Abstract
The growth of axons is fundamental to the development and repair of brain circuitry. We show here that Mst3b, a neuron-specific homolog of the yeast kinase Ste20, is critical for axon outgrowth. Mst3b is activated in response to trophic factors, and suppressing its expression (via siRNAs) or its function (by a dominant-negative mutant) blocks axon outgrowth. Inosine, a purine nucleoside that stimulates axon outgrowth, activates Mst3b kinase activity, whereas 6-thioguanine, a purine analog that blocks outgrowth, inhibits the activity of this kinase. These findings place Mst3b as a key regulator of axon outgrowth and help explain the purine sensitivity of this process.
Keywords: inosine, neural development, regeneration, signal transduction
Axon outgrowth begins shortly after neurons undergo final cell division, and it ends when axon terminals reach their appropriate targets. Although this process is not normally reactivated in the mature CNS after injury, some degree of CNS regeneration has been achieved experimentally by altering neurons' intrinsic growth state and by counteracting inhibitory signals associated with myelin, the perineuronal net, or a glial scar (1–5). Thus, understanding the mechanisms that underlie axon growth not only affords insight into how the brain's circuitry develops but may also enable us to improve functional outcome after stroke, trauma, or neurodegenerative disorders.
The purine nucleoside inosine stimulates axon outgrowth in certain types of neurons in culture and in vivo after CNS injury (6–10). The purine analog 6-thioguanine (6-TG), on the other hand, blocks outgrowth induced by neurotrophic factors (9, 11, 12), and this effect is paralleled by the inhibition of a previously unidentified 45–50 kDa serine-threonine protein kinase (13). Inosine competitively reverses the inhibitory effects of 6-TG on outgrowth (7, 9), suggesting that it may act as an agonist of the 6-TG-sensitive kinase. We now identify the purine-sensitive kinase as mammalian Ste20-like protein kinase-3b (Mst3b) and show that it is a key regulator of axon outgrowth.
Results
Isolation of the Purine-Sensitive Kinase.
The observation that 6-TG blocks axon outgrowth in primary neurons (9, 12) suggested to us that the kinase it inhibits might be widely expressed in the brain. We prepared a high-speed supernatant fraction from neonatal bovine cortex and applied this material to a sulfopropyl–Sepharose column, which was previously shown to yield a partial purification of a 6-TG-sensitive kinase from PC12 pheochromocytoma cells (13). Subsequent purification was achieved by using an adenine nucleotide-affinity column (Cibacron blue) (Fig. 1A), two cycles of reversed-phase HPLC, and SDS/PAGE. Throughout, a protein band of ≈50 kDa exhibited the properties previously described for the kinase of interest, i.e., a capacity to phosphorylate histone protein HF1 in a 6-TG-sensitive, Ca2+-, Mg2+-, and Mn2+-insensitive fashion (11–14). Proteolytic fragments of the purified kinase, when sequenced by LC-MS, exactly matched portions of human Mst3 (15). Mst3 is a member of the Ste20 family of serine–threonine kinases (16); other family members are involved in diverse aspects of cellular differentiation (17). Two splice variants of Mst3 have been described that differ in their N termini (Fig. 1A). One of these variants, Mst3b, has been shown to be neuron-specific in its expression (18), although no specific functions have been ascribed to it as yet. For clarity, we shall refer to the variant discovered first as Mst3a. By using 5′ RACE with a rat brain cDNA library, we isolated rat cDNAs that encode N-terminal sequences that are highly homologous to those found in mouse and human Mst3a and Mst3b (Fig. 1B). By using this sequence data, we synthesized a peptide representing the distinctive N-terminal region of Mst3b and used it to generate an anti-Mst3b-specific antibody. The resulting antibody bound to a single 50-kDa band on Western blots, and this binding was suppressed by the immunogenic peptide but not by an Mst3a N-terminal peptide (Fig. 1C). Further evidence for the specificity of the antibody is shown below by the loss of the 50-kDa band in cells stably transfected with a plasmid expressing a small hairpin RNA that interferes with Mst3b expression. The anti-Mst3b antibody labeled neurons specifically (Fig. 1 D and E), consistent with earlier reports on the distribution of the mRNA (18). Preincubating the antibody with the Mst3b N-terminal peptide blocked immunostaining (data not shown). Thus, the 6-TG-sensitive protein kinase that we isolated from neonatal cortex is Mst3b, a neuron-specific homolog of yeast Ste20.
