Abstract
Understanding the structure and formation of amyloid fibrils, the filamentous aggregates of proteins and peptides, is crucial in preventing diseases caused by their deposition and, moreover, for obtaining further insight into the mechanism of protein folding and misfolding. We have combined solid-state NMR, x-ray fiber diffraction, and atomic force microscopy to reveal the 3D structure of amyloid protofilament-like fibrils formed by a 22-residue K3 peptide (Ser20-Lys41) of β2-microglobulin, a protein responsible for dialysis-related amyloidosis. Although a uniformly 13C,15N-labeled sample was used for the NMR measurements, we could obtain the 3D structure of the fibrils on the basis of a large number of structural constraints. The conformation of K3 fibrils was found to be a β-strand–loop–β-strand with each K3 molecule stacked in a parallel and staggered manner. It is suggested that the fibrillar conformation is stabilized by intermolecular interactions, rather than by intramolecular hydrophobic packing as seen in globular proteins. Together with thermodynamic studies of the full-length protein, formation of the fibrils is likely to require side chains on the intermolecular surface to pack tightly against those of adjacent monomers. By revealing the structure of β2-microglobulin protofilament-like fibrils, this work represents technical progress in analyzing amyloid fibrils in general through solid-state NMR.
Keywords: 2,2,2-trifluoroethanol; amyloid fibril; dialysis-related amyloidosis; protein misfolding; x-ray fiber diffraction
Amyloid fibrils are highly ordered filamentous aggregates formed by the self-assembly of peptides or proteins (1–4). There are currently ≈20 known diseases associated with deposition of amyloid fibrils, including Alzheimer's disease, type II diabetes, Parkinson's disease, and dialysis-related amyloidosis. Additionally, numerous peptides and proteins not directly related to diseases also can form amyloid-like fibrils in vitro, suggesting that amyloid fibril formation is a generic property of the polypeptide chain (2). To obtain further insight into protein folding and misfolding, it is crucial to clarify the mechanism of fibril formation and the structural stability of amyloid fibrils. Recently, it has been found that amyloid fibrils are, in general, amenable to the most sophisticated magic-angle-spinning (MAS) solid-state NMR methods, providing atomic-level structural information that otherwise has been inaccessible (5–10). These techniques, although focused on monomeric structural properties, shed light on a number of structural details and properties of fibril formation. Most recently, the structural details of whole fibrils have been proposed for amyloid-β fibrils (11). Combining various techniques, including solid-state NMR, closely related structural models for amyloid-β fibrils have been constructed (12, 13).
β2-Microglobulin (β2-m), a component of the type I major histocompatibility antigen, forms amyloid fibrils in patients receiving hemodialysis for long periods (14, 15). Because of its clinical importance and suitable size for examining the relation between protein folding and amyloid formation, β2-m has been a target of extensive study on the relation between protein folding and amyloid fibril formation (14–18). In previous work, we found that a 22-residue K3 peptide (Ser20-Lys41), obtained by digesting β2-m, spontaneously forms amyloid protofilament-like fibrils in vitro (19). The K3 peptide is, therefore, an important system for detailed investigation of the structure and formation of amyloid fibrils.
K3 peptide forms fibrils over a wide range of pH and solvent conditions, both with and without the presence of alcohols (19–23). In particular, in 20% (vol/vol) 2,2,2-trifluoroethanol (TFE) and 10 mM HCl, long and thin fibrillar structures with a homogeneous thickness are formed (21–23). We consider these to be protofilaments, the individual filaments making up the mature amyloid fibrils. Here, we have used solid-state NMR, together with x-ray fiber diffraction and atomic force microscopy (AFM) measurements, to probe the structure of K3 fibrils. Because the K3 fibrils in 20% (vol/vol) TFE do not have a superstructure where several protofilaments associate with one another laterally (3), they are a suitable target for analyzing the whole fibril structure, excluding the contribution of the interfibril interactions. Even with a uniformly 13C,15N-labeled sample, we could propose the 3D structure of the fibrils, leading to an atomic-level understanding the protofibril structure and the types of interactions required to stabilize this structure.
Results
Fibril Morphology and X-Ray Fiber Diffraction.
