Over a wide range of environments, up to five ant species forage every square meter of ground (1). In Amazonian rainforests, the biomass of ants dwarfs that of vertebrates (2), and in many rainforest trees, ants make up a large fraction of individual insects (3). This ecological dominance and the complexity of their societies makes their phylogeny of great interest as a glimpse into the development of the modern world in terms of the relationships between the various groups of ants, how their characteristics evolved, and when they originated. This year we have seen not one but two blockbuster articles examining ant phylogeny and time of origin of the group, one of which is by Brady et al. (4) in this issue of PNAS. The two articles (4, 5) agree in several important respects but disagree in others.
Early thought on ant phylogeny was bedeviled by the belief that all or most of the genera with armored cuticles and strong stings belonged in a single subfamily, the Ponerinae (6). Brown (7) pointed the way forward by suggesting that various other ant subfamilies arose within the ponerines, which are thus paraphyletic; presciently, he proposed a close relationship between the Ectatomminae (then a ponerine tribe) and the giant subfamily Myrmicinae [>4,500 species (8)]. However, he made no nomenclatural change, and subsequent authors tended to treat the ponerines as a single group. This tendency to agglomerate seriously compromised the ability to make sense of ant phylogeny, and for decades the procession of phylogenetic schemes was notable in its diversity rather than its stability. The crucial breakthrough came from Bolton (24), who erected a host of new subfamilies and subdivided the original subfamily Ponerinae into six; although he still placed all of these together, this recognition of difference liberated phylogeneticists to make new findings (4, 5, 9).
Bolton's (24) reorganization of ant systematics joined with the increasing ease of obtaining DNA sequence, a moderately good fossil record, and the rise of phylogenetic methods able to handle large data sets and estimate divergence dates. The first major and convincing effort to elucidate ant phylogeny at a grand scale and set it in temporal context was that of Moreau et al. (5) earlier this year. The study by Brady et al. (4) is even larger, dealing with 162 species from all 20 currently recognized ant subfamilies and 10 outgroups and using 6 kb of DNA sequence from seven nuclear genes. There is much agreement between the two studies. In particular, most subfamilies are monophyletic, and the two trees place them in similar positions. Brown's suggestion of a strong relationship between the Ectatomminae and the Myrmicinae is not contradicted statistically by the new findings.
There is thus now the emergence of the promise of stability in ant phylogeny, with these studies having very similar trees, but this result includes a puzzling anomaly, namely the placement of the Leptanillinae as the sister group to all other ants. Those leptanillines that have been studied are tiny, eyeless subterranean ants with an army-ant lifestyle, preying on geophilomorph centipedes like wolves on elk (10). Their bizarre habit of the queen feeding on hemolymph from her larvae also occurs in the Amblyoponinae (11), and this and morphological similarities raised suspicions that these groups are closely related. Having the Leptanillinae placed at the base of the tree of all ants (4, 5) is therefore very odd. For one thing, eye-balling the resulting tree gives the impression that the ancestral ant was eyeless and lived underground, so that the great majority of ants today must have secondarily regained eyes and moved to hunt in the open air.
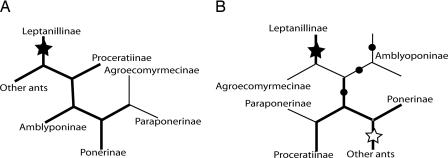
Long branch attraction (12), in which groups at the ends of long branches are wrongly placed together during phylogeny inference, can also lead to spurious rearrangement of the ingroup taxa (13). The problem is mainly one for parsimony and will not occur for maximum likelihood or Bayesian analysis when the substitution model has been correctly specified (14), but the models now available may not reflect reality sufficiently well to avoid it in some small, but unknown, number of cases (15). To paraphrase Li (16), substitution models are naturally artificial despite the attempt to be artificially natural. Brady et al. (4) surmised that long branch attraction might have affected the placement of the ant groups and thus repeated the analysis with the outgroups omitted. Significant differences appeared between the two analyses (Fig. 1). In particular, the poneroids, a group of morphologically similar subfamilies, which had formed a monophyletic assemblage in the rooted tree, no longer did so when the outgroups were omitted. Next, Brady et al. tested nine hypotheses for the rooting of the ant tree by constraining each such link in turn and found that only four of these were eliminated statistically. The most likely one of the remaining five still placed the Leptanillinae as the sister group to the rest of antdom, but the second most likely of them nests them near the Amblyoponinae and allows the interpretation of the ancestral ant as an above-ground forager with eyes. Thus, an ant tree in accordance with morphological expectation is among those fitting the molecular data, but it is not a done deal.