Fig. 1.
Identification of a purine-sensitive protein kinase. (A) A 6-TG-sensitive protein kinase was isolated from neonatal bovine brain and was visualized by using in-gel kinase assays. After adenine-nucleotide affinity chromatography, column fractions were separated by SDS/PAGE in gels containing an experimental substrate (HF1) and incubated with [γ-32P]ATP in Ca2+- and Mg2+-free, Mn2+-containing buffer in the presence (+) or absence (−) of 6-TG. The arrow indicates the presence of a 50-kDa, 6-TG-sensitive kinase. (B) Further purification and sequencing by LC-MS revealed that the purine-sensitive kinase is an isoform of Mst3. Mst3a and Mst3b are identical except at their N termini (sequences shown in red and blue, respectively). K65 lies in the ATP-binding region of the common kinase domain (KD). The asterisk denotes a region not found in other Ste20 family members that was used to generate an Mst3 C-terminal antibody. (C) Adult rat cortex was solubilized in Laemmli buffer, and the proteins were separated on a 4–20% acrylamide gel and transferred to a poly(vinylidene difluoride) membrane. The N-terminal Mst3b antibody was incubated without (−) or with a 10-fold molar excess of the antigenic peptide (3b) or a peptide representing the N terminus of Mst3a (3a) and used to probe the poly(vinylidene difluoride) membrane. (D) The Mst3b N-terminal antibody stains neurons in the dentate gyrus (DG) and in the CA1, CA2, and CA3 fields of the adult rat hippocampus. (E) The Mst3b antibody stains neuronal layers of the occipital cortex. [Note relative absence of staining in the white matter (wm) and molecular layer (ml), which contain few neuronal somata.]
Mst3b Displays the Known Properties of the Purine-Sensitive Kinase.
Because the initial characterization of the 6-TG-sensitive kinase was done in PC12 pheochromocytoma cells, we used this cell line for our initial studies. As expected (11, 14, 19), PC12 cells extended neurites in response to the nerve growth factor (NGF, 50 ng/ml), and this effect was inhibited by the purine analog 6-TG (10 μM) (Fig. 2A). Inosine (1 mM) reversed the inhibitory effect of 6-TG. Although by itself inosine has only a modest effect on PC12 cells (19), it induces strong outgrowth in goldfish retinal ganglion cells (7, 9), mammalian peripheral ganglionic cells (6), and embryonic rat cortical neurons (see below). Neither 6-TG nor inosine altered cell survival (Fig. 2B).
Fig. 2.
Mst3b exhibits the known properties of the purine-sensitive kinase. (A) Purine sensitivity of neurite outgrowth in PC12 cells. NGF-induced outgrowth is inhibited by 10 μM 6-TG and restored with the addition of 1 mM inosine (Ino). (Outgrowth is defined as the percentage of cells with processes ≥2 cell diameters after 48 h.) ∗∗∗, P < 0.001. (B) None of these manipulations affected cell survival (viable cells per well). (C) Effects of NGF and 6-TG on Mst3b kinase activity. By using the N-terminal-specific antibody, we immune-precipitated Mst3b from control PC12 cells or from PC12 cells exposed to NGF for the indicated intervals. Kinase activity was assayed by using histone HF1 as a substrate. (D) Mst3b protein levels in PC12 cells stably expressing shRNA constructs directed against the 5′ end of mst3b, vector alone, the 5′ end of mst3b with two nucleotides altered (mst3bc), or the 5′ end of mst3a. Western blots were probed with the Mst3b-specific antibody and an antibody to GAPDH as a control for loading. (E) Blocking Mst3b expression with mst3b shRNA eliminates NGF-induced, purine-sensitive kinase activity. IP kinase assays, performed as in C, were carried out by using homogenates of PC12 cells stably transfected with plasmids expressing a control shRNA or an shRNA specific to the 5′ end of mst3b mRNA. Identical results were obtained with either the Mst3b-specific antibody or the anti-C-terminal antibody that recognizes both isoforms of Mst3. (F) (Upper) Inosine (200 μM) reverses the inhibitory effect of 6-TG on Mst3b activity in vitro. The IP kinase assay used HF-1 as a substrate. (Lower) The same gel stained for total HF-1 protein.
