Abstract
Prion diseases or transmissible spongiform encephalopathies are characterized histopathologically by the accumulation of prion protein (PrP) ranging from diffuse deposits to amyloid plaques. Moreover, pathologic PrP isoforms (PrPSc) are detected by immunoblot analysis and used both as diagnostic markers of disease and as indicators of the presence of infectivity in tissues. It is not known which forms of PrP are associated with infectivity. To address this question, we performed bioassays using human brain extracts from two cases with phenotypically distinct forms of familial prion disease (Gerstmann-Sträussler-Scheinker P102L). Both cases had PrP accumulations in the brain, but each had different PrPSc isoforms. Only one of the brains had spongiform degeneration. Tissue from this case transmitted disease efficiently to transgenic mice (Tg PrP101LL), resulting in spongiform encephalopathy. In contrast, inoculation of tissue from the case with no spongiform degeneration resulted in almost complete absence of disease transmission but elicited striking PrP-amyloid deposition in several recipient mouse brains. Brains of these mice failed to transmit any neurological disease on passage, but PrP-amyloid deposition was again observed in the brains of recipient mice. These data suggest the possible isolation of an infectious agent that promotes PrP amyloidogenesis in the absence of a spongiform encephalopathy. Alternatively, the infectious agent may be rendered nonpathogenic by sequestration in amyloid plaques, or PrP amyloid can seed amyloid accumulation in the brain, causing a proteinopathy that is unrelated to prion disease. Formation of PrP amyloid may therefore not necessarily be a reliable marker of transmissible spongiform encephalopathy infectivity.
Keywords: amyloid, Gerstmann-Sträussler-Scheinker, transmissible spongiform encephalopathy, neurodegeneration
Prion diseases are characterized by the conversion of a host-encoded cellular isoform of prion protein (PrPC) into a pathologic PrP isoform (PrPSc) that is thought by many to be infectious in the absence of nucleic acids (1, 2). PrPSc is distinguished from PrPC by its relative resistance to protease digestion and its altered sedimentation properties, although protease-sensitive PrPSc isoforms have also been described (1–3). Immunohistochemical analyses of PrP in tissues of patients with prion diseases have showed differing patterns of PrP accumulation in the brain, ranging from diffuse deposits to amyloid plaques (4, 5). In this study, all abnormal PrP isoforms are referred to as PrPSc. Congophilic or thioflavin S fluorescent PrPSc deposits are referred to as PrP amyloid.
Gerstmann-Sträussler-Scheinker (GSS) disease is a familial prion disease associated with several mutations in the prion protein gene (PRNP), P102L being the most common (4). This mutation may be associated with one of two pathologic phenotypes; both have diffuse deposits of PrPSc and PrP-amyloid plaques in the brain, but only one type has spongiform degeneration (6, 7). Previous studies of GSS P102L have shown 21- and 8-kDa PrPSc fragments in brain extracts from patients with spongiform degeneration, whereas an 8-kDa PrPSc but not a 21-kDa PrPSc fragment was detected in patients without spongiform degeneration (6, 7). Whether the different phenotypes seen in patients with GSS P102L correspond to two different strains associated with a single point mutation in PRNP has yet to be determined.
The proposition that PrPSc is not only an abnormal protein central to the pathogenesis of disease but is also the infectious agent itself has been based on the correlation between the presence of PrPSc and the development of neurological symptoms, pathologic changes, and increase in infectivity titers (8, 9). However, brain tissue from PrP-null mice adjacent to prion-infected neurografts did not develop neuropathologic changes, suggesting that PrPC must be expressed by cells undergoing pathologic changes and that PrPSc might not be neurotoxic (10). Infectivity has been found in brains containing no detectable PrPSc, suggesting that PrPSc and infectivity may not correlate in all models of disease (11–13). Conversely, most GSS variants have been more difficult to transmit to animals than other forms of prion disease (14, 15). Although the absence of detectable infectivity in such diseases could be caused by low infectivity titer or a species barrier effect between humans and animals used to bioassay the infectivity (14, 15), these findings demonstrate that the relationship between PrPSc and infectivity is still far from understood. Mutations in the PRNP gene might conceivably lead to a noninfectious neurological disease associated with protein misfolding and at the same time render the carrier more susceptible to infection. This explanation would account for the marked differences in clinical and pathological phenotypes observed in GSS patients having the same P102L mutation. Therefore, it is possible that PrPSc isoforms might be nonpathogenic, pathogenic without being infectious, or pathogenic and infectious. PrPSc might therefore accumulate in both transmissible and nontransmissible prion diseases. If PrPSc isoforms not associated with infectivity exist, it is important to define them, because, in the absence of transmission studies, the detection of PrPSc is the main criterion used to assess the presence of infectivity in animals and humans. Transmissible spongiform encephalopathies (TSEs) might represent only a portion of the conditions called prion diseases, and identifying the difference between the transmissible and nontransmissible diseases would be important not only for disease diagnosis but also for assessing the risk of secondary infections.