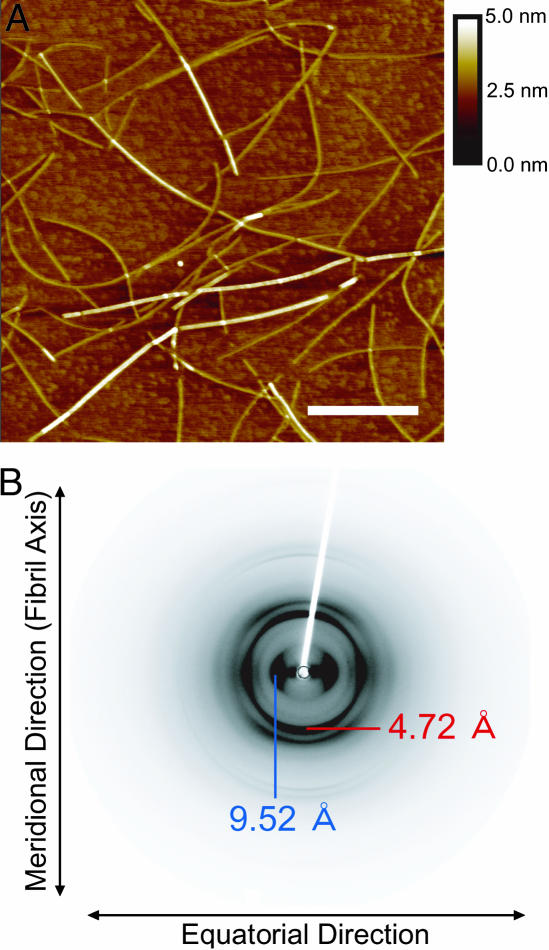
The K3 peptide forms two types of fibrillar structures with distinct morphology and secondary structure: “f210 fibrils” and “f218 fibrils,” differing in the location of the minimum of the far-UV circular dichroism (CD) spectrum (23). The AFM images revealed a thin and long morphology (Fig. 1A), and the CD showed a strong negative ellipticity at 210 nm (Fig. 6, which is published as supporting information on the PNAS web site), confirming that the fibrils prepared in the present article correspond to the f210 fibrils (23). The thickness of the fibrils was homogeneous without a helical twist; fibril diameter ranged from 1.0 to 2.1 nm with a statistical average of 1.5 nm (±0.3). The x-ray fiber diffraction showed a typical cross-β pattern (Fig. 1B). Strong bands with a characteristic spacing of 4.5–4.7 Å, typically observed for amyloid fibrils, were assigned to the distance between adjacent peptide chains in the β-sheets that comprise a cross-β structure. Other strong bands corresponding to a characteristic spacing of 9.4–9.8 Å represented the distance between the laminated β-sheets where peptide side chains are packed (24). Although several additional peaks were observed, they are difficult to assign because of their low quality (Fig. 7, which is published as supporting information on the PNAS web site).
Fig. 1.
AFM images and x-ray fiber diffraction of K3 fibrils. (A) AFM images of K3 fibrils formed in 20% (vol/vol) TFE/10 mM HCl. The scan was performed with a 25-fold diluted sample on a freshly cleaved mica surface. The white scale bar represents 500 nm, and the scan size is 2.5 × 2.5 μm with 512 × 512 points. (B) X-ray fiber diffraction of the K3 fibrils with incident beam perpendicular to the fibril axis. The data shows a typical cross-β pattern. The diffractions corresponding to 4.72 Å (red) and 9.52 Å (blue) indicate the distance between β-strands in the β-sheet and β-sheet layers in the laminated structure, respectively.
Signal Assignments.
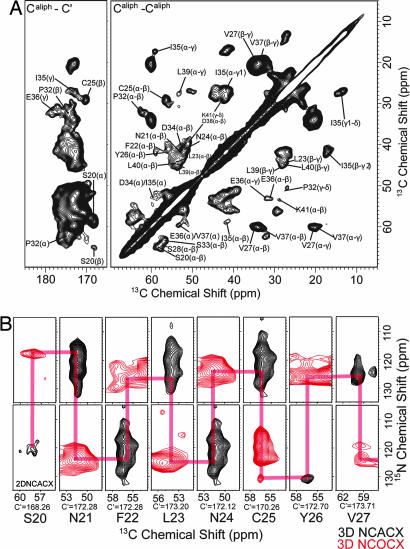
To assign sequential resonances of K3 fibrils, we made a set of 2D and 3D 15N–13C and 13C–13C correlation experiments. Fig. 2A shows a 2D 13C–13C correlation spectrum obtained for fully labeled K3 fibrils with an rf-driven recoupling (RFDR) sequence at 4.0-ms mixing time. Cys, Gly, His, Pro, Ile, Glu, and Lys each appear once in K3. Pro32, Ile35, and Glu36 resonances were easily characterized with their characteristic chemical-shift pattern. In addition, sequential correlations were observed in the spectrum at 4.0-ms mixing time. Intramolecular peaks were distinguished from intermolecular peaks by comparing the spectra of the fully labeled sample with that of the spin-diluted sample in which a fraction of the labeled peptide was 1/3 (see below). These peaks enabled the assignment of overlapped peaks (Asn21, Phe22, Asp34, and Val37). Ser residues were well separated but could not be distinguished without sequential correlation data. Gly29 gave only the C′–Cα resonance overlapped with the C′–Cβ resonance of Leu residues. To distinguish multiple bond correlations, we also performed a 2D 13C–13C supercycled POST-Cz (SPCz) 5 double-quantum experiment at a mixing time of 1.5 ms, yielding a spectrum that distinguishes one- and two-step dipolar correlations (Fig. 8, which is published as supporting information on the PNAS web site). The spectrum clearly indicated the C′–Cα peak of Gly29.
Fig. 2.
13C–13C and 15N–13C correlation NMR spectra of K3 fibrils. (A) 2D broadband 13C–13C correlation spectra for intraresidue correlations. The figure shows the expansion of the cross-peak regions for aliphatic-carbonyl carbons and aliphatic-aliphatic carbons. This spectrum was obtained at 16.4 T, with a MAS frequency of 16.0 kHz and a 4.00-ms mixing period during which an RFDR pulse sequence was applied. (B) 2D slices of the 3D 15N–13C intraresidue (NCACX) and sequential (NCOCX) correlation spectra. Black and red spectra indicate intraresidue and interresidue correlations, respectively. These spectra were obtained at 11.8 T and a MAS frequency of 12.5 kHz with 1.25-ms and 2.00-ms mixing periods during which the RFDR pulse sequence was applied for NCACX and NCOCX, respectively. Red lines indicate the sequential assignments.
