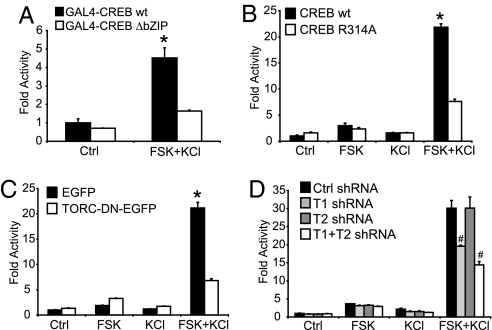
The characterization of CREB–TORC interaction has shown that TORCs bind to the CREB bZIP DNA-binding domain in an arginine 314-dependent manner (1, 3). We thus transfected cortical neurons with CREB constructs either lacking the bZIP domain or with an Arg314Ala mutation, and compared their activity to the wild-type CREB constructs (Fig. 3 A and B). Calcium and cAMP poorly activate these mutant forms of CREB unable to interact with TORCs, suggesting again that CREB Ser-133 phosphorylation is not sufficient to detect the coincidence of these signals. We then designed a specific dominant-negative form of TORC, which consisted of the first 147 aa of TORC1, comprising its nuclear localization signal and the CREB-binding domain (3), fused to EGFP. The expression of this protein drastically decreased the cAMP- and Ca2+-mediated enhancement of CREB-dependent transcription, whereas EGFP expression had no effect (Fig. 3C). Finally, we used RNA interference to diminish the expression of endogenous TORC1 and TORC2. We generated short hairpin RNA (shRNA)-producing plasmids, which efficiently inhibit the expression of TORC1 and TORC2, as shown in SI Fig. 8. TORC1 shRNA decreased by 35% the synergism triggered by FSK and KCl, whereas silencing of TORC2 was without effect (Fig. 3D). Combining TORC1 and TORC2 shRNA further reduced CREB-mediated transcription to 50%, suggesting that the low levels of TORC2 in neurons may marginally activate CREB when TORC1 is silenced. Nevertheless, these data suggest that TORC1 is playing a major role in the cAMP/Ca2+ coincidence detection in neurons, whereas the participation of TORC2 in this process in unclear. The partial abolition of the synergism triggered by FSK and KCl is most probably caused by an incomplete silencing of TORC1. Taken together, these data constitute a compelling body of evidence that, in neurons, TORC1 is required for the synergistic activation of CREB-mediated transcription by Ca2+ and cAMP.
Fig. 3.
TORC1 mediates the synergistic activation of CREB-mediated transcription by calcium and cAMP in neurons. (A) Stimulation of CREB activity by calcium and cAMP requires its bZIP domain. Cortical neurons were transfected with a GAL4-luciferase reporter, and either full-length GAL4-CREB [wild-type (wt)] or truncated GAL4-CREB ΔbZIP (amino acids 4–283). (B) A CREB mutant defective in TORC binding is not activated synergistically by calcium and cAMP. Cortical neurons were transfected with a CRE-luciferase reporter in combination with either wild-type CREB or a CREB mutant with an arginine-to-alanine substitution at position 314 within the bZIP domain (R314A). (C) Effect of a TORC dominant-negative (TORC-DN-EGFP) on the synergistic activation of CREB-mediated transcription by FSK and KCl. Expression of EGFP was used as a control. (D) Silencing of TORC1 inhibits the synergistic effects of calcium and cAMP signals on CREB activation. Cortical neurons were transfected with a CRE-luciferase reporter gene and shRNA producing plasmids against TORC1 (T1) and TORC2 (T2) or a nonsilencing negative control (Ctrl). Results are displayed as the mean (±SEM) of fold luciferase activity (n = 3) relative to control. ∗, Significantly different from all of the other conditions (two-way ANOVA followed by Bonferroni's post hoc test; P < 0.05). #, Significantly different from the Ctrl shRNA FSK plus KCl condition (four-way ANOVA followed by Bonferroni's post hoc test; P < 0.05).
Synergistic Activation of bdnf Gene Expression Requires Calcineurin Activity.