Previous experiments have shown that gene-targeted transgenic (Tg) mice, which express murine Prnp P101L (analogous to PRNP P102L in humans), do not develop any spontaneous neurological disorder but do show increased susceptibility to infection with the agent extracted from brains of patients with GSS having the P102L mutation and spongiform degeneration (13). These Tg mice, therefore, represent an ideal model for studying the two phenotypes of GSS associated with the P102L mutation and determining the relationship between PrPSc and infectivity. To address these issues, we inoculated brain extracts from two patients with GSS P102L (each with a distinct pathologic phenotype) into Tg mice homozygous for PrP-P101L (Tg 101LL). Here, we show that challenge with brain extracts from the patient with spongiform degeneration resulted in an efficient transmission of disease. In contrast, inoculation of brain extracts from the patient with no spongiform degeneration caused almost no clinical disease but induced striking PrP-amyloid deposition in brains of several recipient mice; extracts of those brains failed to transmit neurological disease on further passage but again induced PrP-amyloid plaques in recipient mice. Thus, PrP amyloid can accumulate and indeed induce production of further PrP amyloid without resulting in spongiform degeneration of the brain or neurological disease.
Results
Correlation Between PrPSc Isoforms and Infectivity.
GSS P102L brain extracts were derived from two patients (one with spongiform degeneration and the other without spongiform degeneration) by purification of detergent-insoluble PrP in the absence of proteinase K (PK) digestion to ensure that both PK-resistant and PK-sensitive PrPSc species were present. Immunoblot analysis confirmed the presence of 21-kDa PrPSc in the brain extract obtained from the patient with spongiform degeneration and the 8-kDa PrPSc fragment in the brain extracts obtained from the patient without spongiform degeneration [supporting information (SI) Fig. 4]. We designated the brain extract from the GSS patient with spongiform degeneration as PrP-21 and the brain extract from the GSS patient without spongiform degeneration as PrP-8 to signify the difference between the forms of PrPSc present in the two inocula. Each extract was inoculated intracerebrally into Tg 101LL mice and control 129/Ola WT mice. All of the Tg 101LL mice inoculated with PrP-21 (Tg 101LL-21) developed neurological symptoms and were culled at the terminal stages of disease between 245 and 330 days postinoculation with a mean incubation time of 290 ± 4 days (Table 1). Histopathologic examination showed spongiform degeneration of the brain and accumulation of variable amounts of PrPSc deposits (SI Table 3). Mice expressing WT PrP that were inoculated with fraction PrP-21 showed no clinical or pathologic signs of prion disease up to 691 days of age (Table 1).
Table 1.
Transmission of disease to Tg 101LL and WT mice
| Inoculum | Mouse strain | Prion disease* | Incubation time, days ± SEM |
|---|---|---|---|
| PrP-21 | 101LL | 23/23 | 290 ± 4 |
| PrP-21 | 101PP | 0/22 | NA (≥ 690) |
| PrP-8 | 101LL | 1/22 | 622 |
| PrP-8 | 101PP | 0/20 | NA (≥ 830) |
NA, not applicable, no illness at cull.
*Mice with clinical signs and spongiform degeneration.
In sharp contrast to the results obtained with PrP-21 inocula, 21 of 22 Tg 101LL mice inoculated with PrP-8 remained asymptomatic (Tg 101LL-8a mice) and were either killed at the end of their normally expected lifespans (up to 814 days postinoculation) or culled because of intercurrent illness. None of these animals showed neurological signs or had spongiform degeneration in the brain at necropsy. Similarly, 20 mice expressing WT PrP that were inoculated with PrP-8 remained asymptomatic, without spongiform degeneration, and were culled up to 832 days postinoculation (Table 1). Unexpectedly, 622 days after inoculation, one Tg 101LL-8 mouse developed neurological signs (Tg 101LL-8s mouse) and was euthanized (Table 1). Histopathologic studies showed spongiform degeneration and accumulation of diffuse deposits of PrPSc in the thalamus of Tg 101LL-8s (Table 1 and SI Table 3).
Brains of Asymptomatic Tg 101LL-8 Mice Contain PrP-Amyloid Plaques.