To assign the side-chain resonances, we also performed a double-quantum/single-quantum correlation experiment (INADEQUATE; Fig. 9, which is published as supporting information on the PNAS web site). However, because of small chemical-shift differences in some residues, their peaks were overlapped. To separate signal sets in an additional dimension, 3D 15N–13C correlation experiments were conducted by using SPECIFIC CP transfers and RFDR 13C–13C mixing blocks (Fig. 2B). Analysis of a series of 13C–13C (Fig. 10, which is published as supporting information on the PNAS web site) and 15N–13C 2D and 3D spectra led to the main-chain signal assignments of all residues. For some residues, we confirmed the chemical shift by 13C–13C spin-diffusion experiments with dipolar-assisted rotational resonance (DARR) at short mixing times (<20 ms). The summary of obtained chemical shifts is shown in Table 2, which is published as supporting information on the PNAS web site.
Constraints Based on the Chemical Shifts.
The N, Cα, Cβ, and C′ chemical shifts were analyzed with the program TALOS, which predicts dihedral angles from secondary chemical shifts (25). For Asn21-Ser28 and Ser33-Leu40, satisfactory convergence was obtained, enabling the ϕ and ψ values to be predicted. These predicted angles were in the β-strand region of the Ramachandran plot. Moreover, the conformation of the peptide bond between His31 and Pro32 could be determined from the chemical-shift data. The difference of 5.3 ppm between the 13Cβ and 13Cγ chemical shifts for Pro32 in K3 fibrils indicates that the His31-Pro32 peptide bond assumes a trans conformation (26). Although this isomer is common in peptides and globular proteins, the native β2-m has a cis conformation at the His31-Pro32 peptide bond, which plays an important role in the slow refolding of β2-m (27).
Tertiary Contacts in the K3 fibrils.
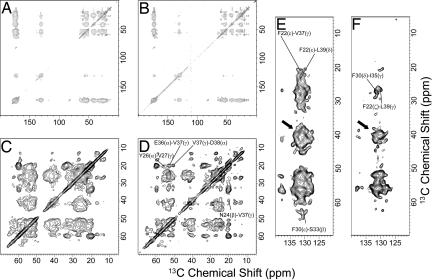
To reveal the tertiary structure of the K3 fibrils, a series of 13C–13C spin-diffusion experiments with DARR were applied to both the fully labeled and spin-diluted samples. Various spin-diffusion spectra of K3 fibrils were obtained with mixing times ranging between 2.5 ms and 500 ms. Fig. 3A–D shows spectra of both the fully labeled and the spin-diluted fibrils at a mixing time of 350 ms. Comparison of the spectra revealed that each monomer makes contact with adjacent monomers at many sites. Although many overlapping peaks were observed in the spectra of fully labeled samples, the spectra of spin-diluted samples, which were dominated by intramolecular correlations, were relatively well separated. Most strong cross-peaks corresponded to intraresidue correlations, but some peaks corresponded to long-range contacts. In particular, we observed cross-peaks that can be assigned to the F22ζ-L39γ, F30δ-I35γ, and N24β-V37γ correlations. These peaks represent intramolecular packing, indicating that K3 has a bend ranging from Gly29 to Pro32 (Fig. 3). These cross-peaks were observed at the mixing time of 350 ms as reported for the interresidue contact in β-amyloid (11). On the other hand, several intermolecular cross-peaks also were identified by comparing the peak intensities of fully labeled fibrils with those of spin-diluted fibrils (Fig. 11, which is published as supporting information on the PNAS web site). The spectra at a mixing time of 350 ms showed the contact of the Phe22 side chain with Leu39 Cδ of an adjacent K3 monomer. The contact of the Phe22 side chain with Val37 Cγ also was indicated by the spin-diffusion spectra at 350 ms.
Fig. 3.
2D 13C–13C spin-diffusion experiments (DARR) with the fully labeled fibrils and spin-diluted fibrils. These spectra were recorded at 16.4 T with a 350-ms mixing period for the spin-diffusion experiment at a 16.0–kHz MAS frequency. (A, C, and E) The 13C–13C correlation spectrum obtained with the fully labeled fibrils. (B, D, and F) The 13C–13C correlation spectrum obtained with the spin-diluted fibrils. (C and D) Expansions of aliphatic regions. (E and F) Expansions of the cross-peak regions between aliphatic and aromatic carbons. The arrows indicate the regions of MAS side bands of aromatic carbon signals.
1H-1H Spin-Diffusion (CHHC) Experiments.