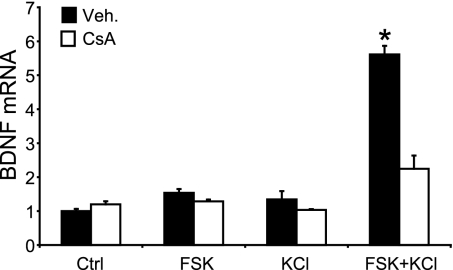
Heterosynaptic LTP in the CA1 region of the hippocampus serves as an important and biologically relevant model of how neurons establish association. Repeated tetanic stimulation of an input to the CA1 cells triggers the synthesis of factors that can be intracellularly distributed, and captured by synaptic tags at other, weakly stimulated synapses that subsequently become potentiated (16). Whereas establishment of the synaptic tag does not require de novo protein synthesis (16), the expression of the diffusible plasticity factors appears to be controlled by CREB (12). BDNF, a bona fide CREB target gene product (17), was identified as one of these intracellular factors (18), which produces a long-lasting enhancement of synaptic transmission in the hippocampus (19). Moreover, BDNF-deficient transgenic mice show a reduced L-LTP in the Schaffer collateral–CA1 pathway (18, 20). These findings prompted us to test whether TORC1 is involved in the regulation of BDNF gene expression. As shown by real-time RT-PCR, Ca2+ and cAMP synergistically increased the levels of BDNF mRNA in neurons, and CsA treatment strongly reduced this effect (Fig. 4). These results support the notion that TORC1 is required for the activation of neuronal CREB target genes by Ca2+ and cAMP, and raise the intriguing possibility that TORC1 could be involved in homosynaptic and heterosynaptic hippocampal LTP.
Fig. 4.
The CREB target gene bdnf is synergistically activated by calcium and cAMP in a calcineurin-dependent manner. BDNF mRNA levels in mouse cortical neurons exposed to FSK and/or KCl for 90 min, in the absence (Veh.) or presence of CsA (measured by real-time RT-PCR). Levels of BDNF mRNA are normalized to β-actin and represented in fold increase relative to control. Results are displayed as the mean ± SEM (n = 3). ∗, Significantly different from all of the other conditions (two-way ANOVA followed by Bonferroni's post hoc test; P < 0.05).
TORC1 Is Involved in Hippocampal L-LTP.
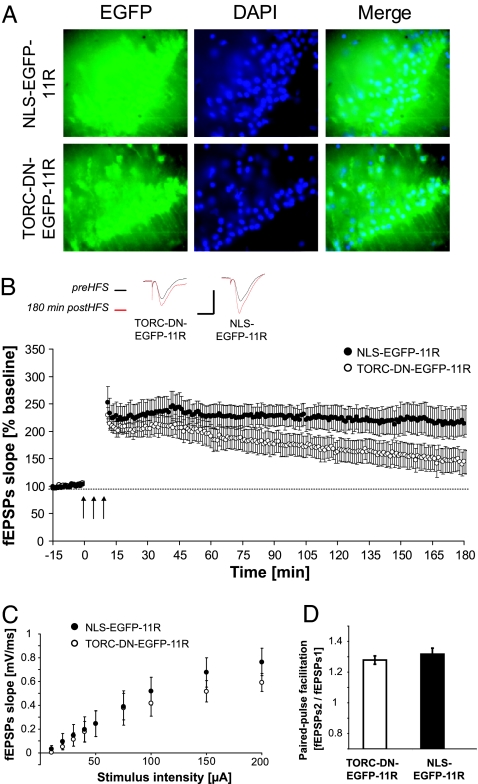
To directly test whether L-LTP can be inhibited by interfering with endogenous TORC1 function, we added a protein transduction domain, consisting of 11 arginine residues (21), to the TORC dominant-negative used in Fig. 3C and purified recombinant protein (TORC-DN-EGFP-11R) for delivery to acute brain slices. As a control protein, we used EGFP fused to a nuclear localization signal and the 11 arginines (NLS-EGFP-11R) (Fig. 5A). Three trains of tetanic stimulation (1 s, 100 Hz, 5 min between trains) resulted in a strong potentiation of the field excitatory postsynaptic potentials, which persisted up to 3 h in the presence of the control protein NLS-EGFP-11R. In contrast, the dominant-negative TORC-DN-EGFP-11R provoked a statistically significant decay of this potentiation 75 min after the tetanic stimulations (Fig. 5B). The input/output curve and the paired-pulse facilitation were similar for both conditions, indicating no gross abnormalities in synaptic transmission and presynaptic function (Fig. 5 C and D). These results constitute strong evidence that TORC1 is involved in the maintenance of L-LTP at the Schaffer collateral–CA1 synapses in the hippocampus.
Fig. 5.