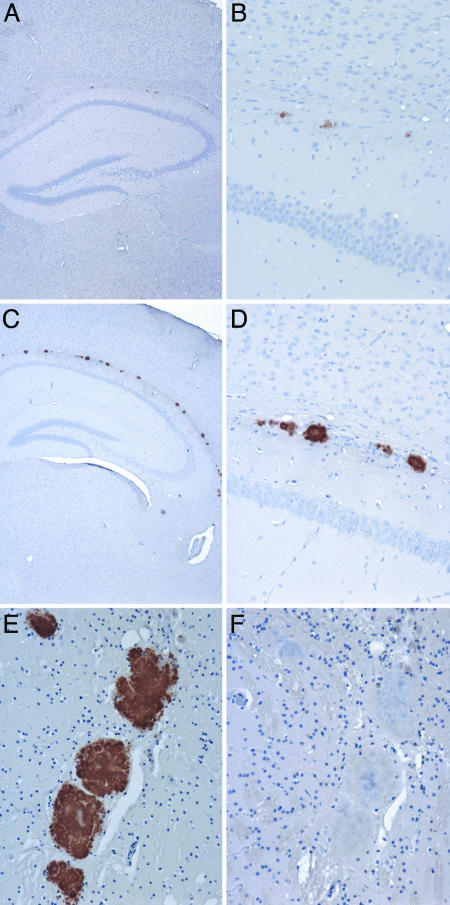
Brain sections from both Tg 101LL-21 mice (with neurological signs and spongiform degeneration) and Tg 101LL-8a mice (without neurological signs or spongiform degeneration) were analyzed by immunohistochemistry to characterize the pattern of PrPSc deposition in affected mice and look for evidence of PrPSc accumulation in asymptomatic animals. Surprisingly, both groups of mice showed PrPSc-immunopositive ring-shaped deposits in the brain (SI Table 3). Tg 101LL-21 mice showed small plaques (≈5 μm in diameter) in the corpus callosum and surrounding areas (Fig. 1 A and B). In contrast, Tg 101LL-8a mice showed large multicentric plaques (up to ≈80 μm in diameter) in the corpus callosum and vicinity (Fig. 1 C and D). The deposits were thioflavin S fluorescent and green-gold birefringent when stained with Congo red, confirming the presence of PrP amyloid (SI Fig. 5). We speculated that PrP amyloid formed in Tg 101LL mice could have resulted from (i) the aggregation of endogenous (mouse) mutant PrP in aged animals, (ii) residual inoculum (human PrPSc), or (iii) the recruitment of mutant mouse PrP by the inoculum to form PrP amyloid. To study the first possibility, we analyzed 39 uninoculated Tg 101LL mice culled between 615 and 880 days of age. Microscopic examination of the brain indicated that these animals did not show spongiform degeneration, amyloid plaques, or PrPSc accumulation. To study the second and the third possibilities, we conducted immunohistochemical studies using species-specific mAbs to PrP on brain sections from selected animals inoculated with PrP-21 and PrP-8. Amyloid plaques were labeled with antibodies directed to mouse PrP but not with antibodies directed to human PrP (Fig. 1 E and F); therefore, PrP-amyloid plaques must have been aggregates of mouse PrP and were not accumulations of residual inoculum (i.e., human PrPSc).
Fig. 1.
Immunohistochemistry of the cerebrum of Tg 101LL mice showing PrP accumulation in the vicinity of the corpus callosum. (A and B) Mouse inoculated with PrP-21. (C and D) Mouse inoculated with PrP-8. Higher-power magnification of plaques in the corpus callosum are shown in B and D. (E and F) Serial sections from a mouse inoculated with PrP-8 stained with mAb 6H4 (E) and mAb 3F4 (F). (A and B) Brains from animals developing neurological signs were examined at the terminal stages of the disease (310 days postinoculation. (C–F) Animals without neurological disease were analyzed at the end of their lifespan 562 days postinoculation (C and D) and 731 days postinoculation (E and F). (A–E) PrP deposits were detected by immunohistochemical analysis using mAb 6H4 that recognizes mouse PrP. (F) No immunopositivity was observed with mAb 3F4 that recognizes human PrP. (Magnifications: ×4, A and C; ×20, B and D–F.)
PrPSc Is Present in Tg 101LL-8a Mice.
