Abstract
Cancer/testis (CT) antigens are immunogenic proteins expressed in normal gametogenic tissues and in different types of tumors. CT antigens are promising candidates for cancer immunotherapy, and the identification of novel CT antigens is a prerequisite for the development of cancer vaccines. We have identified a CT antigen, named CTSP-1, with partial similarity to the breast differentiation antigen NY-BR-1. CTSP-1 presents several splicing and polyadenylation variants and has a very restricted expression pattern among normal tissues. CTSP-1 is exclusively expressed in normal testis and is aberrantly expressed in 47.6% (10 of 21) of tumor cell lines and in 44.4% (75 of 169) of tumors from different histological types. The highest percentages of positive expression were observed in melanomas (59.0%) followed by prostate (58.0%) and lung (57.0%) tumors. CTSP-1 is part of a highly conserved gene family, and members of this family also have a restricted expression pattern and similar protein structure. Antibodies against members of this gene family were detected in 10% (14 of 141) of plasma samples from patients with a wide spectrum of tumors. The highest percentages of antibody response were observed in patients with prostate (20.8%), thyroid (20.0%), and breast (16.6%) tumors. Because of its very restricted expression pattern in normal tissues and immunogenicity in different types of tumors, CTSP-1 should be considered a promising candidate for cancer immunotherapy.
Keywords: immunotherapy, tumor antigen
Cancer/testis (CT) antigens are predominantly expressed in normal gametogenic tissues as well as in different histological types of tumors (1–4). In testis, CT antigens are expressed exclusively in cells of the germ cell lineage, although there is a marked variation in the protein expression pattern during different stages of sperm development. Likewise, a heterogeneous expression is observed in tumors (1, 4). The methylation status of the promoter region seems to be the main, but not the only, regulator of their specific expression pattern (1, 3, 4).
Most CT antigens have no defined biological function, but their involvement in signaling, transcription, translation, and chromosomal recombination has been proposed (3, 4). It has also been proposed that the aberrant expression of CT antigens in tumors recapitulates the germ-line gene expression program, and that it is related to some characteristics of the neoplastic phenotype such as immortality, invasiveness, immune evasion, and metastatic capacity (3, 5).
Because of their restricted expression pattern, CT antigens are considered ideal targets for cancer immunotherapy (1, 6). Indeed, a small subset of patients immunized with the known CT antigens MAGE-A and NY-ESO-1 have shown clinical benefits after immunization (7–10). However, because CT antigens are expressed in only a small subset of human tumors and in only a fraction of cases of a given tumor type, the identification of additional CT antigens is crucial for improving current immunotherapy protocols. Presently, 44 distinct CT-antigen families have been described, of which several have multiple members resulting in a total of 89 transcripts (3).
During the process of identification of genes located on human chromosome 21 (HC21) (11), we found a gene (C21ORF99) that was predominantly expressed in testis and had partial similarity to the 5′region of the breast differentiation antigen NY-BR-1 gene (12). In this work, we refined the characterization of C21ORF99 gene structure and renamed it CTSP-1. We found that CTSP-1 has a restricted expression pattern in normal tissues characteristic of CT antigens and is expressed at a high frequency in tumor cell lines and tumor samples. We also found that CTSP-1 is part of a highly conserved gene family, and that some members of this family also have an expression pattern characteristic of CT antigens. Antibodies against members of this gene family were detected in plasma samples from patients with a wide spectrum of tumors.
Results and Discussion
CTSP-1 Gene Structure and Polyadenylation Variants.
Using the information generated by alignments between ESTs and the sequence of the HC21 (11), we identified a gene, named C21ORF99 (AF427490), which was predominantly expressed in normal testis and showed partial similarity to the 5′ region of the breast differentiation tumor antigen NY-BR-1 gene (AF269087) and its paralog NY-BR-1.1 (AF269088), located at chromosomes 10 and 18, respectively (12).
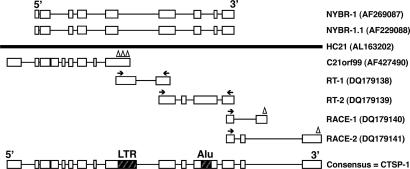
C21ORF99 sequence was obtained by sequencing the full insert of cDNA clones from which the ESTs aligning to HC21 were derived (11). However, based on the mapping of NY-BR-1 and NY-BR-1.1 to HC21 sequence, we suspected that the initial sequence obtained for C21ORF99 (derived from cDNA clones containing both polyA signal and tail) corresponded to a shorter polyadenylation variant (Fig. 1).
Fig. 1.
Schematic representation of the strategies used in the generation of CTSP-1 consensus sequence. NY-BR-1, NY-BR-1.1, and C21ORF99 sequences were aligned to the genomic sequence of the human chromosome 21 (HC21). Exons are represented as boxes, and lines correspond to introns. Primers are represented with arrows, and triangles correspond to polyadenylation signals. Repetitive elements (LTR and Alu) are also represented.
To extend the initial C21ORF99 sequence and verify the existence of additional polyadenylation variants, we used a combination of RT-PCR and 3′-RACE experiments. First, we designed two pairs of primers specific for HC21 sequence in regions that showed high similarity to NY-BR-1 and NY-BR-1.1. RT-PCR products obtained with these primers were cloned and sequenced, and the original C21ORF99 sequence was extended as illustrated in Fig. 1.