TORC1 is involved in L-LTP maintenance in the Schaffer collateral–CA1 pathway. (A) Transduction of the recombinant TORC dominant-negative protein (TORC-DN-EGFP-11R) or the control recombinant protein (NLS-EGFP-11R) in hippocampal slices. Many cells contain recombinant protein in the nucleus, as evidenced by the colocalization of EGFP and DAPI staining. (B) Effect of TORC-DN-EGFP-11R on L-LTP induced by three trains of 100-Hz pulses (1-s duration) separated by 5-min intervals (HFS). Seventy-five minutes after the tetanic stimulation, LTP became significantly smaller in slices treated with TORC- DN-EGFP-11R (n = 7, 7 rats) than in control slices (n = 9, 9 rats) treated with NLS-EGFP-11R (t test; P < 0.05). An arrow indicates a stimulation of a 1-s train at 100 Hz. Traces show examples of postsynaptic responses before (preHFS; black) and 180 min after (180 min postHFS; red) the tetanic stimulation. [Scales for these traces: 1 mV (vertical), 10 ms (horizontal).] HFS was applied ≈1.5 h after the end of the incubation with the recombinant proteins. (C) Transduction of TORC-DN-EGFP-11R did not have any significant effect on the input/output curve. There was no significant effect of treatment after a two-way ANOVA on postsynaptic responses with treatment as an independent variable and concentration as a repeated measure (P > 0.05). N as in B. (D) Transduction of TORC-DN-EGFP-11R did not interfere with the presynaptic function as evidenced by unchanged paired-pulse facilitation (t test; P > 0.05). N as in B.
Discussion
Our study demonstrates that calcium and cAMP synergistically activate CREB-mediated transcription in neurons through the recruitment of a newly described CREB coactivator, called TORC1. This represents a novel mechanism of cross talk between calcium and cAMP pathways in neurons. These second messengers do not always act cooperatively and can even have antagonistic effects in these cells. For instance, phosphorylation of dopamine- and cAMP-regulated phosphoprotein 32 at Thr-34 is stimulated by cAMP, but inhibited by calcium, leading to a differential regulation of protein phosphatase-1 (22). Moreover, it was recently shown that cAMP inhibits the calcium-mediated activation of the transcription factor myocyte enhancer factor 2D in hippocampal neurons (23). CREB, on the other hand, was found to be a substrate for both PKA and Ca2+/calmodulin-dependent protein kinase II, soon after its cloning (24, 25). Therefore, it was speculated that CREB could detect coincidences between calcium entry and elevations in cAMP concentrations triggered by action potentials and G protein-coupled receptor activation, respectively. Consequently, it was hypothesized that the convergence of the two second messenger pathways onto CREB could participate in long-term associative synaptic plasticity (24). Theoretically, two kinases directly phosphorylating CREB at the same residue could have at best additive effects on transcription. However, according to our present results, calcium and cAMP exert a synergistic, rather than additive effect on CRE-dependent transcription, which should allow neurons to detect coincidences at a better signal-to-noise ratio. Mechanistically, such a synergism is likely to reside in the fact that TORC1 and TORC2 rely on the simultaneous activation of a phosphatase and inhibition of a corresponding kinase to detect coincidences. The extracellular signal-regulated kinase that participates in the cascade leading to CREB phosphorylation (8) could also serve as coincidence detector (26). Indeed, calcium activates extracellular signal-regulated kinase through Ca2+/calmodulin-dependent protein kinase IV (27), whereas cAMP promotes its nuclear translocation through PKA (28). Therefore, to investigate the role of TORCs in the synergistic effects of calcium and cAMP on CRE-mediated transcription, we used a specific dominant-negative protein and we silenced endogenous TORC1 and TORC2 genes by using RNA interference. Our results demonstrate that TORC1 plays a major role in detecting calcium/cAMP coincidences in neurons. However, it remains to be determined whether CREB phosphorylation and the resulting recruitment of CBP are always involved in CRE-regulated transcription in addition to TORC-mediated transactivation, or whether TORCs could activate CREB without CBP recruitment in certain conditions. Of note, CREB phosphorylation could still be indispensable for activity-dependent transcription of genes that require CBP-mediated histone acetylation and convergent action of different kinases on CREB could convey information to the nucleus about stimulus duration and amplitude (8).