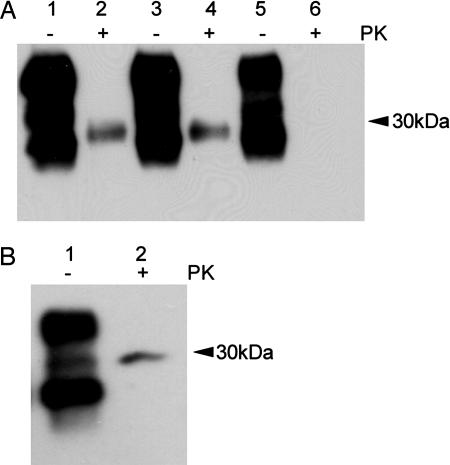
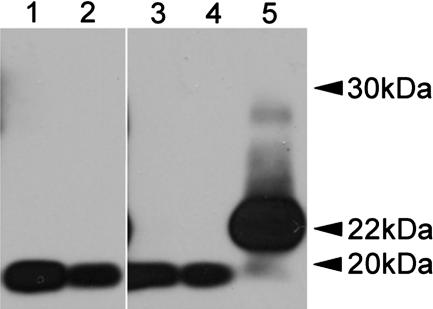
Although no clinical disease or spongiform degeneration was observed in Tg 101LL-8a mice, the PrP-amyloid plaques observed in several of these mice suggested that the brains contained PrPSc isoforms that could be identified by immunoblot analysis. Brain homogenates from Tg 101LL-21 and Tg 101LL-8 mice were incubated with PK under standard conditions (20 μg/ml for 1 h at 37°C), and digests were analyzed by immunoblot. Levels of PrPSc were low (Fig. 2A, lanes 2 and 4), or undetectable (SI Fig. 6, lanes 3 and 8) in extracts from brains of mice with neurological disease and spongiform degeneration. As expected, a low level of PrPSc was also detected in the brain of one Tg 101LL-8a mouse with striking PrP-amyloid plaques (Fig. 2B). However, PrPSc was not detected by immunoblot in the brain of four other Tg 101LL-8a mice that contained PrP-amyloid plaques (Fig. 2A, lane 6, and SI Fig. 6, lanes 1, 2, 6, and 7). The discrepancy between detection of PrP-amyloid plaques by immunohistochemistry and the absence of PrPSc in immunoblot analysis in some Tg 101LL-8a mouse brains might result from dilution of PrPSc below the limit of detection in these samples or the presence of PK-sensitive PrPSc (3, 16–18). We did not observe PrPSc after (i) differential precipitation of PrPSc with sodium phosphotungstic acid (NaPTA) (SI Fig. 6), (ii) a series of digestion reactions using reduced levels of PK (1–20 μg/ml), or (iii) limited digestion with PK at 4°C (designated “cold” PK treatment) followed by removal of glycan side chains from PrP with PNGaseF (Fig. 3) (3, 18). In addition, we did not detect PrPSc by time-resolved fluorescence immunoassay (DELFIA, PerkinElmer, Waltham, MA) after differential extraction with guanidine hydrochloride (Gdn·HCl) (19). Hence, although PrPSc was identified by immunoblot in one 101LL-8a mouse with large amyloid plaques, no evidence of large accumulations of PK-sensitive PrPSc or Gdn·HCl-insoluble PrPSc was found in several of the 101LL-8a mice, despite the presence of PrP-amyloid deposits as identified by immunohistochemistry.
Fig. 2.
Immunoblot analysis of brain homogenates from mice inoculated with PrP-21 and PrP-8. (A) Lanes 1 and 2, Tg 101LL-21 (mouse with neuropathologically confirmed prion disease, 277 days postinoculation); lanes 3 and 4, Tg 101LL-8s (mouse with neuropathologically confirmed prion disease, 622 days postinoculation); and lanes 5 and 6, Tg 101LL-8a (asymptomatic mouse, without spongiform degeneration showing multicentric amyloid plaques, 703 days postinoculation). Samples in lanes 2, 4, and 6 were treated with 20 μg/ml PK. The film was overexposed to show the low levels of PK-res PrPSc present in the samples shown in lanes 2 and 4. (B) Low levels of PrPSc were identified in one 101LL-8a mouse after treatment with 20 μg/ml PK. PrP was detected with mAb 7A12.
Fig. 3.
Cold-PK digestion of brain homogenate from Tg 101LL-8 mice and controls. Lane 1, Tg 101LL-8a (asymptomatic mouse with multiple PrP-amyloid plaques, 633 days postinoculation); lane 2, Tg 101LL-8a (asymptomatic mouse with multiple PrP-amyloid plaques, 716 days postinoculation); lane 3, uninfected Tg 101LL mouse; lane 4, uninfected 129/Ola control mouse; and lane 5, ME7-scrapie-infected 129/Ola control mouse. The 22- to 24-kDa PrP band (corresponding to “cold PK”-resistant fragment of PK-sensitive PrPSc) is present only in the ME7 scrapie-infected control mouse. All samples were treated with 250 μg/ml PK on ice for 1 h and deglycosylated with PNGase F. ME7 scrapie control was loaded at ≈25% of the concentration of lanes 1–4 to allow comparison. The blot was probed with mAb 7A12. The image was cropped from a single blot to remove lanes with irrelevant samples.
Infectivity Is Not Detected in Tg 101LL-8a Mice with PrP Amyloid.
Although Tg 101LL-8a mice showed no neurological signs or spongiform degeneration, the presence of PrP-amyloid plaques in the brain could indicate a subclinical disease, with low levels of infectivity. Looking for an asymptomatic carrier state, we serially passaged brain homogenate from a Tg 101LL-8a mouse with amyloid plaques. Brain homogenates from 101LL-21 and 101LL-8s mice were also passaged as controls. Homogenates from Tg 101LL-21 and Tg 101LL-8s animals transmitted disease to Tg 101LL mice after incubation times that were shorter than those after primary passage, indicating that the infectious agent had adapted to mice. Although the inoculum from the Tg 101LL-21 mouse also transmitted disease to WT mice, inoculum from Tg 101LL-8s mice failed to transmit disease to WT mice (Table 2). Thus, expression of the normal mouse Prnp gene seemed to prevent replication of the infectious agent present in Tg 101LL-8s mice; alternatively the incubation time for that infection might have exceeded the normal lifespan of WT mice.