Because the 3′ end of NY-BR-1 (from nucleotide 3,199 to 4,458) and NY-BR-1.1 (from nucleotide 3,220 to 3,673) did not show significant similarity to the HC21 sequence, we have also performed 3′-RACE experiments to generate a full-length sequence. Two distinct 3′-RACE fragments corresponding to two additional polyadenylation variants were amplified and sequenced (Fig. 1). All 3′-ESTs available in public databases and corresponding to CTSP-1 are derived from the shortest variant, suggesting it is the most abundantly expressed variant (data not shown).
A consensus mRNA sequence of 3,915 bp, organized in 15 exons, was obtained by assembling the C21ORF99 sequence, the RT-PCR products, and the longest 3′-RACE fragment (Fig. 1) and was named CTSP-1. This sequence is provided as Data Set 1, which is published as supporting information on the PNAS web site. The alignment between the CTSP-1 consensus sequence and NY-BR-1 nucleotide sequence is not continuous but presents seven regions of high similarity (average identity of 86.6%) that encompass 1,223 of the 3,915 nucleotides (31%) of the CTSP-1 sequence.
Careful analysis of the CTSP-1 mRNA consensus sequence revealed the presence of two transcribed repetitive elements located within exons 9 (LTR repeat) and 12 (Alu repeat), which could not be found in the NY-BR-1 and NY-BR-1.1 sequences (Fig. 1). Because the polyadenylation sites associated with the shorter polyadenylation variant are contained within the LTR repeat, it is tempting to speculate that the presence of this repetitive element within exon 9 is associated with the occurrence of alternative polyadenylation. Supporting this hypothesis, it has recently been reported that the insertion of retroelements in the human genome can modulate gene expression by introducing intragenic polyadenylation signals as well as by disrupting gene structure (13).
CTSP-1 Gene Family.
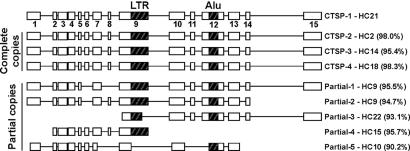
Recent duplication events have been described for many CT antigens such as NY-ESO-1 and SSX, resulting in two or more identical copies with identical coding sequences (14, 15). To identify additional CTSP family members, the CTSP-1 consensus sequence was aligned to the human genome. Three complete copies of highly similar genes were found on chromosomes 2 (CTSP-2, 98.0% identity), 14 (CTSP-3, 95.4% identity), and 18 (CTSP-4, 98.3% identity). The exon–intron structure seems highly conserved among these four members, including the presence of both LTR and Alu repeats, suggesting they are products of recent gene-duplication events (Fig. 2). However, because the exon–intron structure for these loci were predicted based on the alignment of CTSP-1 to the genomic sequence, confirmation of this structure will depend on the generation of expressed sequences covering the full extension of all these genes. ESTs and/or cDNA clones corresponding to all these family members can be found in public databases, suggesting that all of them are transcribed. As for CTSP-1, all ESTs and cDNA clones corresponding to these family members represent the first nine exons and are probably derived from the shorter polyadenylation variant.
Fig. 2.
Schematic representation of the exon/intron structure of the CTSP-1 family members. Complete and partial copies are represented with their corresponding chromosomal location and similarity to HC21 genomic sequence.
In addition to these complete copies, two partial copies containing the LTR and Alu repeat were identified on chromosome 9. However, their exon–intron structure is slightly different, and exons 6, 8, and 15 are missing when compared with the complete copies (Fig. 2). Additionally, partial copies covering <58% of the CTSP-1 mRNA length and with a lower similarity (<96%) were found on chromosomes 22, 15, and 10 (Fig. 2), and they probably result from failed duplication events, as has also been observed for other CT-gene families, such as SSX. An extended analysis of the evolution and conservation of this gene family among primates will be presented elsewhere.
Expression Profile and Alternative Splicing.
Because one of the criteria for identifying CT-antigen genes is their specific expression in tumors, but not in normal tissues except in testis, the mRNA expression pattern of CTSP-1 was determined by RT-PCR. Primers used in the expression analysis were manually designed to ensure specific amplification, and RT-PCR products were sequenced to confirm their specificity.
CTSP-1 mRNA expression was first examined in normal testis. A pair of primers located in exons 3 and 8 was used in RT-PCR with 40 cycles of amplification. Three predominant fragments of 239, 376, and 402 bp were obtained (Fig. 3A), and the whole amplification product was cloned without prior gel purification. Clones with different insert sizes were selected for sequencing. Using this strategy, we were able to identify at least eight splicing variants in which exons 4, 5, and 7 were alternatively spliced, as illustrated in Fig. 3B. No alternative splicing variants involving exons 1, 2, and 9 were observed when RT-PCR analysis was carried out using primers located on exons 1 and 9.
Fig. 3.
CTSP-1 splicing variants. (A) Southern blot of RT-PCR products amplified with CTSP-1-specific primers. Splicing variants (a–h) are indicated with arrows. cDNA samples used were as follows: normal testis tissue (lane 1), tumor cell lines A172 (lane 2), A2058 (lane 3), H1155 (lane 4), breast tumor samples (lanes 5 and 6), prostate tumor samples (lanes 7 and 8), and no cDNA-negative control (lane 9). (B) Schematic representation of CTSP-1 splicing variants (a–h). The coding exons are represented as gray boxes. Primers used in RT-PCR are represented as arrows, and the size of the putative ORF for each splicing variant is provided in amino acids (aa).