As evidenced by the present study, synergistic activation of CRE-dependent target genes is accompanied by the nuclear translocation of transfected and endogenous TORCs. However, we observed a clear difference between TORC1 and TORC2 with respect to their readiness to translocate to the nucleus. Inhibition of CRM-1/exportin1-mediated nuclear export by LMB was necessary to demonstrate the nuclear accumulation of transfected TORC1, as well as endogenous TORC1 that is predominantly expressed in neurons. On the contrary, TORC2 translocated in the absence of LMB, but is much less abundant than TORC1 in these cells. We speculate that the resistance of TORC1 to translocation may contribute to filter out stimuli that do not need to trigger a transcriptional response leading to synaptic changes.
Our finding that the synthesis of BDNF and hippocampal plasticity requires TORC1 suggest that both calcium and cAMP, which have an established role in L-LTP (4, 29), have to be present in CA1 cells within a defined time window to allow enhanced synaptic transmission. Under physiological conditions, the coincidence of Ca2+ and cAMP may reflect repeated arrival of input signals. Calcium-stimulated adenylate cyclases have been shown to convert Ca2+ entry into cAMP signals; therefore, subsequent inputs exciting the same CA1 cell might result in Ca2+ entry that coincides with PKA activity triggered by the preceding stimulus. Indeed, the combined deletion of calcium-stimulated adenylate cyclases AC1 and AC8 prevents the late phase of homosynaptic LTP in the Schaffer collateral–CA1 pathway (30). Another interesting possibility is that the requirement for a Ca2+/cAMP coincidence in CA1 cells reflects the finding that D1/D5 dopamine receptor activation is necessary for hippocampal L-LTP (31). Dopamine release in hippocampal slices has been shown to be evoked by conventional tetanization protocols and is thought to be necessary, together with NMDA receptor activation, to trigger the synthesis of the cell-wide distributed, diffusible plasticity factors that can be captured by synaptic tags (32). In vivo in behaving animals, novelty is reported to facilitate hippocampal LTP in the Schaffer collateral–CA1 pathway in a D1/D5 receptor-dependent manner (33). From these studies, a mechanism emerges that relies on mesencephalic dopaminergic input to transform hippocampal early- phase LTP into L-LTP. Our study provides a cellular basis for this process by identifying TORC1 as a neuronal Ca2+/cAMP coincidence detector and a transcriptional coactivator for CREB-regulated gene expression.
We postulate that, besides its role in hippocampal L-LTP, TORC1 could be a key player of several neuronal processes that require Ca2+ and cAMP coincidence detection. Given the importance of CREB-mediated transcription in memory consolidation, addiction, neuronal survival, circadian rhythmicity, and developmental plasticity, future studies may endow TORC1 with a multitude of functions in the central nervous system.
Materials and Methods
Cell Culture and Transfection.
Primary cultures of neurons were prepared from 17-day-old Swiss mice embryos and cultured as described (34, 35). After 6 days in vitro, plasmid DNA was transfected by using Nupherin-neuron (Biomol, Plymouth Meeting, PA) and TransFectin (Bio-Rad, Hercules, CA) as described (35).
Plasmids.
pcDNA3-TORC1-myc was constructed by cloning the brain-derived mouse TORC1 cDNA into a pcDNA3-myc vector. pcDNA3-FLAG-TORC2 (1) was provided by M. Montminy (The Salk Institute, La Jolla, CA). CRE-luciferase and pUB6-LacZ (Invitrogen, Carlsbad, CA) have been described (36). pcDNA3-TORC11–147-EGFP was constructed by fusing the first 147 aa of mouse TORC1 to EGFP. R314A mutation was introduced into pRc/RSV-FLAG-CREB (37) via site-directed mutagenesis (Stratagene, La Jolla, CA). The Gal4-Luc reporter gene is described in ref. 38. pcDNA3-GAL-CREB constructs are described in SI Materials and Methods.
Pharmacology and Cell Stimulation.
Neuronal stimulation was usually performed 20 h after transfection. To reduce endogenous synaptic activity and prevent calcium entry through NMDA receptors, cortical neurons were pretreated for 30 min with 1 μM tetrodotoxin (Alexis, San Diego, CA), 100 μM d-(−)-2-amino-5-phosphonopentanoic acid (Sigma, St. Louis, MO), and 40 μM 6-cyano-7-nitroquinoxaline-2,3-dione (Sigma). To increase the specificity of the stimulations, neurons not depolarized with KCl were exposed to 10 μM nifedipine (Sigma) and neurons not stimulated with FSK were treated with 20 μM H-89 (Calbiochem, San Diego, CA). The effect of these inhibitors on CREB-mediated transcription is shown in SI Fig. 9. When indicated, neurons were exposed 30 min before stimulation to either 5 μM CsA (Calbiochem), FK506 (LC Laboratories, Woburn, MA), or rapamycin (Calbiochem). Thirty minutes after the addition of the inhibitors, cells were stimulated with 10 μM FSK (Calbiochem) and/or 30 mM KCl.
