Abstract
PURPOSE In 2004, a commentary by Merenstein was published in JAMA describing how he was sued for engaging a patient in shared decision making for prostate cancer screening. The article sparked considerable debate on the impact of litigation on medical care. A natural experiment (a study assessing shared decision making under way at the practice that was sued) enabled us to evaluate whether physicians changed their prostate cancer screening behavior after the lawsuit.
METHODS As part of a randomized controlled trial conducted between January 2002 and November 2004, patients and physicians completed exit questionnaires about prostate cancer screening discussions after health maintenance examinations. We compared responses before, during, and after physicians became aware of the lawsuit.
RESULTS A total of 432 of 497 patients completed questionnaires (180 before the practice became aware of the lawsuit, 87 as knowledge of the case diffused through the practice, and 165 after publication of Merenstein’s commentary). Comparing patients’ responses over the 3 time periods, there were no changes in the average locus of decision-making control, time spent discussing screening, number of screening topics discussed, knowledge scores, or decisional conflict. The frequency with which physicians reported performing prostate-specific antigen testing increased (before vs after: 84% vs 90%; P = .03), and physicians were more likely to report that they, rather than the patients, had made the screening decision (before vs after: 3.3% vs 11.1%; P = .003).
CONCLUSIONS The physicians in closest proximity to this well-known legal case continued to engage patients in shared decision making and to let patients decide whether to be screened. Prostate-specific antigen testing increased during this period.
Keywords: Prostatic neoplasms, decision making, malpractice, guideline adherence/statistics & numerical data, prostate-specific antigen/blood, mass screening/methods
INTRODUCTION
A commentary by Merenstein1 published by JAMA in January 2004 sparked considerable debate. He poignantly recalled his experience as a family medicine resident, when he was sued for letting a patient decide whether to be screened for prostate cancer after engaging him in shared decision making, as current guidelines recommend. The patient declined screening, was later found to have prostate cancer, and successfully sued the practice, in essence, for encouraging shared decision making. Dr Merenstein was exonerated, but his residency program was found liable for teaching him to engage patients in shared decision making to decide whether to screen for prostate cancer.
Merenstein’s prediction of a chilling effect on shared decision making resonated with clinicians, promulgating letters to JAMA,2–7 articles elsewhere,8,9 sessions at national medical conferences,10 and international legal analyses.11,12 The prevailing view was that clinicians will likely forgo shared decision making and paternalistically order screening tests, such as the measurement of prostate-specific antigen (PSA), to avoid legal vulnerability. We were able to explore this hypothesis at the practice involved in the Merenstein case, where a controlled trial of a shared decision-making tool was under way at the time of the lawsuit.
METHODS
Between January 2002 and November 2004, Merenstein’s former residency training site (a northern Virginia family practice center serving a suburban patient population) was the setting of a randomized trial of Web-based and paper-based decision aids for prostate cancer screening, the results of which are reported elsewhere.13 All male patients aged 50 to 70 years undergoing a health maintenance examination were eligible. Patients who had prostate cancer, who had been previously enrolled in the study, or who did not have Internet access were excluded. Patients were stratified into groups corresponding to their physician and then assigned randomly, within their strata, to receive no previsit education, the Web-based decision aid, or the brochure decision aid. Outcomes were measured to determine whether exposure to the decision aids increased elements of shared decision making.
Merenstein saw the patient in July 1999. We launched our trial on decision aids in January 2002. In September 2002, 2 practice clinicians became aware of the lawsuit, and the courtroom verdict was delivered in July 2003. Merenstein’s article appeared in JAMA in January 2004. Of the 497 patients enrolled in our trial, 200 were enrolled before the entire practice learned of the lawsuit (before period: January 2002 through June 2003), 100 were enrolled as knowledge of the case diffused through the practice (diffusion period: July 2003 through December 2003), and 197 were enrolled after Merenstein’s JAMA commentary was published (after period: January 2004 through November 2004). All faculty and resident physicians were fully aware of the details of the case by the time of the Merenstein publication.