CTSP-1 mRNA expression was then analyzed in cDNA samples derived from 20 additional normal tissues and 21 tumor cell lines. To favor the detection of all splicing variants, RT-PCR products were transferred to nylon membranes and hybridized with a cDNA probe corresponding to exon 3, which is present in all splicing variants. Among the 21 normal tissues, CTSP-1 expression was restricted to testis and could also be detected in 47.6% (10 of 21) of all analyzed tumor cell lines (Fig. 4). Interestingly, although the expression of several splicing variants was detected in normal testis cDNA, a less complex pattern of alternative splicing could be visualized in cDNA derived from tumor cell lines (Fig. 3A). The shortest variant, in which exons 4, 5, and 7 are skipped, appears to be the most abundant and frequently expressed variant. Although we do not have a biological explanation for such diversity in testis, our results are in agreement with the general view that a higher proportion of splicing variants are present in testis, and that alternative splicing is particularly important in testis development and spermatogenesis (16).
Fig. 4.
CTSP-1 mRNA expression pattern in normal tissues and tumor cell lines. Southern blot of RT-PCR products amplified with CTSP-1-specific primers. (A) Normal cDNA samples used were as follows. Lanes: 1, testis; 2, lung; 3, prostate; 4, small intestine; 5, breast; 6, brain; 7, heart; 8, uterus; 9, bone marrow; 10, placenta; 11, colon; 12, fetal brain; 13, liver; 14, fetal liver; 15, thymus; 16, salivary gland; 17, spinal cord; 18, kidney; 19, spleen; 20, skeletal muscle; 21, adrenal gland; and 22, no cDNA-negative control. (B) cDNA samples from tumor cell lines used were: 1, Caski; 2, HeLa; 3, A172; 4, T98G; 5, HL-60; 6, K562; 7, H358; 8, H1155; 9, Du145; 10, PC3; 11, SCABER; 12, IM9; 13, FADu; 14, MCF-7; 15, MDA-MB-436; 16, MDA-MB-231; 17, SW-480; 18, SAOS-2; 19, A2058; 20, SKmel-25; 21, HEPG2; and 22, no cDNA-negative control. GAPDH amplification was used as positive control for cDNA synthesis.
The expression profile of CTSP-1 was then analyzed in 169 tumor samples derived from 10 different histological types of tumors. Positive expression was detected in 44.4% (75 of 169) of the samples (Table 1). The highest percentages of positive expression were observed in melanomas (59.0%) followed by prostate (58.0%) and lung (57%) tumors. As observed in the tumor cell lines, the shorter variant was the most abundant and frequently expressed (data not shown). This expression pattern is partially in agreement with the classification proposed by Scanlan et al. (2). In the proposed classification, melanomas and lung tumors were defined as tissues with higher CT expression; breast, prostate, and esophagus were classified as having moderate and stomach and colon as having low CT expression. Gliomas, thyroid, and uterus tumors were not classified in this analysis due to the insufficient number of samples analyzed.
Table 1.
Frequency of mRNA expression of CTSP family members in tumor samples
| Tumor | CTSP-1 (%) | CTSP-2 (%) | CTSP-4 (%) | |||
|---|---|---|---|---|---|---|
| Breast | 9/25 | (36.0) | 1/6 | (16.5) | 0/6 | (0.0) |
| Colon | 7/18 | (39.0) | 0/14 | (0.0) | 0/14 | (0.0) |
| Esophagus | 2/5 | (40.0) | 2/4 | (50.0) | 0/4 | (0.0) |
| Glioblastoma | 6/13 | (46.0) | Not done | Not done | ||
| Lung | 8/14 | (57.0) | 2/11 | (18.8) | 0/11 | (0.0) |
| Melanoma | 10/17 | (59.0) | 1/9 | (11.0) | 0/9 | (0.0) |
| Prostate | 14/24 | (58.0) | 8/17 | (47.0) | 0/17 | (0.0) |
| Stomach | 4/9 | (44.0) | 1/3 | (33.3) | 0/3 | (0.0) |
| Thyroid | 7/24 | (29.0) | 0/6 | (0.0) | 0/6 | (0.0) |
| Uterus | 8/20 | (40.0) | 4/14 | (28.5) | 0/14 | (0.0) |
| Total | 75/169 | (44.4) | 19/84 | (22.6) | 0/84 | (0.0) |
The expression pattern of the other three family members was also analyzed by RT-PCR. Primers used in the expression analysis were manually designed to ensure specific amplification and RT-PCR products were sequenced to confirm their specificity. Although the expression of the other CTSP-1 family members was also restricted among normal tissues, they were not frequently expressed in tumor cell lines and tumor samples. This fact has also been observed for other CT-antigen members of the MAGE and SSX families (17–19). A positive expression in normal testis was detected for CTSP-2 and -4, the first also being expressed in brain, fetal brain, and spinal cord among the 21 normal tissues analyzed (data not shown). Expression of CTSP-2 and -4 was not detected in any of the tumor cell lines (data not shown), although CTSP-2 expression was detected in tumor samples at a lower frequency when compared with CTSP-1 (Table 1). CTSP-3 expression was not detected in any of the samples analyzed. Splicing variants were also detected for CTSP-2 and -4.
An important element in the induction of CT-antigen gene expression is promoter demethylation. It has been shown that demethylation of CT gene promoters with 5-Aza-2′deoxycytidine induces antigen expression in cells that do not normally produce them (20–22). CTSP-1 gene expression was induced in the MCF-7 breast tumor cell line after treatment with 5-Aza-2′deoxycytidine, suggesting that methylation indeed plays an important role in CTSP-1 gene expression regulation (Fig. 7, which is published as supporting information on the PNAS web site).
CTSP-1 Protein.