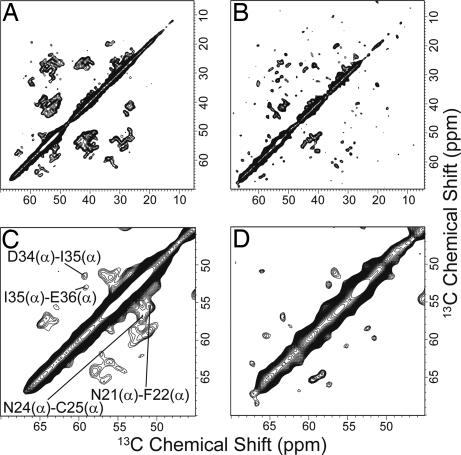
To determine the sheet topology and the registry of hydrogen bonds in the fibrils, we carried out 1H–1H spin-diffusion experiments. With a 1H mixing time of ≈250 μs, antiparallel β-sheets give strong single-bond Cα-Cα cross-peaks, whereas parallel β-sheets give much weaker peaks (28, 29) (Fig. 12, which is published as supporting information on the PNAS web site). Fig. 4A and B shows the 2D correlation spectra obtained from the 1H–1H spin-diffusion experiment at a 1H mixing time of 210 μs for the fully labeled and spin-diluted fibrils, respectively. In the spin-diluted fibrils, the ratio of the labeled and unlabeled samples was 1:2. Some interresidue Cα–Cα peaks between residues i and i + 1, which were much weaker than one-bond correlation peaks, were observed in the spectra of fully labeled fibrils, whereas these were strikingly reduced in the same experiment for the spin-diluted fibrils. The Cα–Cα peaks reflect the correlation between residues i and residues i + 1 of adjacent K3 molecules as well as sequential correlations. The result is consistent only with a parallel β-sheet geometry in which each residue in the fibril is in-register and each monomer is in nearly the same local conformation.
Fig. 4.
2D 13C–13C correlation spectra obtained with 1H–1H spin-diffusion (CHHC) experiments. (A) The spectrum obtained with fully labeled fibrils. The correlation between Cα–Cα carbons is indicated. (B) The spectrum obtained with spin-diluted fibrils. (C) Expansion of the Cα carbon region in the spectrum of fully labeled fibrils. (D) Expansion of the Cα carbon regions in the spectrum of spin-diluted fibrils. Note that the Cα–Cα cross-peaks are significantly reduced. This reduction indicates that the cross-peaks contain intermolecular correlations. These spectra were obtained at 14.1 T with a 210-μs mixing period for the 1H–1H spin-diffusion experiment at a MAS frequency of 14.0 kHz.
Calculation of the 3D Structure.
The 34 dihedral angle restraints from the TALOS prediction, along with distance constraints from the other NMR measurements, yield a total of 55 restraints (Table 1). Among the 27 13C distance restraints from the spin-diffusion experiments, there are 13 and 14 interresidue and intermolecular restraints, respectively. In the present study, we can exclude the contacts between protofilaments, because the K3 fibrils consist of only one protofilament (see Discussion). Although we can distinguish intra- and intermolecular correlations for the two β-strands in a molecule, the obtained correlations are not sufficient to specify an interacting molecule in a group of molecules in the vicinity. This insufficiency allows the possibility of the strand assemblies with different degrees and directions of stagger (11, 12). Here, we assumed the structures in STAG(+1) and STAG(−1) following the definition of Petkova et al. (11). When the N- and C-terminal β-strands of each K3 molecule are located back and front, respectively, the C-terminal β-strand in the STAG(+1) model is displaced upward by a half of the interstrand distance (i.e., 4.7 Å) (see figure 7 of ref. 11). On the other hand, in the STAG(−1) model, the C-terminal β-strand is displaced downward by a half of the interstrand distance. Additionally, intermolecular restraints attributable to hydrogen bonds were added in β-strand regions predicted by TALOS. The restraints were arranged such that each residue in the fibril paired in-register in a parallel β-sheet orientation.
Table 1.
Summary of restraints for K3 amyloid fibrils
| Restraints | Value |
|---|---|
| Total experimental restraints | 61 |
| Total distance restraints | 27 |
| Total 13C–13C restraints | 27 |
| Intramolecular restraints | 13 |
| Intermolecular restraints | 14 |
| Restraints in the class 2.0–3.5 Å | 2 |
| Restraints in the class 3.5–4.5 Å | 15 |
| Restraints in the class 4.5–6.5 Å | 10 |
| Total backbone dihedral angle restraints | 34 |
| Chemical shift-based restraints (TALOS) | 34 |
| Energies kcal/mol | |
| Final energies | 106.0 |
| Energies of bonds | 3.3 |
| Energies of angles | 65.6 |
| Energies of impropers | 2.5 |
| Energies of van der Waals | 31.8 |
| rms deviation | |
| Bonds, Å2 | 0.02 |
| Angles, ° | 0.42 |
| Impropers, ° | 0.15 |
| Backbone of the tetramer | 1.67 |
| Backbone of the middle monomer | 1.43 |
The 13C–13C restraints were obtained with 2D spin-diffusion experiments. The energy and rms deviations are averaged value of the 10 lowest-energy structures among 50 calculated structures.