Fig. 1.
Certainty becomes managed uncertainty. Analyzing the complete sequence ant data set together with outgroups yielded tree A, with the outgroups joining at the filled star, implying that the Leptanillinae are the sister group to all other ants. Analyzing just the ant sequences led to a significantly different result, tree B. Testing nine hypotheses (dots or stars) for rooting the ant tree eliminated four but left five as statistically not separable. The most likely of these, shown with a filled star, remains on the branch to the Leptanillinae, but the second most likely, shown with an open star, falls on the branch to the rest of the ants, implying that the leptanillines are closely related to the Amblyponinae, with which they share some striking characteristics. Thick lines denote branches with posterior probability of at least 0.95. The instability of the ingroup according to whether outgroups are included in the analysis may have resulted from long branch attraction.
Given the thoroughness of the phylogenetic analysis, it is striking that this sophistication did not extend to comparative analysis, for which no details have been given. Powerful Bayesian methods are now available, which, unlike earlier approaches, take uncertainty in the phylogeny into account (17, 18). Given the wealth of morphological, ecological, social, and behavioral characteristics ants present, the data now available promise a further revolution in understanding all features of ant biology.
But when did ants arise? Most modern ants (and many predatory wasps) have adults subsisting mostly on floral nectar or hemipteran exudates while hunting prey (or carrion) for the young. These characteristics speak for an association with angiosperms, which has been suggested as important to the origin of ants (19). Stemming from winged stinging ancestors, they have reduced the winged stage to a dispersal phase and adapted to life on or in the ground by females casting off their wings once they have mated. Speculating a little, dispersing, mating, and settling on the ground predisposed such insects to form small family groups, leading naturally to a strong influence of kin selection fostering the further transition to the differentiation between queens and workers (20, 21). Losing wings for foraging not only opened up the ground and its surface, it also opened the night; many ants forage at night, but exceedingly few flying social insects manage this (22).
It is in dating the origin of ants that the emphases of this year's blockbuster ant articles differ most sharply. Moreau et al. (5) explored a number of dating techniques, but both Moreau et al. and Brady et al. (4) settled on the method of penalized likelihood (23) and used the same set of ant fossil ages. Whereas Moreau et al. appear to have used only the minimum ages of ant fossils in their analysis, Brady et al. also assigned two different fixed ages (145 and 185 Mya) to the most basal node, basically marking the origin of the aculeate Hymenoptera. In each article, a maximum and minimum age was estimated for the ants. As did some earlier analyses (including one by myself and some by members of the Brady et al. team), Moreau et al. concluded that a Jurassic age for the ants is plausible; the confidence limits for these estimates range from 132.6 to 176.4 Mya. Brady et al. used their three “best” trees for dating, the tree resulting from analyzing all of their sequences in a single analysis (Fig. 1A) and the trees with the highest likelihood resulting from adding the outgroups to various points on the tree analyzed for ants alone (starred in Fig. 1B). The most divergent of these dates and their confidence limits yields the range 105.6–143.2 Mya. The two ranges overlap, but whereas that of the earlier study overlaps the Jurassic, that of Brady et al. (4) does not. However, Brady et al. regard the older ages as problematic and hence stress a mid-Cretaceous age for the most recent common ancestor for ants. Even so, Brady et al. concede that there may have been ants on Earth even earlier, in the form of the apparent ant ancestors, the enigmatic fossil-only Sphecomyrminae. Where this uncertainty leaves the association with the angiosperms is a little unclear; it seems less problematic to have ants diversifying in the presence of rich angiosperm forests than before these arose, but homopterans would have provided the sugary secretions consumed by adults in preangiosperm times.
There is now the emergence of the promise of stability in ant phylogeny.