The CTSP-1 sequence was translated to amino acid sequence, and the presence of a stop codon was detected in exon 4, generating a 115-aa-long putative protein, which is much shorter than the NY-BR-1 (1,341 aa) and NY-BR-1.1 (1,011 aa) proteins. The presence of “premature” stop codons was also detected in all splicing variants, generating small “truncated” proteins of size ranging from 115 to 202 aa (Fig. 3B). Interestingly, the longest ORF was predicted from the shortest splicing variant (without exons 4, 5, and 7), which is the most frequently expressed variant in testis, tumor cell lines, and tumor samples (Fig. 3). Although restricted to the N-terminal region of the NY-BR-1, protein similarity between the CTSP-1 longest ORF and the NY-BR-1 protein is high [identities = 159 of 249 (63%), positives = 180 of 249 (72%), and gaps = 46 of 249 (18%)]. All other members of the family seem to have “premature” stop codons as evaluated by aligning the CTSP-1 consensus sequence to the genomic sequence of chromosomes 2, 14, and 18.
Motif analysis of the amino acid sequence corresponding to the longest ORF of CTSP-1 identified four tandem ankyrin repeats (14–47, 48–80, 81–103, and 114–146 aa). However, the bZIP site and the tandem repetitive elements located at the C-terminal region of the NY-BR-1 protein could not be found in the CTSP-1 sequence. A similar protein structure is present in the other CTSP-1 family members.
The existence of the CTSP-1 protein was confirmed by Western blot by using a mouse polyclonal antibody against the CTSP-1 recombinant protein. As shown in Fig. 5A, a single band with the expected molecular mass (≈23 kDa) was detected in total protein extracts from normal testis. No higher molecular mass bands were detected. Preimmune serum and serum raised against CTSP-1 but depleted of anti-CTSP-1 Igs were used as controls of serum specificity.
Fig. 5.
CTSP-1 protein expression in normal testis and in prostate tissue. (A) CTSP-1 protein was detected by Western blot in protein extract from normal testis by using a CTSP-1 polyclonal antibody (1). Preimmune serum (2) and CTSP-1 polyclonal antibody depleted of anti_CTSP-1 Igs (3) were used as negative controls. Molecular weight markers are indicated (M). (B) Immunohistochemistry staining of CTSP-1 protein using the anti-CTSP-1 polyclonal antibody normal testis (1), CTSP-1 mRNA-positive prostate tumor (4), normal adjacent prostate tissue (5), and CTSP-1 mRNA-negative prostate tumor (6). Normal testis incubated with preimmune sera (2) and CTSP-1 polyclonal antibody depleted of anti-CTSP-1 Igs (4) were used as negative controls.
CTSP-1 protein was also analyzed by immunohistochemistry in normal testis. Again, preimmune serum and serum raised against CTSP-1 but depleted of anti-CTSP-1 Igs were used as controls of serum specificity. CTSP-1 exhibited an intense staining in testis, and the staining was restricted to germ cells. Among the germ cells, an intense staining was detected in both the nucleus and the cytoplasm of the cells in later stages of differentiation, such as spermatocytes and spermatids (Fig. 5B). It should be noted, however, that spermatogonias were mostly negative. A similar expression pattern has been observed for other non-X CT antigens (CT antigens mapped outside chromosome X; ref. 3).
CTSP-1 protein expression was then analyzed in prostate tissues. Prostate tumor samples, some of them expressing CTSP-1 mRNA, and normal prostate samples, were analyzed by IHC-immunohistochemistry. In the CTSP-1 mRNA-positive prostate tumors, staining was restricted to the tumor cells (Fig. 5B). No reactivity was observed in the CTSP-1 mRNA-negative prostate tumors, as well as in normal prostate samples, confirming the specificity of the anti-CTSP-1 antibody (Fig. 5B). However, because of the high similarity observed among all family members, we cannot rule out that the Western blot and immunohistochemistry results represent the accumulated expression of all CTSP members.
Humoral Response Against CTSP-1 in Cancer Patients.
To evaluate the presence of antibodies against the CTSP proteins in plasma from cancer patients, we established an immunoblotting assay by using recombinant his-tagged CTSP-1 (≈32 kDa). Another his-tagged unrelated recombinant protein, purified under the same conditions, served as internal negative control.
A total of 141 plasma samples from cancer patients with different histological types of tumors were used in the immunoblotting assay (Table 2, Fig. 6). Among the 141 plasmas analyzed, 14 (10.0%) were reactive to the CTSP-1 recombinant protein. Plasma samples from 50 healthy donors were used as negative controls. Reactivity against CTSP-1 recombinant protein was detected in only one sample from this control group (data not shown). Antibodies against CT antigens have been detected in patients with autoimmune disorders such as lupus eritrematosus (23) and vitiligo (24) and in men subjected to vasectomy (25). In addition, we cannot exclude the possibility that we have identified a not yet diagnosed cancer patient within the healthy control group.
Table 2.
Frequency of anti-CTSP-1 antibodies in plasma samples from cancer patients
| Tumor | Antibody response (%) | |
|---|---|---|
| Breast | 3/18 | (16.6) |
| Colon | 0/20 | (0.0) |
| Esophagus | 0/4 | (0.0) |
| Lung | 1/13 | (8.0) |
| Melanoma | 1/22 | (4.5) |
| Prostate | 5/24 | (20.8) |
| Stomach | 1/8 | (12.5) |
| Thyroid | 2/10 | (20.0) |
| Uterus | 1/22 | (4.5) |
| Total | 14/141 | (10.0) |
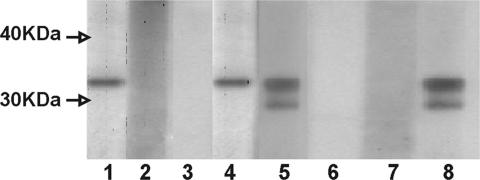
Fig. 6.