To investigate whether Mst3b might mediate the response of PC12 cells to growth factors, we first carried out immunoprecipitation (IP) kinase assays. When PC12 cells were in a resting state, the Mst3b-specific antibody precipitated a kinase that exhibited only low levels of HF1-phosphorylating activity. Exposing cells to NGF strongly increased the activity of the precipitated kinase. This activity was blocked by the addition of 6-TG to the reaction (Fig. 2C). To verify that the precipitated kinase is Mst3b per se, we transfected PC12 cells with either a plasmid expressing a small hairpin RNA (shRNA) targeted to the distinctive 5′ end of mst3b mRNA or a similar plasmid expressing a control shRNA with nucleotides 8 and 9 mutated: These nucleotides lie halfway down the stem of the 5′ mst3b shRNA sequence. On Western blots, cells expressing the mst3b-specific shRNA showed considerably lower levels of Mst3b than control cells expressing either the vector alone, the mutated mst3b shRNA (mst3bC), or an shRNA directed to the N terminus of mst3a mRNA (Fig. 2D). The latter shRNA blocks the expression of Mst3a in 293 cells transfected with a plasmid expressing Mst3a (data not shown). In IP kinase assays, PC12 cells expressing the mst3b-specific shRNA exhibited lower levels of the NGF-inducible, 6-TG-sensitive kinase activity than cells expressing the control shRNA (Fig. 2E Left). Similar results were obtained when an antibody to the C-terminal region that is shared by Mst3a and Mst3b was used to precipitate the kinase (Fig. 2E Right). This latter result indicates that Mst3b is the dominant NGF-sensitive Mst3 isotype in PC12 cells. Finally, in vitro, inosine reversed the inhibitory effects of 6TG (Fig. 2F), paralleling its effects on outgrowth (Fig. 2A). Thus, in PC12 cells, Mst3b is activated by NGF and is sensitive to purine nucleosides.
Mst3b Is Critical for Neurite Outgrowth in PC12 Cells.
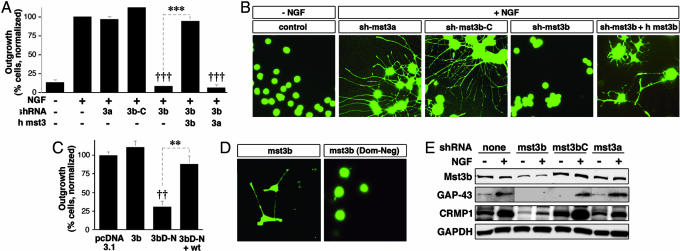
To investigate whether Mst3b is critical for outgrowth, we used two approaches: suppressing its expression or expressing a dominant-negative form of the kinase. Cells stably expressing an shRNA directed to the 5′ region of rat mst3b mRNA showed little outgrowth when treated with NGF (Fig. 3A and B). The response of these cells to NGF was rescued by transfecting them with a plasmid encoding human Mst3b, whose 5′ mRNA sequence differs from that of the rat at 15 of 22 positions; a plasmid expressing Mst3a failed to rescue outgrowth in these cells (Fig. 3 A and B). Thus, Mst3a and Mst3b are functionally distinct from one another. In addition, PC12 cells expressing either an shRNA directed against the 5′ end of mst3a mRNA or the mutated mst3b 5′ shRNA showed a normal response to NGF (Fig. 3 A and B). Further studies on the role of Mst3b were done by using a dominant-negative approach. PC12 cells were transfected with plasmids expressing either wild-type human Mst3b or a mutant human Mst3b in which Lys-65, which lies in the ATP-binding region of the protein, was mutated to a methionine. Mutation of the analogous lysine in Mst3a has previously been shown to produce a kinase-dead mutant (15) that would be expected to interact with the protein's normal substrates and binding partners in an unproductive manner. Whereas overexpression of wild-type Mst3b caused a small increase in NGF responsiveness, expression of the Mst3b-K65 mutant inhibited outgrowth (Fig. 3 C and D). Transfecting cells expressing the kinase-dead mutant with a plasmid overexpressing the wild-type kinase rescued their ability to respond to NGF (Fig. 3C). Together, these studies indicate that Mst3b is essential for NGF-induced outgrowth in PC12 cells.