We obtained outcomes data for the research study from exit questionnaires administered to the patients and their physicians immediately after health maintenance examinations. The questionnaires were designed to measure the quality of the decision-making process. The primary outcome was the Control Preferences Scale of Degner and colleagues,14,15 a single question that measures the respondent’s perception of the locus of decision-making control. Response options range from A to E; choice A represents complete patient control, choice E represents complete physician control, and choice C represents a purely shared decision. Other questions explored additional characteristics of the prostate cancer screening decision-making process, including patients’ and physicians’ perceptions of the duration and number of topics covered in discussions, decisional conflict,16 knowledge about prostate cancer screening,17 and frequency of PSA testing. The methods have been presented previously.18
We performed statistical analyses on data gathered from returned questionnaires using SAS version 9.1.3 (SAS Institute, Cary, NC).19 We report aggregate results of the 3 study groups, based on when patients were seen in relation to the Merenstein case (before, diffusion, or after period). The denominators of all analyses include only survey respondents. We used 2-sided statistical tests for all calculations. We used the Kruskal-Wallis test—a nonparametric companion to analysis of variance20—to make comparisons across all 3 time periods on the locus of decision-making control, decisional conflict, number of topics discussed, and time spent discussing screening. We used the Wilcoxon rank sum test to compare changes between the before and after periods. The Fisher exact test was used to compare proportional responses (knowledge and frequency of PSA testing). We also conducted an analysis of covariance to determine whether the statistical significance of our findings was influenced by the level of training of the physician seen (resident or faculty) or the study group to which the patient was randomized.
RESULTS
Of 1,073 men scheduled for health maintenance examinations, 497 (46%) agreed to participate and were randomized to the control (n = 75), brochure (n = 196), or Web site (n = 226) groups. Questionnaires were completed by 432 (87%) of the patients and 457 (91%) of the physicians, representing the final analytic sample populations. We received completed questionnaires from 180 patients in the before period, 87 in the diffusion period, and 165 in the after period. Patients enrolled in the 3 periods were similar with respect to age, race, education, prior PSA testing, and the proportions randomized to trial groups (Table 1 ▶). The number of patients seen by residents increased over the 3 time periods (23%, 30%, 35%; P = .04).
Table 1.
Characteristics of the Study Population (N = 432)
| Characteristic | Before Period (n = 180) | Diffusion Period (n = 87) | After Period (n = 165) | PValue* |
| Age, mean, years | 58 | 57 | 56 | 0.13 |
| Race, % | ||||
| White | 89 | 91 | 93 | 0.59 |
| African American | 3 | 2 | 2 | 1.00 |
| College education or higher, % | 85 | 85 | 82 | 0.64 |
| Prior testing for PSA, % | 70 | 70 | 65 | 0.41 |
| Type of physician seen, % | 0.02† | |||
| Faculty | 77 | 70 | 65 | |
| Second-year resident | 7 | 12 | 18 | |
| Third-year resident | 16 | 18 | 16 | |
| Group randomized to, % | 0.39† | |||
| Control | 17 | 16 | 13 | |
| Brochure decision aid | 39 | 32 | 43 | |
| Web-based decision aid | 44 | 52 | 44 | |
PSA = prostate-specific antigen.
* Calculated across the 3 time periods using the Kruskal-Wallis test.
† Aggregate P value for population characteristic.
Patients’ perceptions of the locus of decision-making control (the study’s primary outcome measure) did not differ significantly before and after the diffusion period (Figure 1 ▶). In proportions that were statistically indistinguishable in the before, diffusion, and after periods, most patients (91%, 87%, and 90%, respectively) reported having substantial control over the screening decision (choice A, B, or C), and approximately one third (34%, 37%, and 38%, respectively) described a shared locus of decision control with the physician (choice C). Also, the low frequency with which patients reported complete physician control (choice E) did not change (4%, 8%, and 7%, respectively). The minority of physicians who reported that they (the physicians) made the decision alone (choice E) increased, however, comparing responses before and after the diffusion period (from 3.3% to 11.1%; P = .003). The frequency with which physicians reported choices A through D, and the aggregate locus of decision control as perceived by physicians did not differ or change significantly across the 3 time periods.