Detection of antibodies against CTSP-1 recombinant protein in plasma samples from cancer patients. A Western blot using CTSP-1 recombinant protein and plasma samples from prostate cancer patients (lanes 1–7) is shown. An anti-HisTag antibody was used as positive control (lane 8). Molecular weight markers are indicated.
The highest percentages of antibody response were observed in patients with prostate (20.8%), thyroid (20.0%), and breast (16.6%) tumors (Table 2). With the exception of NY-ESO-1, humoral immune responses to CT antigens are relatively rare, occurring in <10% of the cancer patients (26–29).
For 121 of the 141 patients included in this study, a correlation between CTSP-1 mRNA expression and the presence of a humoral immune response could be analyzed because both RNA and plasma samples were available (Table 3, which is published as supporting information on the PNAS web site). A significant percentage (15.5%) of patients bearing CTSP-1-expressing tumors developed a humoral immune response against the protein. However, antibodies against CTSP-1 were also detected in 6.3% of patients with CTSP-1-negative tumors. A possible explanation for this apparent discrepancy would be the presence of an immune response against other CTSP family members. The expression of CTSP-2 and -4 in these CTSP-1-negative tumors was then assayed by RT-PCR. Of the CTSP-1-negative tumors analyzed, only one showed expression of CTSP-2, and none of them showed expression of CTSP-4. We have also analyzed CTSP-2 and -4 mRNA expression in 84 of the 121 samples from which the corresponding plasma was available. The small percentage of positive expression of CTSP-2 (22.6%) and CTSP4 (0%) in these samples suggests that the immune response observed in these patients is likely to be directed to CTSP-1. Alternative explanations for the discrepancy between CTSP-1 expression and antibody response would be the presence of tumor heterogeneity not represented in the small tissue fragment analyzed by RT-PCR; the presence of undetected metastases expressing CTSP-1 or the clearance of CTSP-1-positive tumor cells by the immune system.
Concluding Remarks.
The identification of genes that are selectively expressed in tumors and code for proteins inducing a specific immune response in cancer patients is a prerequisite for the development of efficient immunotherapeutic approaches to cancer. Proteins coded by these antigens could be used to develop polyvalent cancer vaccines, overcoming obstacles associated with tumor heterogeneity and immune escape and increasing the number of cancer patients eligible for vaccination (30, 31).
We have identified a CT antigen, which is frequently expressed in different types of tumors and is capable of eliciting a humoral immune response in cancer patients. Because antibodies detected against CTSP-1 are of the IgG class, the activation of CD4+ T cell response to CTSP-1 could also be present in these patients. Further experiments are ongoing to test whether the CTSP-1 protein is able to induce CD4+ and CD8+ T cell responses in cancer patients.
Materials and Methods
Generation of CTSP-1 Full-Length Sequence.
cDNA sequencing.
C21ORF99 sequence was obtained by sequencing the full insert of cDNA clones from which the ESTs aligning to HC21 were derived. cDNA clones (IMAGE 1461135 and 2909444) were obtained from Research Genetics (Invitrogen, Carlsbad, CA) and were sequenced directly by using the vector's primers.
RT-PCR.
NY-BR-1 and NY-BR-1.1 sequences were aligned to HC21 genomic sequence by using the BLASTN program. Primers for RT-PCR were designed based on the HC21 genomic sequence in regions that showed similarity to NY-BR-1 and NY-BR-1.1 and that were not covered by the C21ORF99 sequence. RT-PCRs were carried out in 25 μl containing 1 μl of first-strand testis cDNA, 1× TaqDNA polymerase buffer (Invitrogen), 0.1 mM dNTP, 2 mM MgCl2, 1 unit of TaqDNA polymerase (Invitrogen), and 6 pmol of the following primers: CTSP-F1 (5′-CTGAAAGCTTGGTGGAAAG-3′) and CTSP-R1 (5′-GTTCCTTCTTCCAAAACTTC-3′) or CTSP-F2 (5′-AAGACTGAATGAGTGGCAG-3′) and CTSP-R2 (5′-CTGATTCAAATTACTTCTTACAG-3′). Amplification conditions were: initial denaturation for 4 min at 94°C followed by 40 cycles of 45 sec at 94°C, 45 sec at 58°C, and 1 min at 72°C. PCR products were analyzed on 8% silver-stained polyacrylamide gels and cloned for sequencing.
RACE.
3′-RACE was performed on normal testis poly(A)+ RNA by using the Marathon cDNA Amplification Kit (Clontech, Mountain View, CA). Amplification reactions were performed in 25 μl by using 5 μl of cDNA, 0.2 mM dNTPs, 0.2 μM CTSP-1-specific primer (SP-RACE, 5′-CCATGGCTCACACCTGTAATCTCATCAC-3′), 0.2 μM adaptor primers, 1 unit of Advantage TaqDNA polymerase. PCR conditions were: 1 min at 94°C followed by 5 cycles of 5 sec at 94°C and 4 min at 70°C, 5 cycles of 5 sec at 94°C and 4 min at 68°C, and 25 cycles of 5 sec at 94°C, 30 sec at 65°C with a final extension step of 4 min at 68°C. Nested PCR was carried out by using 1 μl of the first reaction product and the following primers: SP-RACE3N (5′-GAAAAAGTTAGAAGTGAAGCAACTTGAG-3′) and nested adaptor primers. PCR products were cloned and sequenced as described above.
Computational Analysis.