Table 2.
Subpassage of mouse adapted GSS infectivity in Tg 101LL and WT mice
| Inoculum | Mouse strain | Prion disease* | Incubation time, days ± SEM |
|---|---|---|---|
| 101LL-21 | 101LL | 18/18 | 173 ± 4 |
| 101LL-21 | 101PP | 13/14 | 264 ± 5 |
| 101LL-8s | 101LL | 17/17 | 203 ± 2 |
| 101LL-8s | 101PP | 0/17 | NA (≥ 615) |
| 101LL-8a | 101LL | 0/14 | NA (≥ 740) |
| 101LL-8a | 101PP | 0/17 | NA (≥ 745) |
101LL-21, 101LL-8s inocula from mice with spongiform degeneration of the brain culled at 277 and 622 days postinoculation, respectively. 101LL-8a, inoculum from a mouse culled 703 days postinoculation, with PrP amyloid and no spongiform degeneration of the brain. NA, not applicable, no illness at cull.
*Mice with clinical signs and spongiform degeneration.
Tg 101LL and WT mice inoculated with brain homogenates from a Tg 101LL-8a mouse (with PrP-amyloid plaques and no spongiform encephalopathy) did not develop neurological disease or spongiform degeneration during their lifespan (up to 745 days postinoculation; Table 2). However, immunohistochemical analyses again showed multicentric PrP-amyloid plaques in the corpus callosum and adjacent areas of several recipient Tg 101LL mice (SI Fig. 7 and SI Table 4). These results suggest that synergistic interactions between amyloidogenic molecules present in the inoculum and the endogenous 101L mutant PrP generated in Tg mice promoted PrP fibrillogenesis but did not cause TSE defined as a transmissible infectious disease.
Discussion
In this study we have shown that PrP amyloid accumulated in the brain and induced production of further PrP amyloid without resulting in clinical disease or the spongiform degeneration usually associated with prion diseases. These results might be interpreted in several ways: (i) that low levels of infectivity were present in Tg mice with amyloid plaques, (ii) that there was dissociation between PrP amyloid and infectivity, (iii) that PrP-amyloid formation may be protective by sequestering infectious particles into an inert aggregate, or (iv) that 101L-PrP aggregates and precipitates, causing a “proteinopathy” due to protein misfolding but does not have the other properties of a self-replicating transmissible agent/prion (i.e., causing a fatal neurodegenerative disease).
Low Levels of Infectivity Are Not Detected in 101LL-8a Tissue.
The apparent dissociation between the presence of PrP amyloid and transmissible disease in 101LL-8a mice might be caused by low levels of infectivity, leading to subclinical disease in the recipient mice. In other models of TSE disease, the presence of small amounts of agent and subclinical disease has been demonstrated by performing a secondary passage, transmitting overt disease to recipient mice (12, 20, 21). However, brain homogenate from a Tg 101LL-8a mouse containing PrP-amyloid plaques did not transmit a spongiform encephalopathy to Tg mice, although PrP amyloid was detected postmortem in the brains of several asymptomatic very old recipient Tg 101LL mice. The 101L mutation in the murine Prnp gene produces Tg mice highly susceptible to infection with the PrP-21 isolate of agent on both primary and subpassage (13) and displays all of the typical findings of a prion disease. Therefore Tg 101LL mice should provide a sensitive bioassay for agent replication in humans and mice and offer an excellent model for studying the relationship between different kinds of PrPSc and infectivity. We have to date successfully transmitted disease from many different prion isolates to Tg 101LL mice, always followed by significantly shorter incubation times and marked spongiform degeneration after subpassage to 101LL mice (refs. 13 and 22 and unpublished data). Although the observations reported here suggest that infectivity did not replicate after primary inoculation of Tg 101LL-8a mice or after mouse-to-mouse passage, further studies will determine whether clinical signs of illness and spongiform degeneration eventually appear after additional passages.
Is PrP Amyloid Protective?
Inefficient transmission of disease to animals injected with PrP-8 might conceivably be caused by a prion strain that promotes amyloidogenesis and does not cause prion disease. Alternatively, production of amyloid may somehow inhibit propagation of infectivity; if so, the disease seen in one animal (Tg 101LL-8s) would represent a failure of this protective mechanism. Others (23) have suggested that the formation of amyloid fibrils might be protective against illness by sequestering harmful PrP oligomers. In addition, it has been shown that disaggregation of PrP amyloid correlated temporally with increases in infectivity titers and that the most infectious units are smaller than amyloid fibrils (24, 25). Whether dissociation of PrP amyloid in brains of asymptomatic Tg 101LL-8a mice would result in the efficient transmission of a spongiform encephalopathy remains to be determined. However, our results show that Tg 101LL mice inoculated with PrP-8 provide a unique model for studying the generation of PrP amyloid in the absence of clinical disease and spongiform degeneration. Importantly, this phenotype was maintained upon serial passage in Tg 101LL mice, indicating that any cofactors needed to form amyloid must be present in these mice.