Reporter Assays.
Four hours after stimulation, transfected cells were lysed and tested for luciferase and β-galactosidase activities as described (35).
Immunocytochemistry.
Mouse cortical neurons were cultured on 12-mm glass coverslips. For the detection of endogenous TORCs and TORC1-myc, 10 ng/ml LMB (Sigma) was added 30 min before stimulation. Cortical neurons were stimulated with 10 μM FSK and 50 mM KCl, and 30 min later, cells were washed twice in PBS and fixed with 4% paraformaldehyde. All washing steps thereafter were performed by using 0.1% Triton X-100 and 0.2% BSA in PBS. Cells were permeabilized and blocked by using 0.5% Triton X-100 and 2% normal goat serum. The following primary antibodies were used: rabbit pan-TORC (amino acids 1–42 of human TORC1) antiserum (1, 3) (1:1,500) for endogenous TORCs, mouse anti-microtubule-associated protein 2 (1:8), mouse anti-myc (1:1,000; Cell Signaling Technology, Danvers, MA) for TORC1-myc, and mouse anti-FLAG M2 (1:600; Sigma) for FLAG-TORC2. Secondary antibodies were anti-mouse-Cy3 (1:600; Jackson Immunoresearch Laboratories, West Grove, PA) and anti-rabbit-Alexa 488 (1:200; Molecular Probes, Carlsbad, CA). Coverslips were mounted with a DAPI-containing mounting medium (Vector Laboratories, Burlingame, CA) and analyzed either with a Zeiss Axioskop 2 mot plus microscope or a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss Light Microscopy, Göttingen, Germany).
Western Blotting.
Thirty minutes, 1 h, or 2 h after stimulation, cortical neurons were lysed in 50 μl of hot (95°C) 3× SDS/PAGE buffer, and boiled for 10 min. The samples were briefly sonicated, clarified by centrifugation, and subjected to SDS/PAGE electrophoresis. After transfer to a nitrocellulose membrane, phospho-CREB and CREB were detected by using the PhosphoPlus CREB (Ser-133) antibody kit (Cell Signaling) following the manufacturer's instructions. Quantification of band intensity was performed with Scion (Frederick, MD) Image analysis software. Phospho-CREB intensity was normalized to total CREB for the corresponding lane. All experiments were performed in triplicate. Results are displayed as the mean ± SEM (n = 3) relative phospho-CREB levels.
RNA Interference.
Details on the pSUPER shRNA producing plasmids are given in the SI Materials and Methods. Mouse cortical neurons were transfected at 5 days in vitro with 0.2 μg of CRE-luciferase, 0.15 μg of pUB6-LacZ, and either 0.4 μg of pSUPER-Control, or 0.2 μg of pSUPER-TORC1 and/or 0.2 μg of pSUPER-TORC2. When only one TORC isoform was silenced, 0.2 μg of pSUPER-Control was cotransfected to keep the total amount of shRNA-producing plasmids constant. At 7 days in vitro, cell stimulation and reporter assays were performed as described above.
Real-Time Quantitative RT-PCR.
Total RNA was extracted from primary neurons or brain samples, and analyzed by real-time RT-PCR as described (35). The oligonucleotide primers for TORC1, TORC2, and BDNF, as well as the methods of quantification are described in SI Materials and Methods.
Expression and Purification of Recombinant Proteins.
The construction of the plasmids driving the expression of TORC-DN-EGFP-11R and NLS-EGFP-11R is described in SI Materials and Methods. Production of recombinant proteins in Escherichia coli BL21-AI (Invitrogen) was induced with 0.2% l-(+)-arabinose (Sigma) for 3.5 h. Bacteria were lysed in 50 mM phosphate buffer (pH 7.6), 0.3 M NaCl, 5% glycerol, 0.1% Triton X-100, 0.4 mM PMSF, 2 mM 2-mercaptoethanol, and 20 mM imidazole. Recombinant proteins were purified on a Ni-NTA agarose affinity column (Qiagen, Valencia, CA) and washed twice with 20 mM and eluted with 100 mM imidazole in lysis buffer.