A rich interplay between systematics, morphology, and molecular phylogeny can be traced. As noted above, the systematic decision (24), based on morphology, to disaggregate the various groups then classified as tribes within the Ponerinae freed molecular phylogeneticists trying to relate the subfamilies, making possible the studies that have appeared. Molecular phylogenetic results, on the other hand, so closely linked one of Bolton's new subfamilies, the Apomyrminae, with one of the others, the Amblyponinae (9), that systematists merged the two (25). The findings include some of molecular evolutionary interest (why did the Leptanillinae evolve so much faster than other ants?), but this work has the most interest to those fascinated by the biology and evolution of this socially sophisticated and ecologically dominant group.
Footnotes
The author declares no conflict of interest.
See companion article on page 18172.
References
- 1.Room PM. Aust J Zool. 1975;23:71–89. [Google Scholar]
- 2.Fittkau EJ, Klinge H. Biotropica. 1973;5:2–14. [Google Scholar]
- 3.Mody K, Bardoz HA, Linsenmair KE. In: Arthropods of Tropical Forests: Spatio-Temporal Dynamics and Resource Use in the Canopy. Basset Y, Novotny V, Miller SE, Kitching RL, editors. Cambridge, UK: Cambridge Univ Press; 2003. pp. 198–212. [Google Scholar]
- 4.Brady SG, Schultz TR, Fisher BL, Ward PS. Proc Natl Acad Sci USA. 2006;103:18172–18177. doi: 10.1073/pnas.0605858103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreau CS, Bell CD, Vila R, Archibald SB, Pierce NE. Science. 2006;312:101–104. doi: 10.1126/science.1124891. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler WM. The Social Insects: Their Origin and Evolution. New York: Harcourt, Brace, and Co; 1928. [Google Scholar]
- 7.Brown WL. Insect Soc. 1954;1:21–31. [Google Scholar]
- 8.Bolton B. J Nat Hist. 1995;29:1037–1056. [Google Scholar]
- 9.Saux C, Fisher BL, Spicer GS. Mol Phylogenet Evol. 2004;33:457–468. doi: 10.1016/j.ympev.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Masuko K. Insectes Soc. 1990;37:31–57. [Google Scholar]
- 11.Masuko K. Behav Ecol Sociobiol. 1986;19:249–255. [Google Scholar]
- 12.Felsenstein J. Syst Zool. 1978;27:401–410. [Google Scholar]
- 13.Holland BR, Penny D, Hendy MD. Syst Biol. 2003;52:229–238. doi: 10.1080/10635150390192771. [DOI] [PubMed] [Google Scholar]
- 14.Mar JC, Harlow TJ, Ragan MA. BMC Evol Biol. 2005;5:8. doi: 10.1186/1471-2148-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson FE, Swofford DL. Mol Phylogenet Evol. 2004;33:440–451. doi: 10.1016/j.ympev.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Li CC. Biometrics. 1967;23:397–484. [PubMed] [Google Scholar]
- 17.Huelsenbeck JP, Nielsen R, Bollback JP. Syst Biol. 2003;52:131–158. doi: 10.1080/10635150390192780. [DOI] [PubMed] [Google Scholar]
- 18.Schultz TR, Cocroft RB, Churchill GA. Evolution (Lawrence, Kans) 1996;50:504–511. doi: 10.1111/j.1558-5646.1996.tb03863.x. [DOI] [PubMed] [Google Scholar]
- 19.Wilson EO, Hölldobler B. Proc Natl Acad Sci USA. 2005;102:7411–7414. doi: 10.1073/pnas.0502264102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster KR, Wenseleers T, Ratnieks FLW. Trends Ecol Evol. 2006;21:57–60. doi: 10.1016/j.tree.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 21.Pamilo P. J Theoret Biol. 1991;149:75–95. doi: 10.1016/s0022-5193(05)80073-6. [DOI] [PubMed] [Google Scholar]
- 22.Taylor RW. Mem Am Entomol Inst. 2006;77:595–619. [Google Scholar]
- 23.Sanderson MJ. Mol Biol Evol. 2002;19:101–109. doi: 10.1093/oxfordjournals.molbev.a003974. [DOI] [PubMed] [Google Scholar]
- 24.Bolton B. Mem Am Entomol Inst. 2003;71:1–370. [Google Scholar]
- 25.Engel MS, Grimaldi DA. Am Mus Novit. 2005;3485:1–23. [Google Scholar]