Figure 1.
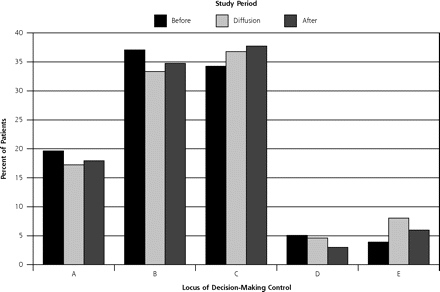
Patient-reported locus of decision-making control in relation to the Merenstein case (N = 431 question respondents).
Note: The figure shows patients’ responses to the survey question, “How was the decision made today on whether to do a PSA blood test? (A) I made the decision on whether to order a PSA test. (B) I made the decision about whether to order a PSA test after seriously considering my doctor’s opinion. (C) My doctor and I shared the responsibility for deciding whether to order a PSA test. (D) My doctor made the final decision about whether to order a PSA test after seriously considering my opinion. (E) My doctor made the decision whether to order a PSA test.” Before period = January 2002 through June 2003; diffusion period = July 2003 through December 2003; after period = January 2004 through November 2004. The differences across the 3 time periods are not significant (P = .54).
Comparisons of responses in the before, diffusion, and after periods showed no significant changes in other key parameters of the prostate cancer screening discussion, such as patient knowledge (average percentage of correct answers, 76%, 79%, and 74%, respectively; P = .86), average Decisional Conflict Scale score (1.53, 1.47, and 1.61; P = .23), average number of topics discussed (6.0, 6.4, and 6.1; P = .37), or average time spent addressing screening (5.4, 4.7, and 4.9 minutes; P = .20) (Figure 2 ▶). Comparing the before and after time periods, the proportion of men receiving a PSA test increased from 84% to 90% (P = .03).
Figure 2.
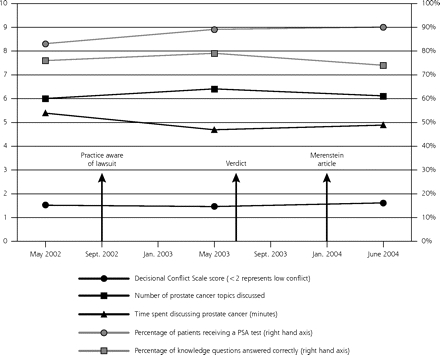
Patient-reported elements of the prostate cancer screening discussion in relation to the Merenstein case (N = 432).
PSA = prostate-specific antigen.
Note: The figure shows temporal trends of measured elements of the prostate cancer screening process in relation to the Merenstein case (before, diffusion, and after periods) as reported by 432 patients who returned a completed questionnaire. The left-hand axis and black lines represent an ordinal scale from 1 to 10. The right-hand axis and gray lines represent a percentage scale from 0% to 100%. Comparing the before and after time periods, the differences are not significant for decisional conflict (P = .23), number of topics discussed (P = .37), time spent on discussion (P = .20), and knowledge (P = .86). The percentage of patients receiving a PSA test increased, however (84% vs 90%; P = .03).
The differences across the 3 time periods did not change in magnitude even after adjusting for the physician’s level of training and the study group. The adjusted values did not differ significantly for locus of decision-making control (P = .44), screening knowledge (P = .10), time spent discussing screening (P = .24), and number of topics discussed as reported by the patient (P = .07). Likewise, the difference in frequency of PSA testing in the before and after periods remained significant (P = .04) after a similar adjustment.