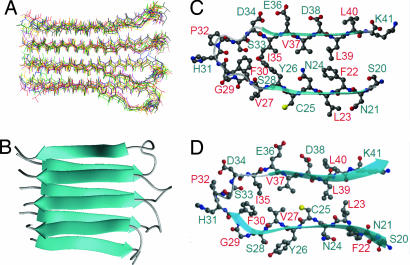
The tetrameric structure of the K3 fibril was calculated by using the above restraints in a simulated annealing molecular dynamics structure optimization with the program CNS. Fig. 5A shows 10 lowest energy structures among 50 calculated structures. The calculation showed that only the STAG(+1) model, as is obvious in Fig. 5B, is consistent with our experimental restraints; probably, trans isomerism of Pro32 is important for assuming this staggered conformation. The main-chain root mean square deviation for these 10 structures was 1.68 Å for the K3 tetramers and 1.43 Å for the two K3 molecules located in the interior. The high degree of divergence of these structures was not attributable to the flexibility of fibrils but to the lack of long range structural constraints. The overall 3D structure of K3 in the fibrillar state is β(Asn21-Ser28)–loop(Gly29-Pro32)–β(Ser33-Lys40) in a parallel β-sheet assembly in the STAG(+1) mode. Importantly, the calculated structure is different from the native structure in that nonpolar residues (Val27, Cys25, and Leu23) buried in the interior of the Ig fold are exposed to the surface (see Discussion).
Fig. 5.
3D structures of tetrameric K3 and monomeric K3 in the fibrillar state. The conformation of K3 in the fibrillar state obtained by simulated annealing molecular dynamics by using CNS. (A) Calculated ensemble of tetrameric structures of K3 fibrils. (B) Ribbon model representation of tetrameric K3 in parallel STAG(+1) conformation. (C) The conformation of one K3 structure in the fibrillar state. (D) Comparison of the conformation of the K3 region in the crystal structure of native β2-m. Notably, the residues between Phe22 and Ser28 are flipped relative to the crystal structure of native β2-m in the fibrillar state.
Mutational Analysis of Phe Residues.
Aromatic amino acids have been suggested to be important for amyloid fibril formation (30). The aromatic residues of K3 are Phe22, Tyr26, and Phe30. Assuming a β-strand–loop–β-strand conformation with a parallel β-sheet topology, π–π interactions between the same aromatic residues of adjacent peptides might be important for fibril formation. To investigate the role of Phe side chains in K3 fibril formation, two mutants with Phe22-Ala or Phe30-Ala substitutions were synthesized. Although wild-type K3 formed fibrils spontaneously in 20% (vol/vol) TFE and 10 mM HCl with a large negative intensity at 210 nm (i.e., f210 fibrils), the CD spectrum (Fig. 6) indicates that these mutants did not form fibrils. These results might be attributable to an increased barrier of fibril nucleation. Thus, seed-dependent fibrillization assays of K3 mutants also were performed by using wild-type K3 seeds. However, no notable fibrillation was observed, indicating that the removal of Phe22 or Phe30 strikingly inhibits the fibril formation.
Discussion
K3 Fibrils Consist of Two-Layered, Parallel, and Staggered β-Sheets.
The goal of the present study was to determine the 3D structure of K3 fibrils formed in 20% (vol/vol) TFE and 10 mM HCl. Mature amyloid fibrils consist of several β-sheets stabilized by hydrophobic interactions (4, 10). In analogy with the structural hierarchy of globular proteins, Petkova et al. (11) defined primary, secondary, tertiary, and quaternary structures of amyloid fibrils. Although primary structure is defined similarly in globular proteins and amyloid fibrils, secondary structure in amyloid fibrils is mostly β-sheet, and the determination of secondary structure corresponds to the identification of the location of β-sheet regions. Tertiary structure in amyloid fibrils involves the parallel or antiparallel β-sheet orientation, and quaternary structure relates to the position and orientation of β-sheets relative to one another.
Thus, to construct the 3D structure of K3 fibrils, it is necessary to identify the number of β-sheet layers constituting the fibrils (i.e., quaternary structure). The x-ray fiber diffraction data indicated that the distances between adjacent peptide chains in the β-sheet and the laminated β-sheets are 4.5–4.7 Å and 9.5–10.0 Å, respectively, implying that the K3 fibrils consist of the least two β-sheet layers. If the fibrils have three layers of β-sheets, the fibril diameter would be >20 Å. Considering that the average diameter of K3 fibrils was ≈15 Å in AFM measurements, only a structure made of two layers of β-sheets is consistent. Most likely, the 5-Å difference between AFM measurements and the diffraction originates from the volume of exposed side chains: the distance between β-sheets is defined in terms of the backbone–backbone distance and does not include side chains. We conclude that the K3 fibril is made of two β-sheet layers, where each K3 molecule consists of two β-strands and a connecting loop. This minimal quaternary structure is an ideal system for analyzing the atomic structure and stabilization of amyloid fibrils.
The tertiary structure (i.e., orientation of β-sheets) also is required for complete structural determination. Solid-state 1H–1H spin-diffusion experiments revealed that K3 fibrils consist of parallel β-sheets with intermolecular hydrogen bonds formed between the same residues. To date, many proposed amyloid fibril models consist of parallel β-sheets (6, 11–13, 31), suggesting that the parallel orientation is preferred over the antiparallel orientation. However, it also has been reported that both parallel and antiparallel β-sheets exist even for fibrils formed from the same peptides (8, 32), suggesting that the orientation preference depends on the experimental conditions. The secondary structure of K3 fibrils as determined by TALOS was found to be β(Asn21-Ser28)–loop(Gly29-Pro32)–β(Ser33-Lys40). The distribution of secondary structures is basically consistent with reports of various fragments corresponding to the K3 region analyzed by vibrational spectroscopy (33, 34).