The CTSP-1 consensus sequence was obtained by assembling the C21ORF99 sequence the two RT-PCR fragments and 3′-RACE fragments using Phred/Phrap/Consed (32). Repetitive elements along the CTSP-1 sequence were identified with RepeatMasker (A. F. A. Smit, R. Hubley, and P. Green, RepeatMasker, http://repeatmasker.org). CTSP family members and partial copies were identified by aligning CTSP-1 consensus sequence against the human genome (Mar 2006) using BLAT (33). Consensus sequences including all predicted exons were translated in all six reading frames and putative proteins were aligned by using ClustalW (34). Protein motif searches were performed with PROSITE (35) and Pfam databases (36).
Expression Analysis Samples.
Human tumor cell lines A172 and T98G (glioblastoma); FaDu (squamous cell carcinoma); SW480 (colorectal adenocarcinoma); Skmel-28 and A2058 (malignant melanoma); DU145 and PC3 (prostate carcinoma); HeLa and CasKi (cervix adenocarcinoma); MDA-MB-231, MCF-7, and MDA-MB-436 (breast ductal carcinoma); IM9 (B transformed lymphoblasts); HL60 and K562 (lymphocytes); Help G2 (hepatocarcinoma); H1155 and H358 (lung carcinoma); SCABER (urinary bladder carcinoma); and SAOS-2 (osteosarcoma) were obtained from American Type Culture Collection (Manassas, VA). Tumor samples were collected from patients treated at Hospital A. C. Camargo. All samples were collected after explicit informed consent and with local ethical committee approval. Total RNA derived from 21 normal human tissues (testis, lung, prostate, small intestine, breast, brain, heart, uterus, bone marrow, placenta, colon, fetal brain, liver, fetal liver, thymus, salivary gland, spinal cord, kidney, spleen, skeletal muscle, and adrenal gland) was purchased from Clontech (Mountain View, CA).
RNA extraction, cDNA synthesis, and RT-PCR.
Total RNA was extracted by the conventional CsCl-guanidine thiocyanate method (37). RNA samples were checked for integrity by agarose gel electrophoresis, and 2 μg was used for cDNA synthesis. Reverse transcription was performed with DNA-free RNA by using SUPERSCRIPT II Reverse Transcriptase (Invitrogen) and oligo(dT). Primers used for expression analysis of CTSP-1, -2, -3, and -4 were CT1F (5′-GCTGTCCATTATGCTGTTAAC-3′), CT1R (5′-TTTTGAGAATTTTTAGATATC-3′), CT2F (5′-CCTGTGGTGCAGACATCG-3′), CT2R (5′-ATTCCAAAAGTTGTTGATGAAC-3′), CT3F (5′-CTGTCCATTATGCTGTTTATG-3′), CT3R (5′-AATTTTTAGATATCTTTTGTTTG-3′), CT4F (5′-TGCTGATCCAAATATTGTAGG-3′), and CT4R (5′-CATTTAAACTTATCAACTGCAA-3′). PCR fragments were analyzed either on agarose gels followed by Southern blot analysis or on 8% silver-stained polyacrylamide gels. PCR fragments were cloned and sequenced as mentioned above to confirm their specificity.
5-aza 2′deoxycytidine treatment.
MCF-7 breast tumor cell line was treated with 30 μM 5-Aza 2′deoxycytidine for 48 h. After treatment, the cells were harvested and used for RNA extraction and cDNA synthesis as described above. RT-PCR amplifications were carried out by using the same CTSP-1 primers as for gene expression analysis.
CTSP-1 Protein Detection.
CTSP-1 recombinant protein.
CTSP-1 longest ORF (202 amino acids) was amplified from normal testis cDNA by using the following primers: CTSP1RecF (5′-CGGGATCCATGAAGA AGACGACAATG-3′) and CTSP-1 RecR (5′-CGGAATTCCTATTCGTCAGGTGTTCT-3′). The PCR product was digested with BamHI and EcoRI and cloned into the expression vector pET28a (Stratagene, La Jolla, CA). After sequencing, the recombinant plasmid pET28a/CTSP-1 was transformed into Escherichia coli BL-21 A494. After induction with 1 mM isopropyl β-d-thiogalactoside at 37°C for 4 h, the CTSP-1 recombinant protein fused with a 6His-tag (≈32 kDa) was purified by Ni2+ affinity chromatography by using the NiNTA agarose resin (Invitrogen). The purified protein was analyzed by Western blot by using an anti-His-tag monoclonal antibody (Amersham Biosciences, Piscataway, NJ) to confirm the purification specificity.
CTSP-1 polyclonal antibody.
C57 mice (3-month-old females) were immunized s.c. with 25 μg of CTSP-1 recombinant protein by using Freund's Complete Adjuvant (Sigma, St. Louis, MO) and Mg(OH)2. Three weeks later, the mice were boosted with an s.c. injection of 25 μg of the recombinant protein by using Freund's Incomplete Adjuvant, and a final boost was repeated 3 weeks later. Two weeks after the final boost, the sera were collected and analyzed by ELISA to determine antibody titer. Mouse serum raised against CTSP-1 but depleted of anti-CTSP-1 Ig was used as a control of serum specificity. For depletion, 100 μg of the His-tagged CTSP-1 recombinant protein diluted in PBS was immobilized on Ni-NTA agarose beads (Qiagen, Valencia, CA) for 3 h at room temperature. Beads were then incubated for 16 h at 4°C with mouse serum raised against CTSP-1. Depleted serum was recovered from beads by centrifugation at 500 × g for 10 min and used in Western blot (1:1,000 dilution) and IHC (1:10 dilution) experiments.