DISCUSSION
The circumstances that occurred at the practice involved in this study presented a unique and, to our knowledge, unprecedented opportunity to examine the impact of adverse litigation on practice behavior. A fortuitous natural experiment became possible because our decision aid study, in progress at the time of the lawsuit, was examining the very process (shared decision making) and context (prostate cancer screening) over which the case was lost. A large body of literature addresses concerns with our current malpractice system.21–25 Prior research reports describe defensive medicine—the practice of performing nonrecommended tests or medical interventions for fear of liability—although these results are based primarily on clinician surveys or observed associations between clinicians’ behaviors and past exposure to litigation.26–29 Conversely, using hypothetical patient cases, Glassman et al30 demonstrated that unnecessary defensive test ordering did not increase among clinicians who had been sued. Our study, however, is perhaps the first study to prospectively capture the patient’s perspective on whether physicians’ decision-making processes were affected by losing a malpractice case.
We found that these physicians did not appear to measurably change their approach to engaging patients in decision making for prostate cancer screening. Patients were equally well informed about prostate cancer screening and had low Decisional Conflict Scale scores over the observed time periods. As reported by patients, physicians did not retake control over the decision and continued to spend time discussing issues related to screening. This perspective contrasts with the physicians’ perspective, which suggested that control over the decision increased after the case. This difference, of uncertain clinical importance, may have arisen because the physicians, and not the patients, were aware of the lawsuit.
We did observe a 6% increase in PSA testing. Whether a similar increase was occurring at other primary care practices is unknown. Nor can we determine whether this trend, at our study site or elsewhere, was a reaction to the Merenstein case or represented an unrelated secular trend. We do know that a temporal trend was not observed nationally during this time period. PSA testing among US men aged 40 years or older was 54% in 2002 and 52% in 2004.31
Interestingly, our patient population had a higher rate of PSA testing, both before and after the lawsuit, than is reported nationally. This difference may be explained by our sampling frame—men aged 50 to 70 years who were coming to the physician for a health maintenance examination—an age group and setting for which PSA testing is more common. Several studies evaluating decision aids with a similar sampling frame also reported high screening rates, ranging from 82% to 100%.32–34 Our high baseline screening rate may introduce a ceiling effect, however, limiting how much screening can increase over time. It is unclear whether the 6% increase in PSA testing that we observed underestimates the increase in testing that might occur when practices with lower baseline rates experience adverse litigation.
To the extent that our findings reflect a true causal link between litigation and increased PSA testing, malpractice losses could have broad implications for difficult medical decisions such as those that involve shared decision making. Shared decision making is a complex process that relies on the unbiased sharing of information.35–37 Several key domains of the decision-making process that we measured were not altered by the malpractice case, as assessed by metrics frequently used to evaluate decision aids and decision processes in general.14,16,17 What we could not measure was the quality and the nature of patient-clinician discussions, which may have been fundamentally altered by the malpractice case in ways that our study could not discern. Our metrics, which focused on information sharing and decision-making control, might not have detected subtle shifts in physician biases that ultimately altered the patient’s decision. The effect of clinician bias on shared decisions is a key argument for including decision aids as part of the process to decide whether to receive prostate cancer screening,38 such as the resources that we evaluated in our main study, or through the assistance of “decision counselors,” nonbiased third parties dedicated to helping patients make difficult decisions.13,39
Our temporal analysis has at least 6 limitations, notably that the natural experiment examines a question that was not the subject of an a priori hypothesis. First, the conclusions are derived from a study that was designed prospectively to measure the effect of decision aids, not of a malpractice case. Second, outcomes questionnaires relied on self-report and were oriented toward measuring the elements of shared decision making, as required for our clinical trial; direct observation would have yielded insights on other aspects of the quality and character of discussions between patients and physicians. Third, if the a priori hypothesis of this study were to assess the impact of litigation on physician behavior, we could have collected and analyzed additional data on physician characteristics that might influence test-ordering behavior, such as risk perception, risk aversion, perceived value of PSA testing, and individual experiences with this and other malpractice cases. Fourth, as described elsewhere,10 the instrument of Degner and colleagues14,15 that we used to measure shared decision making lacks precision in defining whether decisions are shared.13,36 Fifth, the practice experienced intense exposure to the Merenstein case and may not be representative of other primary care practices, although our null findings would suggest an even more negligible effect elsewhere. Our data on locus of control, drawn from a study population with high screening rates at baseline, may not be generalizable to settings where screening is less common. To the extent that patients predisposed to screening were overrepresented in our sample, our data may lack external validity for more undecided patients. Sixth, we did not power the study to evaluate temporal trends across the 3 time periods; our sample sizes were established for the aims of the original study.