The 3D structure of the K3 tetramer was calculated by using the above hierarchical restraints, revealing that K3 in the fibrillar state assumes a β-strand–loop–β-strand conformation with each K3 molecule stacked in a parallel orientation with the STAG(+1) mode. The location of secondary structures are similar to those of the native structure: β(Ser20-Phe30)–loop(His31-I35)–β(Glu36-Lys41). To our surprise, the calculated structure was different from the native structure in that nonpolar residues (Val27, Cys25, and Leu23) buried in the interior of the Ig fold are exposed to the surface. In the fibril form, aromatic Phe22 and Tyr26 and polar Gln24 and Ser28 are buried between the β-strands. Nevertheless, this structure is consistent with our previous UV absorption study, which indicated Tyr26 was buried in the interior of the fibrils (23). Additionally, the structure with exposed hydrophobic residues (Leu40, Val27, and Leu23) is consistent with the report that the K3 fibrils prepared in 20% (vol/vol) TFE assemble laterally into mature fibrils after being transferred to a solution without TFE (22). The exposed hydrophobic side chains clearly play an important role in assembling protofibrils to form the mature fibrils.
Protofilaments Stabilized by Intermolecular Interactions.
The validity of the calculated structure was examined by mutagenesis of K3 fragment. There are several reports on the role of side chains in the stabilization of amyloid structures (8, 11, 12, 30, 31, 35). We focused on the role of aromatic Phe side chains. In the calculated structure, the Phe rings are aligned and separated by a distance of 4.5–4.7 Å (i.e., the interstrand spacing), a distance favorable for π–π interactions (36). The mutational analysis showed that the Phe to Ala substitutions inhibited the amyloid fibril formation, even though Phe30 is located in a loop region in the calculated structure (Fig. 6C).
Moreover, the role of Tyr26 buried between the β-sheets is intriguing: it is conceivable that the Tyr26 residues also are stabilized by π–π interactions, although such interactions are not obvious from the calculated structure: the phenol ring of Tyr26 runs parallel to the plane of the β-sheets. Another side-chain interaction that has been described previously is the “Asn ladder” formed by Asn24 (31, 37). According to the calculated fibrillar structure, the side chain of Asn24 is located in the interior of the fibrils, namely, in a hydrophobic environment not thermodynamically favorable for the isolated polar amide group. To avoid this energetic disadvantage, the amide groups of aligned Asn24 residues are likely to form an intermolecular hydrogen-bond network with adjacent molecules.
These characteristics of the calculated structure and the results of mutagenesis indicate that the fibrillar structure assembles so as to optimize intermolecular interactions between neighboring K3 peptides rather than intramolecular packing, as seen in globular proteins. This consideration is supported by the conformational difference between the fibrillar and native β2-m structures (Fig. 5 C and D). The K3 peptide corresponds to β-strands B and C with the connecting B–C loop in the native β2-m. Although a similar β-strand–loop–β-strand geometry is observed in the fibrils, the residues between Phe22 and Ser28 are flipped relative to the fibrillar state. This marked difference in the interstrand interactions probably leads to a less tightly packed interior of K3 fibrils in comparison with that of the native structure.
Conclusions
Recent 2D and 3D solid-state NMR methods provide sufficient inter- and intraresidue correlations for nearly complete signal assignment of this 22-residue peptide in a noncrystalline state. The resulting chemical shifts yield important dihedral angle constraints. Distance information can be obtained by 13C-NMR detection of 1H-1H and 13C-13C spin diffusion in a uniformly labeled fibril. The intra- and intermolecular distances can be distinguished by isotope dilution with natural-abundance molecules. Together, even with a uniformly 13C,15N-labeled sample, it was possible to construct the 3D structure of the fibrillar form of K3. The fibrillar state consisted of a β-strand–loop–β-strand conformation with the K3 molecules stacked in a parallel manner with the STAG(+1) mode, where maximization of the intermolecular interactions, in particular aromatic π–π interactions, is achieved rather than optimal intramolecular packing. The overall structure resembles the fibril structures of Aβ(1–40) (11), Aβ(1–42) (12), and the HET-s fragment (6), suggesting that it is a common structural motif of amyloid protofibrils. Together with thermodynamic studies of full-length β2-m (38, 39), it is likely for side chains on the intermolecular surface to be tightly packed with adjacent monomers, otherwise loose packing with cavities would be observed.
Materials and Methods
K3 and Mutant Peptides.
Recombinant human β2-m and 13C,15N-labeled β2-m was expressed in Escherichia coli and purified as described previously (27, 40). K3 peptide was obtained by digestion of β2-m with lysyl endopeptidase (Achromobacter protease I) as described in ref. 19. K3 and its mutant peptides also were synthesized by the Fmoc method in a step-wise fashion on 0.1 mmol of preloaded Fmoc-Ala-PEG-polystylene resin with a Pioneer peptide synthesizer (Applied Biosystems, Foster City, CA). Details are provided in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Fibril formation of K3 was performed for one day at 25°C by using a final peptide concentration of 100 μM in 20% (vol/vol) TFE, 10 mM HCl, and 1 mM NaCl (23). For the spin-diluted fibrils for solid-state NMR, a mixture of the labeled and nonlabeled K3 peptides at a molar ration of 1:2 was dissolved in 10 mM NaOH and polymerized in the same way.