Is PrP Amyloid Infectious?
The formation of PrP-amyloid plaques without clinical prion disease has previously been described in scrapie-infected Tg mice expressing PrP lacking the normal glycophosphatidylinositol (GPI) membrane anchor (GPI−/− mice), in which PrP is present but not located on the cell surface (26). Infected GPI−/− mice accumulated a large amount of PrP amyloid but did not become ill. However, replication of the infectious agent did occur in GPI−/− mice because typical scrapie was transmitted from their brains to WT mice. In the present study, there was no apparent propagation of a pathogenic infectious agent associated with PrP-amyloid accumulation in the brains of Tg 101LL-8a mice as evidenced by failure to transmit disease to either Tg 101LL or WT mice on subpassage. In our model, PrP in 101LL mice is normally attached to the cell membrane. Whether the different cellular locations of PrP affect the ability of mice to propagate infectivity remains to be established. Parallel studies using GPI−/− mice and our Tg 101LL-mouse model may help to dissect the contribution of PrP amyloid to prion diseases and infectivity.
Although the PrP-8 inoculum contained readily detectable levels of PrPSc, it is possible that the inefficient transmission of disease (to a single Tg 101LL-8s animal) was associated with an unstable and readily degradable form of PrPSc. However, brains of many mice inoculated with the original PrP-8 inoculum contained amyloid plaques, indicating that PrPSc in the inoculum was not degraded or rapidly cleared after inoculation. Our interpretation of these data are that PrPSc in the PrP-8 inoculum was stable and induced the refolding of mouse PrP to form amyloid. The detection of large PrP-amyloid plaques in very old asymptomatic Tg 101LL mice (601–814 days postinoculation) indicates that late amyloid formation was induced by PrP-8 in otherwise disease-free animals. A similar phenomenon has recently been described for Alzheimer's disease (AD). Although there is currently no epidemiological evidence that AD is a transmissible disease, induction of β-amyloidogenesis has been reported in experimental mouse models after the inoculation of brain extracts from patients with AD and Tg mouse models of AD, indicating that amyloidogenesis was accelerated by the presence of preformed amyloid seeds (27). Additionally, others (18) have shown an acceleration of the spontaneous neurological disease observed in Tg mice overexpressing 101L-PrP when asymptomatic animals are inoculated with brain from a sick Tg-101L mouse. Our data therefore suggest that in several 101LL Tg mice the inoculation of PrP-8 might have induced a PrP-linked proteinopathy propagated by seeding that is distinct from a TSE. Amyloid formation may therefore be caused purely by PrP misfolding and aggregation in the absence of a TSE infectious agent.
The Role of PrP Amyloid in TSE Disease.
PrPSc is considered by many to be the infectious agent in prion diseases, and susceptibility of mice to infection clearly requires the presence of a stable PrPSc that converts PrPC into additional PrPSc efficiently (1). In our model, inocula containing PrP-8 induced the conversion of murine 101L-PrP into amyloid but did not induce spongiform degeneration in the recipient mouse brains. Thus, PrP amyloid itself might not be infectious, explaining the failure to transmit disease from brains of some GSS patients to animals. Whatever the reasons, our findings have profound implications for the common assumption that the presence of PrPSc or PrP amyloid signifies presence of the TSE infectious agent. To assess the risk of transmitting prion diseases from animals to humans or between humans, it is important to understand the true relationship between PrPSc and infectivity. Importantly, the detection of PrP amyloid in the brain may not always be a reliable marker of infectivity.
Materials and Methods
Preparation of the Inoculum and Challenge.
PrPSc was purified from the frontal cortex of two GSS patients with the same PRNP P102L mutation but having different clinical–pathological phenotypes (6). Clinical–pathological and molecular studies of these patients have been reported (6). Patient 1 died at age 33. Pathologic analysis showed severe spongiform degeneration and moderate amount of diffuse PrP deposits and amyloid plaques in the brain. Patient 2 died at age 65. Pathologic analysis showed no spongiform degeneration and moderate amounts of diffuse PrP deposits and amyloid plaques in the brain. Immunoblots with brain extracts from patient 1 showed PrPSc isoforms of 21–30 kDa. In contrast, extracts from patient 2 showed prominent 8-kDa PrPSc fragments but no isoforms of 21–30 kDa. PRNP sequence analyses confirmed that both patients were homozygous for methionine at polymorphic codon 129 and had the same P102L point mutation in one PRNP gene. PrPSc was purified from the brain of both patients as described (28). Similar brain equivalents of purified PrP from both patients were used to prepare the inocula. PrPSc samples were diluted 1/100 in sterile physiological saline for inoculation. Groups of Tg 101LL mice and WT mice were inoculated with extracts of brains from patients 1 and 2 as described (13). All mice were genotyped before inoculation and again postmortem. All experiments were reviewed and approved by the Local Ethical Review Committee and performed under license in accordance with the U.K. Animals (Scientific Procedures) Act 1986.