In conclusion, at least in this limited setting of patient-physician discussions of PSA screening, our data on the shared decision-making process do not support the premise of defensive medicine8—that the threat or experience of adverse litigation will alter clinician behavior. The findings of this natural experiment suggest the need for more formal research to test this question. Innovative study designs and methods to capture prospectively the outcomes of unplanned events will ultimately be necessary to fully understand the impact of the current legal system on the medical care that patients receive.
Acknowledgments
We would like to thank the faculty, residents, and patients of Fairfax Family Practice Center at the VCU School of Medicine/INOVA campus for participation in and assistance with this study.
Conflicts of interest: Dr Krist is a faculty member, practicing physician, and partial owner of Fairfax Family Practice Residency, where the study was conducted.
Funding support: This work was funded by the American Academy of Family Physicians Foundation under the Joint Grant Awards Program (grant G0113).
REFERENCES
- 1.Merenstein D. A piece of my mind. Winners and losers. JAMA. 2004;291(1):15–16. [DOI] [PubMed] [Google Scholar]
- 2.Fleming M. Evidence-based medicine on trial. JAMA. 2004;291(14): 1697–1698; author reply 1698. [DOI] [PubMed] [Google Scholar]
- 3.Hall MA, Green MD, Hartz A. Evidence-based medicine on trial. JAMA. 2004;291(14):1697; author reply 1698. [DOI] [PubMed] [Google Scholar]
- 4.Watts C. Evidence-based medicine on trial. JAMA. 2004;291(14): 1697; author reply 1698. [DOI] [PubMed] [Google Scholar]
- 5.Morse LJ. Evidence-based medicine on trial. JAMA. 2004;291(14): 1697; author reply 1698. [DOI] [PubMed] [Google Scholar]
- 6.Hogan RA. Evidence-based medicine on trial. JAMA. 2004;291(14): 1696–1697; author reply 1698. [DOI] [PubMed] [Google Scholar]
- 7.Bicket DP. Evidence-based medicine on trial. JAMA. 2004;291(14): 1696; author reply 1698. [DOI] [PubMed] [Google Scholar]
- 8.Edsall RL. The evidence-based medicine heresy. Fam Pract Manag. 2004;11(2):13. [PubMed] [Google Scholar]
- 9.Hurwitz B. How does evidence based guidance influence determinations of medical negligence? BMJ. 2004;329(7473):1024–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American College of Preventive Medicine. Highlights of Preventive Medicine 2005. Available at: http://www.medscape.com/viewpro-gram/3960_pnt. Accessed: 16 May 2005.
- 11.Overlawyered. Update: commentary on the Merenstein lawsuit. Available at: http://www.overlawyered.com/archives/001414.html. Accessed: 16 May 2005.
- 12.Deutsches Ärtzeblatt. Medicine on trial. Available at: http://www.aer-zteblatt.de/v4/foren/beitrag.asp?id=45750. Accessed: 16 May 2005.
- 13.Krist A, Woolf S, Johnson R, Kerns J. Patient education on prostate cancer screening and involvement in decision making. Ann Fam Med. 2007;5(2):112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol. 1992;45(9):941–950. [DOI] [PubMed] [Google Scholar]
- 15.Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997;29(3):21–43. [PubMed] [Google Scholar]
- 16.O’Connor A. Decisional Conflict Scale. 4th ed. Ottawa, Ontario: University of Ottawa; 1999.