Fig. 3.
Blocking Mst3b expression or Mst3b activity reduces neurite outgrowth. (A and B) PC12 cells were stably transfected to express shRNA constructs specific to the 5′ end of the mRNA of mst3a, of mst3b, or of mst3b with two nucleotides mutated (mst3bC). PC12 cells were then maintained in the absence or presence of NGF as indicated. (A) Quantitative results. Outgrowth (the percentage of cells with neurites normalized to outgrowth in control-transfected cells treated with NGF) was evaluated after 48 h. In the last two cases, cells also were transfected with plasmids expressing human Mst3b or Mst3a. †††, Decrease relative to control-transfected cells treated with NGF significant at P < 0.001; ∗∗∗, increase significant at P < 0.001. (B) Images of stably transfected PC12 cells as described in A. (C and D) Dominant-negative Mst3b blocks outgrowth. PC12 cells were cotransfected with pEGFP-C1 and either pcDNA3.1 (empty plasmid) or pcDNA3.1 expressing wild-type (wt) Mst3b or kinase-dead mutant (K65) Mst3b (dominant-negative, 3bD-N). Additional experiments investigated the ability of wild-type human Mst3b to rescue outgrowth in the presence of 3bD-N. Cells were treated with NGF for 2 d beginning 24 h after transfection. (C) Quantitative analysis. Outgrowth is assessed and normalized as above. ††, Decrease relative to control-transfected cells treated with NGF significant at P < 0.01; ∗∗, increase significant at P < 0.01. (D) PC12 cells expressing dominant-negative Mst3b plus EGFP show reduced outgrowth compared with cells transfected with wild-type Mst3b. (E) Protein expression in PC12 cells stably expressing shRNAs as indicated. Induction of GAP-43 and CRMP-1 in PC12 cells exposed to NGF was normal in all cell lines except those expressing shRNA for Mst3b.
The effects of Mst3b on outgrowth are paralleled by alterations in gene expression. PC12 expressing mst3b-specific shRNA showed lower levels of both the growth-associated protein GAP-43 and of CRMP1 when treated with NGF than cells stably expressing either shRNA directed to the 5′ end of mst3a mRNA or the mutated mst3b shRNA (Fig. 3E). As a control for the specificity of these changes, expression of the housekeeping protein GAPDH was found to be unaffected by knocking down Mst3b.
Mst3b Regulates Axon Outgrowth in Cortical Neurons.