Subpassages in Tg 101LL and WT Mice.
Brain tissues from terminally ill Tg 101LL mice inoculated with brain extracts from patients 1 and 2 (after incubation periods of 277 and 622 days, respectively) and from an asymptomatic mouse without spongiform degeneration inoculated with tissue from patient 2 (703 days old) were used as inocula. Groups of Tg 101LL and WT mice were inoculated intracerebrally as described above.
Scoring of Clinical TSE Disease.
The presence of clinical prion disease was assessed, and incubation times were calculated by following previously described protocols (29). Mice were killed at the terminal stage of disease, at the end of the normal expected lifespan, or because of intercurrent illness. Half brains were fixed in 10% formol saline. The remaining half brains were frozen at −70°C for biochemical analysis. Fixed brain tissue was processed and tissue sections were prepared as described (13).
Lesion Profiles and Imunohistochemical Analysis.
Tissue sections were assessed for spongiform degeneration as described (30). Selected sections were immunostained with mAb 6H4 (Prionics, Zurich, Switzerland) recognizing residues 143–151 of murine PrP (2 μg/ml) and mAb 3F4 (31) recognizing residues 109–112 of human PrP but not mouse PrP (2.5 μg/ml). Amyloid plaques were visualized with thioflavin S or Congo red (6, 32).
PrP Immunoblotting and DELFIA Assays.
Assays of PrP in mice were performed as described (3, 13, 16, 18). Immunoblots were developed with mAb 7A12 recognizing an epitope located between PrP residues 90 and 145 (50 ng/ml) (33). DELFIA assay was performed as described (19).
Genotyping.
Genotypes of mice were determined as described (13).
Supplementary Material
Acknowledgments
We thank Prof D. W. Melton (University of Edinburgh, Edinburgh, U.K.) for production of the 101LL Tg mouse line; Dr D. Asher for critical reading of the manuscript; V. Thomson, K. Hogan, S. Dunlop, and E. Murdoch for care and scoring of the animals; M. S. Sy (Case Western Reserve University, Cleveland, OH) for the 7A12 mAb; A. Boyle and W. G. Liu for lesion-profile analysis; A. Coghill, S. Mack, C. Plinston, and A. William for tissue processing; and P. Hart for producing the digital figures. These studies were funded by the Biotechnology and Biological Sciences Research Council and National Institutes of Health Grant P30 AG10133.
Abbreviations
- PK
proteinase K
- PrP
prion protein
- PrPC
cellular isoform of PrP
- PrPSc
pathologic PrP isoform
- Tg
transgenic
- TSE
transmissible spongiform encephalopathy
- GSS
Gerstmann-Sträussler-Scheinker
- GPI
glycophosphatidylinositol.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609241104/DC1.
References
- 1.Prusiner SB, editor. Prion Biology and Diseases. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2004. pp. 89–141. [Google Scholar]
- 2.Collinge J. Annu Rev Neurosci. 2001;24:519–550. doi: 10.1146/annurev.neuro.24.1.519. [DOI] [PubMed] [Google Scholar]
- 3.Tremblay P, Ball HL, Kaneko K, Groth D, Hegde RS, Cohen FE, DeArmond SJ, Prusiner SB, Safar JG. J Virol. 2004;78:2088–2099. doi: 10.1128/JVI.78.4.2088-2099.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghetti B, Piccardo P, Frangione B, Bugiani O, Giaccone G, Young K, Prelli F, Farlow MR, Dlouhy SR, Tagliavini F. Brain Pathol. 1996;6:127–145. doi: 10.1111/j.1750-3639.1996.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 5.Aguzzi A, Heikenwalder M, Miele G. J Clin Invest. 2004;114:153–160. doi: 10.1172/JCI22438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccardo P, Dlouhy SR, Lievens PMJ, Young K, Vinters HV, Zimmerman TR, Mackenzie IRAM, Brown P, Gibbs CJ, Jr, Gajdusek DC, et al. J Neuropath Exp Neurol. 1998;57:979–988. doi: 10.1097/00005072-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Parchi P, Chen SG, Brown P, Zou W, Capellari S, Budka H, Hainfellner J, Reyes PF, Golden GT, Hauw JJ, et al. Proc Natl Acad Sci USA. 1998;95:8322–8327. doi: 10.1073/pnas.95.14.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeArmond SJ, Mobley WC, DeMott DL, Barry RA, Beckstead JH, Prusiner SB. Neurology. 1987;37:1271–1280. doi: 10.1212/wnl.37.8.1271. [DOI] [PubMed] [Google Scholar]