- 17.O’Dell KJ, Volk RJ, Cass AR, Spann SJ. Screening for prostate cancer with the prostate-specific antigen test: are patients making informed decisions? J Fam Pract. 1999;48(9):682–688. [PubMed] [Google Scholar]
- 18.Woolf SH, Krist AH, Johnson RE, Stenborg PS. Unwanted control: how patients in the primary care setting decide about screening for prostate cancer. Patient Educ Couns. 2005;56(1):116–124. [DOI] [PubMed] [Google Scholar]
- 19.SAS Version 9.1.3. Copyright 2002–2003. Cary, NC: SAS Institute Inc.
- 20.Hollander M, Wolfe D. Nonparametric Statistical Methods. Indianapolis, Ind: John Wiley & Sons; 1999.
- 21.Woolf SH, Krist A. The liability of giving patients a choice: shared decision making and prostate cancer. Am Fam Physician. 2005;71(10):1871–1872. [PubMed] [Google Scholar]
- 22.Murphy JF. When careful medicine becomes defensive medicine. Ir Med J. 2004;97(10):292. [PubMed] [Google Scholar]
- 23.McLean TR. Why do physicians who treat lung cancer get sued? Chest. 2004;126(5):1672–1679. [DOI] [PubMed] [Google Scholar]
- 24.Malpractice mess. Is this the way out? Med Econ. 2004;81(13):30–33. [PubMed] [Google Scholar]
- 25.Phillips RL Jr, Bartholomew LA, Dovey SM, et al. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13(2):121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luckmann R, Melville SK. Periodic health evaluation of adults: a survey of family physicians. J Fam Pract. 1995;40(6):547–554. [PubMed] [Google Scholar]
- 27.Gostin L. A public health approach to reducing error: medical malpractice as a barrier. JAMA. 2000;283(13):1742–1743. [DOI] [PubMed] [Google Scholar]
- 28.Leape LL. Unnecessary surgery. Health Serv Res. 1989;24(3):351–407. [PMC free article] [PubMed] [Google Scholar]
- 29.Nyquist AC, Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. JAMA. 1998;279(11):875–877. [DOI] [PubMed] [Google Scholar]
- 30.Glassman PA, Rolph JE, Petersen LP, Bradley MA, Kravitz RL. Physicians’ personal malpractice experiences are not related to defensive clinical practices. J Health Polit Policy Law. 1996;21(2):219–241. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. BRFSS—CDC’s Behavioral Risk Factor Surveillance System. Available at: http://www.cdc.gov/brfss. Accessed: 7 December 2005.
- 32.Frosch DL, Kaplan RM, Felitti V. The evaluation of two methods to facilitate shared decision making for men considering the prostate-specific antigen test. J Gen Intern Med. 2001;16(6):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frosch DL, Kaplan RM, Felitti VJ. A randomized controlled trial comparing Internet and video to facilitate patient education for men considering the prostate specific antigen test. J Gen Intern Med. 2003;18(10):781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schapira MM, VanRuiswyk J. The effect of an illustrated pamphlet decision-aid on the use of prostate cancer screening tests. J Fam Pract. 2000;49(5):418–424. [PubMed] [Google Scholar]
- 35.Edwards A, Elwyn G. Evidence-Based Patient Choice. Oxford, Great Britian: Oxford University Press; 2001.
- 36.Sheridan SL, Harris RP, Woolf SH. Shared decision making about screening and chemoprevention. A suggested approach from the U.S. Preventive Services Task Force. Am J Prev Med. 2004;26(1):56–66. [DOI] [PubMed] [Google Scholar]
- 37.Rimer BK, Briss PA, Zeller PK, Chan EC, Woolf SH. Informed decision making: what is its role in cancer screening? Cancer. 2004;101(5 Suppl):1214–1228. [DOI] [PubMed] [Google Scholar]
- 38.O’Connor AM, Legare F, Stacey D. Risk communication in practice: the contribution of decision aids. BMJ. 2003;327(7417):736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woolf SH, Chan EC, Harris R, et al. Promoting informed choice: transforming health care to dispense knowledge for decision making. Ann Intern Med. 2005;143(4):293–300. [DOI] [PubMed] [Google Scholar]