Western blot.
Normal testis tissue was homogenized in sample buffer (240 mM Tris, pH 6.8/0.8% SDS/200 mM 2-mercaptoetanol/40% glycerol/0.2% bromophenol blue). A total of 100 μg of the lysate was loaded on 12% SDS/PAGE gels and after fractionation transferred to Hybond-P PVDF membranes (Amersham Biosciences). After blocking with PBS solution containing 5% low-fat milk, membranes were incubated with sera at a 1:10,000 dilution for 1 h at room temperature. Anti-CTSP-1 antibodies were detected by rabbit anti-mouse IgG HRP conjugate (Amersham Biosciences) and visualized with ECL Western Blotting Detection Reagents (Amersham Biosciences).
Immunohistochemistry.
Normal testis and prostate specimens were fixed with buffered formalin and embedded in paraffin. Briefly, sections were heated at 60°C for 20 min, deparaffinized in xylene, followed by a graded series of alcohols (100–75%), and rehydrated in water. Slides were placed three times in 3% hydrogen peroxide for 5 min and washed in water rinses for 5 min before incubation for 24 h with the primary antibody diluted 1:100. Slides were washed in PBS and incubated with biotinylated goat anti-rabbit IgG for 20 min and then with the streptavidin–biotin peroxidase LSAB kit (Dako, Carpenteria, CA). Immunostaining was performed by incubating slides in diaminobenzidine (Dako) solution containing 1 μl of chromogen for 50 μl of buffer substrate during 5 min. After chromogen development, slides were washed, dehydrated with alcohol and xylene, and mounted with coverslips by using a permanent mounting medium.
Antibody Response in Cancer Patients.
Plasmas were obtained from patients treated at Hospital A. C. Camargo. All samples were collected after explicit informed consent and with local ethical committee approval. In addition, plasma samples from 50 healthy individuals were collected from blood donors at the Hospital A. C. Camargo Blood Center. Antibodies against CTSP-1 recombinant protein were detected by Western blot. Five hundred nanograms of purified CTSP-1 recombinant protein (≈32 kDa) were fractioned on 12% SDS/PAGE and transferred to Hybond-P PVDF membranes. After blocking with PBS solution containing 5% low-fat milk, membranes were incubated for 90 min at room temperature with plasma from healthy individuals or cancer patients at a 1:500 dilution. Plasma antibodies binding to CTSP-1 protein were detected by incubation with goat anti-human IgG HRP conjugate (Amersham Biosciences) and visualized with ECL Western Blotting Detection Reagents.
Supplementary Material
Acknowledgments
We thank Drs. Alexandre Barbuto, Marcello Barcinscky, Erney Camargo, and Natanja Kirschbaum-Slager for critically reading this manuscript and Dr. Gerd Ritter for helpful discussions and experimental support. Valéria Paixão, Anna Christina Salim, Maria Cristina Costa, Suely Nonogaki, Carlos Nascimento, Miyuki da Silva, and Severino Ferreira provided outstanding technical assistance. We also thank Dr. Flávio Henrique Silva for assistance with protein expression and Dr. Mônica C. Poli (Blood Center Hospital A. C. Camargo) for providing the plasma samples from the blood donors. This work was supported by the Ludwig Institute for Cancer Research, São Paulo, Brazil, and by Conselho Nacional de Desenvolvimento Científico e Technológico Grant 506250/04-0. R.B.P. was sponsored by a fellowship from Cordenação de Aperfeiçoamento de Pessoal de Nível Superior; F.B., M.D.V., M.H.L. and W.K.M. were sponsored by fellowships from Fundaçao de Amparo a Pesquisa do Estado de São Paulo.
Abbreviations
- CT
cancer/testis
- HC21
human chromosome 21
Footnotes
References
- 1.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 2.Scanlan MJ, Simpson AJ, Old LJ. Cancer Immunol. 2004;4:1. [PubMed] [Google Scholar]
- 3.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 4.Zendman AJ, Ruiter DJ, Van Muijen GN. J Cell Physiol. 2003;194:272–288. doi: 10.1002/jcp.10215. [DOI] [PubMed] [Google Scholar]
- 5.Old LJ. Cancer Immunol. 2001;1:1. [Google Scholar]
- 6.Bodey B. Exp Opin Biol Ther. 2002;2:577–584. doi: 10.1517/14712598.2.6.577. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Jackson H, Parente P, Luke T, Rizkalla M, Tai TY, Zhu HC, Mifsud NA, Dimopoulos N, Masterman KA, et al. Proc Natl Acad Sci USA. 2004;101:9363–9368. doi: 10.1073/pnas.0403271101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, Chen Q, Dimopoulos N, Luke T, Murphy R, et al. Proc Natl Acad Sci USA. 2004;101:10697–10702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jager E, Gnjatic S, Nagata Y, Stockert E, Jager D, Karbach J, Neumann A, Rieckenberg J, Chen YT, Ritter G, et al. Proc Natl Acad Sci USA. 2000;97:12198–12203. doi: 10.1073/pnas.220413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchand M, van Baren N, Weynants P, Brichard V, Dreno B, Tessier MH, Rankin E, Parmiani G, Arienti F, Humblet Y, et al. Int J Cancer. 1999;80:219–230. doi: 10.1002/(sici)1097-0215(19990118)80:2<219::aid-ijc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 11.Reymond A, Camargo AA, Deutsch S, Stevenson BJ, Parmigiani RB, Ucla C, Bettoni F, Rossier C, Lyle R, Guipponi M, et al. Genomics. 2002;79:824–832. doi: 10.1006/geno.2002.6781. [DOI] [PubMed] [Google Scholar]