- 9.Jendroska K, Heinzel FP, Torchia M, Stowring L, Kretzschmar HA, Kon A, Stern A, Prusiner SB, DeArmond SJ. Neurology. 1991;41:1482–1490. doi: 10.1212/wnl.41.9.1482. [DOI] [PubMed] [Google Scholar]
- 10.Bradner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 11.Lasmézas CI, Deslys JP, Robain O, Jaegly A, Beringue V, Peyrin JM, Fournier JG, Hauw JJ, Rossier J, Dormont D. Science. 1997;275:402–404. doi: 10.1126/science.275.5298.402. [DOI] [PubMed] [Google Scholar]
- 12.Race R, Meade-White K, Raines A, Raymond GJ, Caughey B, Chesebro B. J Infect Dis. 2002;186(Suppl 2):166–170. doi: 10.1086/344267. [DOI] [PubMed] [Google Scholar]
- 13.Manson JC, Jamieson E, Baybutt H, Tuzi NL, Barron R, McConnell I, Somerville R, Ironside J, Will R, Sy MS, et al. EMBO J. 1999;18:6855–6864. doi: 10.1093/emboj/18.23.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown P, Gibbs CJ, Jr, Rodger-Johnson P, Asher DM, Sulima MP, Bacote A, Goldfarb LG, Gajdusek DC. Ann Neurol. 1994;35:513–529. doi: 10.1002/ana.410350504. [DOI] [PubMed] [Google Scholar]
- 15.Tateishi J, Kitamoto T, Hoque MZ, Furukawa H. Neurology. 1996;46:532–537. doi: 10.1212/wnl.46.2.532. [DOI] [PubMed] [Google Scholar]
- 16.Safar J, Willie H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, PrusinerSB Nat Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 17.Hsiao KK, Groth D, Scott M, Yang SL, Serban H, Rapp D, Foster D, Torchia M, DeArmond SJ, Prusiner SB. Proc Natl Acad Sci USA. 1994;91:9126–9130. doi: 10.1073/pnas.91.19.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nazor KE, Kuhn F, Seward T, Green M, Zwald D, Pürro M, Schmid J, Biffiger K, Power AM, Oesch B, et al. EMBO J. 2005;24:2472–2480. doi: 10.1038/sj.emboj.7600717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnard G, Helmick B, Madden S, Gilbourne C, Patel R. Luminescence. 2000;15:357–362. doi: 10.1002/1522-7243(200011/12)15:6<357::AID-BIO621>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 20.Hill AF, Joiner S, Linehan J, Desbruslais M, Lantos PL, Collinge J. Proc Natl Acad Sci USA. 2000;97:10248–10253. doi: 10.1073/pnas.97.18.10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Race R, Raines A, Raymond GJ, Caughey B, Chesebro B. J Virol. 2001;75:10106–10112. doi: 10.1128/JVI.75.21.10106-10112.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barron RM, Thomson V, Jamieson E, Melton DW, Ironside J, Will R, Manson JM. EMBO J. 2001;20:5070–5078. doi: 10.1093/emboj/20.18.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caughey B, Lansbury PT. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 24.Gabizon R, McKinley MP, Prusiner SB. Proc Natl Acad Sci USA. 1987;84:4017–4021. doi: 10.1073/pnas.84.12.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, Caughey B. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chesebro B, Trifilo M, Race R, Meade-White K, Teng C, LaCasse R, Raymond L, Favara C, Baron G, Priola S, et al. Science. 2005;308:1435–1439. doi: 10.1126/science.1110837. [DOI] [PubMed] [Google Scholar]
- 27.Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, Neuenschwander A, Abramowski D, Frey P, Jaton AL, et al. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 28.Hope J, Multhaup G, Reekie LJ, Kimberlin RH, Beyreuther K. Eur J Biochem. 1988;172:271–277. doi: 10.1111/j.1432-1033.1988.tb13883.x. [DOI] [PubMed] [Google Scholar]
- 29.Dickinson AG, Meikle VM, Fraser H. J Comp Pathol. 1968;78:293–299. doi: 10.1016/0021-9975(68)90005-4. [DOI] [PubMed] [Google Scholar]
- 30.Fraser H, Dickinson AG. Nature. 1967;216:1310–1311. doi: 10.1038/2161310a0. [DOI] [PubMed] [Google Scholar]
- 31.Kascsak RJ, Rubenstein R, Merz PA, Tonna-DeMasi M, Fersko R, Carp RI, Wisniewski HM, Diringer H. J Virol. 1987;61:3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puchtler H, Sweat F, Levine M. J Histochem Cytochem. 1962;10:355–364. [Google Scholar]
- 33.Li RL, Wong BS, Pan T, Morillas M, Swietnicki W, O'Rourke K, Gambetti P, Surewicz WK, Sy MS. J Mol Biol. 2000;301:567–573. doi: 10.1006/jmbi.2000.3986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.