Hippocampal Slices.
Male rats (R/WISTAR HAN; Charles River Laboratories, L'Arbresle, France), 4–6 weeks of age, were decapitated. Brains were dissected out and 350-μm-thick horizontal hippocampal slices were made in oxygenated ice-cold artificial cerebrospinal fluid (ACF) with high Mg2+, low Ca2+, and K+ (118 mM NaCl/2 mM KCl/4 mM MgCl2/0.5 mM CaCl2/1.2 mM NaH2PO4/25 mM NaHCO3/10 mM glucose). Slices were submerged in oxygenated ACF (124 mM NaCl/3.5 mM KCl/1.3 mM MgSO4/2.5 mM CaCl2/1 mM NaH2PO4/26 mM NaHCO3/10 mM glucose) at room temperature. After 1 h, slices were superfused with oxygenated ACF containing either 0.37 μM TORC-DN-EGFP-11R or 0.37 μM NLS-EGFP-11R for 1 h, washed out with oxygenated ACF for 5 min, transferred in an interface recording chamber, and superfused with oxygenated ACF at 28°C.
Electrophysiology.
Electrophysiological recording started 30 min after the end of superfusion with recombinant proteins. Extracellular field potentials evoked in the CA1 region by 100-μs current pulse stimulation of the Schaffer collaterals were recorded with a glass electrode filled with ACF and positioned in the stratum radiatum. The stimulation electrode consisted of two twisted Teflon-coated platinum/iridium wires. Experiments were initiated when stability of response (initial slope of field excitatory postsynaptic potentials) evoked by a 0.016-Hz baseline stimulation was maintained for ≈15 min. A dose–response curve was first established. The stimulus intensity was then adjusted to evoke ≈40% of maximal response. Paired-pulse facilitation was assessed with a pair of stimuli separated by a 50-ms interval. LTP was induced by three trains of 100-Hz pulses (1-s duration) separated by 5 min intervals [high-frequency stimulation (HFS)] after a stable response to a 0.016-Hz baseline stimulation was obtained for 15 min. The effect of HFS on the response magnitude was then monitored for 180 min by using a 0.016-Hz baseline stimulation. Typically, HFS was applied ≈1.5 h after the end of superfusion with either TORC-DN-EGFP-11R or NLS-EGFP-11R. Slices that displayed <150% potentiation were discarded.
Supplementary Material
Acknowledgments
We thank Ruth Luthi-Carter for critical reading of the manuscript and helpful comments, Marc Montminy for mouse TORC2 cDNA and pan-TORC antiserum, Jean-Luc Martin for the BDNF real-time RT-PCR primers, Raffaella Guidi and Lionel Breuillaud for constructing pSUPER-Control and pSUPER-TORC2, Florence Dubugnon for excellent technical assistance, Ron Stoop and Anouchka Pickenhagen for their expertise on electrophysiological techniques, and Rudolf Kraftsik for helping with the statistical analyses. This work was supported by Swiss National Science Foundation Grants 3100-64031.00 and 3100A0-105773.
Abbreviations
- ACF
artificial cerebrospinal fluid
- BDNF
brain-derived neurotrophic factor
- CBP
CREB-binding protein
- CRE
cAMP-response element
- CREB
CRE-binding protein
- CsA
cyclosporine A
- FSK
forskolin
- HFS
high-frequency stimulation
- L-LTP
late-phase long-term potentiation
- LMB
leptomycin B
- shRNA
short hairpin RNA
- TORC
transducer of regulated CREB activity.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0607524104/DC1.