AFM, X-Ray Fiber Diffraction, and CD Measurements.
AFM images were obtained by using a dynamic force microscope (Nano Scope IIIa; Digital Instruments, Woodbury, NY). X-ray fiber diffraction of partly aligned K3 fibrils by centrifugation were collected at a SPring-8 (Hyogo, Japan), BL44XU beamline at room temperature. Far-UV CD spectra were measured with an AVIV model 215s spectropolarimeter (AVIV Association, Lakewood, NJ). Details are provided in Supporting Materials and Methods.
Solid-State NMR.
The pellets obtained by centrifugation were dried into films with silica gel at 4°C for 5–7 days. The films were ground down and packed into a 3.2-mm NMR spinner. All of the experiments were performed with Varian/Chemagnetics (Palo Alto, CA) Infinity-500, Infinity-600, and Infinity-700 spectrometers operating at 13C NMR frequencies of 125.6, 150.0, and 175.9 MHz, respectively. The experiments were performed with double (1H,13C)- and triple (1H,13C,15N)-resonance T3 probes equipped with a 3.2-mm spinner module. The MAS frequencies were kept at 12.5, 14.0, and 16.0 kHz, respectively. The probe temperature was maintained at −20 to −30°C. Details are provided in Supporting Materials and Methods.
Structure Calculation.
The structure of the fibrillar conformation of the K3 peptide was calculated by using CNS version 1.1 by applying the molecular dynamics simulated annealing protocol with torsion angles as internal degrees of freedom. Four K3 peptides were used in the simulation. The annealing protocol consisted of high-temperature sampling at 50,000 K for 1,000 steps with 8 ps per step and subsequent cooling to 0 K in 1,000 steps. Experimental carbon–carbon distance restrains, backbone dihedral angle restraints obtained by TALOS, and intermolecular constraints of hydrogen bonds for parallel β-sheet among the β-sheet regions were used for structure refinement. All structures have been energy-minimized, and the 10 lowest-energy structures among 50 calculated structures have been selected and further analyzed. Figures of the final structures were drawn with MOLMOL (41).
Supplementary Material
Acknowledgments
We thank Professor Atsushi Nakagawa and Dr. Takanori Matsuura for x-ray fiber diffraction data collection and Dr. Daron Standley for helpful discussions. This work was supported by Takeda Science Foundation and Grant-in-Aid for Priority Areas 40153770 from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
Abbreviations
- MAS
magic angle spinning
- β2-m
β2-microglobulin
- TFE
2,2,2-trifluoroethanol
- AFM
atomic force microscopy
- RFDR
rf-driven recoupling
- DARR
dipolar-assisted rotational resonance.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
Data deposition: The NMR chemical shifts have been deposited in the BioMagResBank, www.bmrb.wisc.edu (accession no. 10022).
References
- 1.Kelly JW. Curr Opin Struct Biol. 1998;8:101–106. doi: 10.1016/s0959-440x(98)80016-x. [DOI] [PubMed] [Google Scholar]
- 2.Dobson CM. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 3.Jiménez JL, Nettleton EJ, Bouchard M, Robinson CV, Dobson CM, Saibil HR. Proc Natl Acad Sci USA. 2002;99:9196–9201. doi: 10.1073/pnas.142459399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makin OS, Serpell LC. FEBS J. 2005;272:5950–5961. doi: 10.1111/j.1742-4658.2005.05025.x. [DOI] [PubMed] [Google Scholar]
- 5.Jaroniec CP, MacPhee CE, Bajaj VS, McMahon MT, Dobson CM, Griffin RG. Proc Natl Acad Sci USA. 2004;101:711–716. doi: 10.1073/pnas.0304849101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritter C, Maddelein M, Siemer AB, Lührs T, Ernst M, Meier BH, Saupe SJ, Riek R. Nature. 2005;435:844–848. doi: 10.1038/nature03793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heise H, Hoyer W, Becker S, Andronesi OC, Riedel D, Baldus M. Proc Natl Acad Sci USA. 2005;102:15871–15876. doi: 10.1073/pnas.0506109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naito A, Kamihira M, Inoue R, Saitô H. Magn Reson Chem. 2004;42:247–257. doi: 10.1002/mrc.1323. [DOI] [PubMed] [Google Scholar]
- 9.Petkova AT, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F, Tycko R. Proc Natl Acad Sci USA. 2002;99:16742–16747. doi: 10.1073/pnas.262663499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petkova AT, Leapman RD, Guo Z, Yau W, Mattson MP, Tycko R. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 11.Petkova AT, Yau W, Tycko R. Biochemistry. 2006;45:498–512. doi: 10.1021/bi051952q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lührs T, Ritter C, Adrian M, Riek-Loher D, Bohrmann B, Döbeli H, Schubert D, Riek R. Proc Natl Acad Sci USA. 2005;102:17342–17347. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shivaprasad S, Wetzel R. Biochemistry. 2004;43:15310–15317. doi: 10.1021/bi048019s. [DOI] [PubMed] [Google Scholar]