To investigate the role of Mst3b in primary neurons, we used dissociated cells from the developing rat forebrain (embryonic day 15). In response to either BDNF or inosine, neurons extended processes that appear to be axons by virtue of being Tau-1-positive. 6-TG blocked the outgrowth of Tau-1-positive processes, whereas inosine competitively overcame this blockade (Fig. 4A and C). The growth of dendritic processes (Tau-1-negative and Map2-positive) was relatively unaffected by these manipulations (Fig. 4B). In addition, 6-TG had no effect on the survival of cortical neurons [neurons per field expressed as mean ± SEM (n = 8): BDNF, 66.4 ± 3.3; BDNF plus 6-TG, 65.6 ± 4.4]. Thus, purine nucleosides appear to specifically regulate the outgrowth of axons in these neurons. Next, to investigate whether Mst3b mediates the axon-promoting effects of BDNF and inosine, we first carried out IP kinase assays with the Mst3b-specific antibody. Treating embryonic cortical neurons with BDNF increased the activity of Mst3b above baseline, as did treating Mst3b from untreated cells with inosine. BDNF-induced activation was inhibited by 6-TG (Fig. 4D). Thus, the positive effects of BDNF and inosine along with the negative effect of 6-TG on Mst3b kinase activity parallel their effects on outgrowth.
Fig. 4.
Axon outgrowth in embryonic forebrain neurons is mediated through Mst3b. (A and B) Axon outgrowth in cortical neurons is purine-sensitive. (A) Quantitation of axon outgrowth in embryonic-day-16 cortical neurons treated with 50 ng/ml BDNF, 100 μM inosine, or 10 μM 6-TG as indicated (Tau-1-positive processes ≥2 cell diameters in length after 2 d in culture). ∗∗∗, Decrease relative to cells treated with BDNF significant at P < 0.001. (B) Quantitation of dendritic growth. The outgrowth of Map2-positive/Tau-1-negative processes was quantified in the same cells shown in A. (C) Representative images of axon growth. Arrows in merged images point to processes that exhibit strong Tau-1 and weak Map2 staining (axons). (D) Activation of Mst3b by BDNF or inosine (ino). Mst3b was immunoprecipitated from lysates of embryonic-day-16 neurons treated with or without (−) inosine or BDNF as indicated; kinase assays were performed in the absence or presence of 6-TG. (E and F) Role of Mst3b in BDNF-induced axon outgrowth. Embryonic-day-16 cortical cells were transfected with plasmids expressing shRNA constructs and EGFP as indicated; in some cases, cells were cotransfected with plasmids expressing wild-type human mst3b (h-mst3b) or a dominant-negative form of rat Mst3b (d/n mst3b). (E) Quantitation of axon outgrowth (Tau-1-positive processes in EGFP-positive transfected cells). ∗∗∗, Decrease relative to BDNF-treated cells significant at P < 0.001. (F) Images of transfected cells. Asterisks indicate transfected cells (green fluorescence); arrows point to Tau-1+ processes in these cells (yellow fluorescence). First row: Transfected cell expressing EGFP and the mst3b control shRNA with two nucleotides altered. Second row: Transfected cell expressing mst3b shRNA fails to extend Tau-1+ processes, although neighboring untransfected cells do; arrowheads show EGFP+/Tau-1 negative processes, presumably dendrites, in transfected cell. Third row: Outgrowth in a cell expressing shRNA to mst3b is rescued by cotransfection with a plasmid expressing wild-type human Mst3b. Note that Tau1- expression is seen mainly in the distal part of the process and only weakly in the cell body and proximal axon. Fourth row: Cells transfected with a plasmid expressing dominant-negative (K65) Mst3b. (G and H) Role of Mst3b in inosine-induced outgrowth. Embryonic-day-16 cortical neurons were transfected with plasmids expressing shRNA constructs, human Mst3b, or dominant-negative (K65) Mst3b as indicated and were exposed to inosine. (G) Quantitation of axon growth. ∗∗∗, Decrease relative to inosine-treated cells expressing the mutated control mst3b shRNA significant at P < 0.001. (H) Inosine-treated cells transfected with various shRNA constructs and/or other mst3b constructs as indicated. Descriptions are similar to those in corresponding parts of F.