- 12.Jager D, Stockert E, Gure AO, Scanlan MJ, Karbach J, Jager E, Knuth A, Old LJ, Chen YT. Cancer Res. 2001;61:2055–2061. [PubMed] [Google Scholar]
- 13.Roy-Engel AM, El Sawy M, Farooq L, Odom GL, Perepelitsa-Belancio V, Bruch H, Oyeniran OO, Deininger PL. Cytogenet Genome Res. 2005;110:365–371. doi: 10.1159/000084968. [DOI] [PubMed] [Google Scholar]
- 14.Aradhya S, Bardaro T, Galgoczy P, Yamagata T, Esposito T, Patlan H, Ciccodicola A, Munnich A, Kenwrick S, Platzer M, et al. Hum Mol Genet. 2001;10:2557–2567. doi: 10.1093/hmg/10.22.2557. [DOI] [PubMed] [Google Scholar]
- 15.Gure AO, Wei IJ, Old LJ, Chen YT. Int J Cancer. 2002;101:448–453. doi: 10.1002/ijc.10634. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Li J, Lu L, Xu M, Xiao J, Yin L, Zhu H, Zhou Z, Sha J. J Androl. 2005;26:189–196. doi: 10.1002/j.1939-4640.2005.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 17.Gure AO, Tureci O, Sahin U, Tsang S, Scanlan MJ, Jager E, Knuth A, Pfreundschuh M, Old LJ, Chen YT. Int J Cancer. 1997;72:965–971. doi: 10.1002/(sici)1097-0215(19970917)72:6<965::aid-ijc8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Lucas S, Brasseur F, Boon T. Cancer Res. 1999;59:4100–4103. [PubMed] [Google Scholar]
- 19.Pold M, Zhou J, Chen GL, Hall JM, Vescio RA, Berenson JR. Genomics. 1999;59:161–167. doi: 10.1006/geno.1999.5870. [DOI] [PubMed] [Google Scholar]
- 20.Weber J, Salgaller M, Samid D, Johnson B, Herlyn M, Lassam N, Treisman J, Rosenberg SA. Cancer Res. 1994;54:1766–1771. [PubMed] [Google Scholar]
- 21.De Smet C, Lurquin C, Lethe B, Martelange V, Boon T. Mol Cell Biol. 1999;19:7327–7335. doi: 10.1128/mcb.19.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coral S, Sigalotti L, Altomonte M, Engelsberg A, Colizzi F, Cattarossi I, Maraskovsky E, Jager E, Seliger B, Maio M. Clin Cancer Res. 2002;8:2690–2695. [PubMed] [Google Scholar]
- 23.McCurdy DK, Tai LQ, Nguyen J, Wang Z, Yang HM, Udar N, Naiem F, Concannon P, Gatti RA. Mol Genet Metab. 1998;63:3–13. doi: 10.1006/mgme.1997.2639. [DOI] [PubMed] [Google Scholar]
- 24.Rocha IM, Oliveira LJ, De Castro LC, Araujo Pereira LI, Chaul A, Guerra JG, Silvestre MC, Batista KM, Pereira FA, et al. Int J Dermatol. 2000;39:840–843. doi: 10.1046/j.1365-4362.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- 25.Lea IA, Adoyo P, O'Rand MG. Fertil Steril. 1997;67:355–361. doi: 10.1016/S0015-0282(97)81923-1. [DOI] [PubMed] [Google Scholar]
- 26.Stockert E, Jager E, Chen YT, Scanlan MJ, Gout I, Karbach J, Arand M, Knuth A, Old LJ. J Exp Med. 1998;187:1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yakirevich E, Sabo E, Lavie O, Mazareb S, Spagnoli GC, Resnick MB. Clin Cancer Res. 2003;9:6453–6460. [PubMed] [Google Scholar]
- 28.Sugita Y, Wada H, Fujita S, Nakata T, Sato S, Noguchi Y, Jungbluth AA, Yamaguchi M, Chen YT, Stockert E, et al. Cancer Res. 2004;64:2199–2204. doi: 10.1158/0008-5472.can-03-3070. [DOI] [PubMed] [Google Scholar]
- 29.Jager E, Stockert E, Zidianakis Z, Chen YT, Karbach J, Jager D, Arand M, Ritter G, Old LJ, Knuth A. Int J Cancer. 1999;84:506–510. doi: 10.1002/(sici)1097-0215(19991022)84:5<506::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 31.Dunn GP, Old LJ, Schreiber RD. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 32.Gordon D, Abajian C, Green P. Genome Res. 1998;8:195–202. doi: 10.1101/gr.8.3.195. [DOI] [PubMed] [Google Scholar]
- 33.Kent WJ. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins DG, Thompson JD, Gibson TJ. Methods Enzymol. 1996;266:383–402. doi: 10.1016/s0076-6879(96)66024-8. [DOI] [PubMed] [Google Scholar]
- 35.Sigrist CJ, Cerutti L, Hulo N, Gattiker A, Falquet L, Pagni M, Bairoch A, Bucher P. Brief Bioinform. 2002;3:265–274. doi: 10.1093/bib/3.3.265. [DOI] [PubMed] [Google Scholar]
- 36.Bateman A, Coin L, Durbin R, Finn RD, Hollich V, Griffiths-Jones S, Khanna A, Marshall M, Moxon S, Sonnhammer EL, et al. Nucleic Acids Res. 2004;32:D138–D141. doi: 10.1093/nar/gkh121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacDonald RJ, Swift GH, Przybyla AE, Chirgwin JM. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.