- 14.Naiki H, Hashimoto N, Suzuki S, Kimura H, Nakakuki K, Gejyo F. Amyloid. 1997;4:223–232. [Google Scholar]
- 15.Jahn TR, Radford SE. In: Amyloid Proteins. The Beta Sheet Conformation and Disease. Sipe JD, editor. Weinheim: Wiley; 2005. pp. 667–695. [Google Scholar]
- 16.Hoshino M, Katou H, Hagihara Y, Hasegawa K, Naiki H, Goto Y. Nat Struct Biol. 2002;9:332–336. doi: 10.1038/nsb792. [DOI] [PubMed] [Google Scholar]
- 17.Katou H, Kanno T, Hoshino M, Hagihara Y, Tanaka H, Kawai T, Hasegawa K, Naiki H, Goto Y. Protein Sci. 2002;11:2218–2229. doi: 10.1110/ps.0213202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jahn TR, Parker MJ, Homans SW, Radford SE. Nat Struct Mol Biol. 2006;13:195–201. doi: 10.1038/nsmb1058. [DOI] [PubMed] [Google Scholar]
- 19.Kozhukh GV, Hagihara Y, Kawakami T, Hasegawa K, Naiki H, Goto Y. J Biol Chem. 2001;277:1310–1315. doi: 10.1074/jbc.M108753200. [DOI] [PubMed] [Google Scholar]
- 20.Ohhashi Y, Hasegawa K, Naiki H, Goto Y. J Biol Chem. 2004;279:10814–10821. doi: 10.1074/jbc.M310334200. [DOI] [PubMed] [Google Scholar]
- 21.Wadai H, Yamaguchi K, Takahashi S, Kanno T, Kawai T, Naiki H, Goto Y. Biochemistry. 2005;44:157–164. doi: 10.1021/bi0485880. [DOI] [PubMed] [Google Scholar]
- 22.Kanno T, Yamaguchi K, Naiki H, Goto Y, Kawai T. J Struct Biol. 2005;149:213–218. doi: 10.1016/j.jsb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi K, Takahashi S, Kawai T, Naiki H, Goto Y. J Mol Biol. 2005;352:952–960. doi: 10.1016/j.jmb.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 24.Fändrich M, Dobson CM. EMBO J. 2002;21:5682–5690. doi: 10.1093/emboj/cdf573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornilescu G, Delaglio F, Bax A. J Biomol NMR. 1999;13:289–302. doi: 10.1023/a:1008392405740. [DOI] [PubMed] [Google Scholar]
- 26.Sarkar SK, Torchia DA, Kopple KD, VanderHart DL. J Am Chem Soc. 1984;106:3328–3331. [Google Scholar]
- 27.Kameda A, Hoshino M, Higurashi T, Takahashi S, Naiki H, Goto Y. J Mol Biol. 2005;348:383–397. doi: 10.1016/j.jmb.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 28.Tang M, Waring AJ, Hong M. J Am Chem Soc. 2005;127:13919–13927. doi: 10.1021/ja0526665. [DOI] [PubMed] [Google Scholar]
- 29.Tycko R, Ishii Y. J Am Chem Soc. 2003;125:13948–13956. doi: 10.1021/ja0342042. [DOI] [PubMed] [Google Scholar]
- 30.Gazit E. FASEB J. 2002;16:77–83. doi: 10.1096/fj.01-0442hyp. [DOI] [PubMed] [Google Scholar]
- 31.Nelson R, Sawaya MR, Balbirnie M, Madsen AO, Riekel C, Grothe R, Eisenberg D. Nature. 2005;435:773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petkova AT, Buntkowsky G, Dyda F, Leapman RD, Yau WM, Tycko R. J Mol Biol. 2004;335:247–260. doi: 10.1016/j.jmb.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 33.Hiramatsu H, Goto Y, Naiki H, Kitagawa T. J Am Chem Soc. 2004;126:3008–3009. doi: 10.1021/ja0383017. [DOI] [PubMed] [Google Scholar]
- 34.Hiramatsu H, Goto Y, Naiki H, Kitagawa T. J Am Chem Soc. 2005;127:7988–7989. doi: 10.1021/ja050844o. [DOI] [PubMed] [Google Scholar]
- 35.Perutz MF, Finch JT, Berriman J, Lesk A. Proc Natl Acad Sci USA. 2002;99:5591–5595. doi: 10.1073/pnas.042681399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chelli R, Gervasio FL, Procacci P, Schettino V. J Am Chem Soc. 2002;124:6133–6143. doi: 10.1021/ja0121639. [DOI] [PubMed] [Google Scholar]
- 37.Kajava AV, Aebi U, Steven AC. J Mol Biol. 2005;348:247–252. doi: 10.1016/j.jmb.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 38.Kardos J, Yamamoto K, Hasegawa K, Naiki H, Goto Y. J Biol Chem. 2004;279:55308–55314. doi: 10.1074/jbc.M409677200. [DOI] [PubMed] [Google Scholar]
- 39.Chatani E, Kato M, Kawai T, Naiki H, Goto Y. J Mol Biol. 2005;352:941–951. doi: 10.1016/j.jmb.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 40.Chiba T, Hagihara Y, Higurashi T, Hasegawa K, Naiki H, Goto Y. J Biol Chem. 2003;278:47016–47024. doi: 10.1074/jbc.M304473200. [DOI] [PubMed] [Google Scholar]
- 41.Koradi R, Billeter M, Wüthrich K. J Mol Graphics. 1996;14:51–55. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.