To investigate the role of Mst3b in cortical neurons more directly, we transiently transfected these cells with plasmids expressing the shRNAs described above. Expression of the shRNA directed to the 5′ end of mst3b mRNA blocked the growth of Tau-1-positive processes in response to BDNF (Fig. 4 E and F). In contrast, BDNF-induced outgrowth was unaffected by expression of either mst3a-specific shRNA or the mst3b control shRNA with two nucleotides mutated. When cells expressing the mst3b-specific shRNA were cotransfected with a plasmid encoding human Mst3b, the outgrowth of Tau-1-positive axons was restored (Fig. 4 E and F). Together, these results indicate that Mst3b is required for BDNF-induced axon outgrowth in cortical neurons.
The ability of inosine to reverse the inhibitory effects of 6-TG in culture (Figs. 2A and 4A) and in vitro (Fig. 2F) suggests that it may act by stimulating Mst3b. To test this hypothesis, we investigated whether knocking down Mst3b blocks the effect of inosine. The mst3b-specific shRNA prevented inosine from stimulating axon outgrowth (Fig. 4 G and H); cotransfecting cells with a plasmid encoding human Mst3b rescued outgrowth. The control shRNA to mst3b with two nucleotide substitutions did not inhibit the effect of inosine (Fig. 4 G and H). Finally, the dominant-negative mutant form of Mst3b blocked inosine-stimulated outgrowth. Thus, the axon-promoting effect of inosine is mediated by Mst3b.
Discussion
These studies place Mst3b as an important regulator of axon outgrowth. Earlier studies had shown that 6-TG inhibits neurite outgrowth and the activity of a 45- to 50-kDa protein kinase in parallel (11, 13, 14) and that inosine competitively reverses the inhibitory effects of 6-TG and stimulates axon outgrowth on its own in some types of neurons (refs. 6, 7, and 9 and this study). These findings suggested the existence of a purine-sensitive protein kinase that plays a crucial role in axon outgrowth. The present study identifies the purine-sensitive kinase as Mst3b, a 49-kDa protein kinase that is activated in response to growth factors (NGF and BDNF) and that regulates axon outgrowth.
Mst3b is a neuron-specific homolog of Ste20, a serine–threonine kinase that controls bud formation in yeast via a MAP kinase signaling pathway (16, 18). Other Ste20-like kinases control other modular MAP kinase signaling pathways that regulate diverse aspects of cellular differentiation (17). Inhibiting Mst3b in the present study blocked the induction of GAP-43 and CRMP-1, two proteins associated with axonal outgrowth (20, 21). How Mst3b relates to signaling cascades involved with the establishment of cell polarity (22–24), cytoskeletal dynamics (25–29), or neurite extension (30, 31) remains to be investigated.
After CNS injury in the mature rat, inosine stimulates undamaged neurons to extend collateral branches that grow into areas of the brainstem and spinal cord that have lost their normal innervation. After either a unilateral transection of the corticospinal tract or a unilateral stroke, inosine causes layer five pyramidal cells on the undamaged side of the brain to extend axon collaterals that cross the midline into the denervated side of the spinal cord (10), leading to improved performance with the denervated forepaw (8). The possibility that Mst3b mediates these effects suggests that further studies on how this kinase is regulated may not only enhance our basic understanding of axon growth, but may also be of benefit in the treatment of CNS injury.
Materials and Methods
Sample Preparation and Chromatography.
Cortical gray matter from freshly dissected calf brains (age, 1–3 weeks; 4°C) was rinsed and dissected into buffer (10 mM NaF/2 mM EDTA/10 mM MnCl2/50 mM NaCl/2 mM PMSF/1 μg/ml leupeptin/50 mM Hepes, pH 7.3) at 4°C. All reagents were from Sigma (St. Louis, MO) unless noted otherwise. The buffered gray matter was then homogenized and centrifuged for 1 h at 15,000 × g. The supernatant fraction was applied to an SP Sepharose Fast Flow column (Amersham Pharmacia Biotech, Piscataway, NJ) and eluted with 0.3 M NaCl. Chromatography was monitored by UV absorbance (280 nm). The presence of the purine-sensitive kinase in column fractions was visualized by using in-gel kinase assays as described below. Column fractions from the SP column containing the highest levels of the kinase were concentrated and applied to a blue Sepharose 6 Fast-Flow column (Amersham Pharmacia Biotech). Fractions were eluted from this latter column with a stepwise gradient of NaCl. Fractions that were enriched with the 6-TG-sensitive kinase were concentrated and further purified on a C4 Progel-TSK column (Supelco, Bellefonte, PA). C4 column fractions containing the highest 6-TG-sensitive kinase activity were pooled from 10 separate runs and rechromatographed on the same C4 Progel-TSK column. The final step in purification was preparative SDS/PAGE of the fractions from the last column that contained 6-TG-sensitive kinase activity. A Coomassie brilliant blue-stained band corresponding to the 6-TG-sensitive kinase activity was excised and sequenced by LC-MS by the Harvard Microchemistry Facility.