References
- 1.Conkright MD, Canettieri G, Screaton R, Guzman E, Miraglia L, Hogenesch JB, Montminy M. Mol Cell. 2003;12:413–423. doi: 10.1016/j.molcel.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Iourgenko V, Zhang W, Mickanin C, Daly I, Jiang C, Hexham JM, Orth AP, Miraglia L, Meltzer J, Garza D, et al. Proc Natl Acad Sci USA. 2003;100:12147–12152. doi: 10.1073/pnas.1932773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, Guzman E, Niessen S, Yates JR, III, Takemori H, et al. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Kandel ER. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 5.Lonze BE, Ginty DD. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 6.West AE, Griffith EC, Greenberg ME. Nat Rev Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- 7.Silva AJ, Kogan JH, Frankland PW, Kida S. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 8.Impey S, Goodman RH. Sci STKE. 2001;2001:PE1. doi: 10.1126/stke.2001.82.pe1. [DOI] [PubMed] [Google Scholar]
- 9.Mayr B, Montminy M. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 10.Pham TA, Impey S, Storm DR, Stryker MP. Neuron. 1999;22:63–72. doi: 10.1016/s0896-6273(00)80679-0. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki S, al-Noori S, Butt SA, Pham TA. J Comp Neurol. 2004;479:70–83. doi: 10.1002/cne.20310. [DOI] [PubMed] [Google Scholar]
- 12.Barco A, Alarcon JM, Kandel ER. Cell. 2002;108:689–703. doi: 10.1016/s0092-8674(02)00657-8. [DOI] [PubMed] [Google Scholar]
- 13.Rammes G, Steckler T, Kresse A, Schutz G, Zieglgansberger W, Lutz B. Eur J Neurosci. 2000;12:2534–2546. doi: 10.1046/j.1460-9568.2000.00108.x. [DOI] [PubMed] [Google Scholar]
- 14.Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- 15.Bittinger MA, McWhinnie E, Meltzer J, Iourgenko V, Latario B, Liu X, Chen CH, Song C, Garza D, Labow M. Curr Biol. 2004;14:2156–2161. doi: 10.1016/j.cub.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Frey U, Morris RG. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 17.Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- 18.Barco A, Patterson S, Alarcon JM, Gromova P, Mata-Roig M, Morozov A, Kandel ER. Neuron. 2005;48:123–137. doi: 10.1016/j.neuron.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Kang H, Schuman EM. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 20.Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 21.Matsushita M, Tomizawa K, Moriwaki A, Li ST, Terada H, Matsui H. J Neurosci. 2001;21:6000–6007. doi: 10.1523/JNEUROSCI.21-16-06000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greengard P. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- 23.Belfield JL, Whittaker C, Cader MZ, Chawla S. J Biol Chem. 2006;281:27724–27732. doi: 10.1074/jbc.M601485200. [DOI] [PubMed] [Google Scholar]
- 24.Dash PK, Karl KA, Colicos MA, Prywes R, Kandel ER. Proc Natl Acad Sci USA. 1991;88:5061–5065. doi: 10.1073/pnas.88.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheng M, Thompson MA, Greenberg ME. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 26.Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, et al. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enslen H, Tokumitsu H, Stork PJ, Davis RJ, Soderling TR. Proc Natl Acad Sci USA. 1996;93:10803–10808. doi: 10.1073/pnas.93.20.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 29.Frey U, Huang YY, Kandel ER. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 30.Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- 31.Frey U, Matthies H, Reymann KG. Neurosci Lett. 1991;129:111–114. doi: 10.1016/0304-3940(91)90732-9. [DOI] [PubMed] [Google Scholar]
- 32.O'Carroll CM, Morris RG. Neuropharmacology. 2004;47:324–332. doi: 10.1016/j.neuropharm.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Li S, Cullen WK, Anwyl R, Rowan MJ. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- 34.Cardinaux JR, Magistretti PJ, Martin JL. Brain Res Mol Brain Res. 1997;51:220–228. doi: 10.1016/s0169-328x(97)00241-6. [DOI] [PubMed] [Google Scholar]
- 35.Kovacs KA, Steinmann M, Magistretti PJ, Halfon O, Cardinaux JR. J Neurochem. 2006;98:1390–1399. doi: 10.1111/j.1471-4159.2006.03957.x. [DOI] [PubMed] [Google Scholar]
- 36.Impey S, Fong AL, Wang Y, Cardinaux JR, Fass DM, Obrietan K, Wayman GA, Storm DR, Soderling TR, Goodman RH. Neuron. 2002;34:235–244. doi: 10.1016/s0896-6273(02)00654-2. [DOI] [PubMed] [Google Scholar]
- 37.Cardinaux JR, Notis JC, Zhang Q, Vo N, Craig JC, Fass DM, Brennan RG, Goodman RH. Mol Cell Biol. 2000;20:1546–1552. doi: 10.1128/mcb.20.5.1546-1552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kovacs KA, Steinmann M, Magistretti PJ, Halfon O, Cardinaux JR. J Biol Chem. 2003;278:36959–36965. doi: 10.1074/jbc.M303147200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.