In-Gel Kinase Assay.
By using an adaptation of a published method (32), column fractions were concentrated and the proteins were separated by SDS/PAGE in a gel containing histone HF1 protein, an experimental substrate of the 6-TG-sensitive kinase (11, 33). The gel was washed twice with 20% isopropanol/50 mM Hepes, pH 7.4; proteins were denatured by rinsing with 6 M guanidine-HCl/10 mM Hepes, pH 7.4; and renatured in 10 mM Hepes/0.02% Tween 40 (three rinses over 16 h). After two rinses in reaction buffer (10 mM Hepes/10 mM NaF/10 mM MnCl2/2 mM EGTA/5 mM mercaptoethanol), the gel was incubated in the same buffer containing 10 μM ATP and 50 μCi/ml (1 Ci = 37 GBq) [γ-32P]ATP (NEN/Perkin-Elmer, Shelton, CT) with or without 6-TG for 15 min. The gel was then washed with stop solution until no radioactivity could be detected in the solution; then the gel was dried and placed on film. The kinase of interest was visualized as an HF1-phosphorylating band whose activity was inhibited by 6-TG.
Antibodies.
Research Genetics/Invitrogen (Carlsbad, CA) generated an antibody to the peptide ACGGNLGSIEELRGAIY that is present in the C-terminal region of the bovine, rat, mouse, and human proteins of both Mst3 isoforms. An antibody directed against the unique N terminus of Mst3b was made in rabbit, using the peptide SKVPRLGLAKRRKATPL conjugated to KLH (Open Biosystems, Huntsville, AL). Anti-GAP-43 antibody was from Boehringer (Indianapolis, IN); anti-GAPDH was from Abcam (Cambridge, MA); and antibodies to CRMP-1, Map2, and Tau-1 were from Chemicon (Temecula, CA).
Western Blot Analysis.
PC12 cells were grown in 100-mm dishes in the presence or absence of 50 ng/ml NGF for periods ranging from 5 min to 2 d and were then collected and centrifuged. Proteins were separated by SDS/PAGE, transferred to poly(vinylidene difluoride) membranes (Millipore, Bedford, MA), probed with primary antibodies overnight at 4°C, washed in Tris-buffered saline/0.05% Tween 20 or the same buffer with 0.28 M NaCl, and incubated for 2 h at room temperature with an HRP-conjugated secondary antibody followed by ECL reagent (Amersham Pharmacia Biotech). Lysates of adult rat cortex were used to test whether the antigenic peptide (10-fold excess) or an Mst3a-specific peptide (10-fold excess) would diminish antibody binding.
IP and in Vitro Kinase Assay.
After homogenization, cell extracts were precleared by overnight incubation with normal rabbit serum followed by a 2-h incubation with protein A agarose beads. Beads were removed by high-speed centrifugation and the supernatants were incubated overnight with 10 μl of anti-Mst3 (C-terminal) or anti-Mst3b (N-terminal) antibody, followed by a 2-h incubation with protein A agarose beads. The immune complex was washed four times with kinase buffer (50 mM Hepes, pH 7.4/10 mM MnCl2/10 mM DTT/2 mM NaF) before adding histone HF1 as a substrate (1.5 μg per reaction) in kinase buffer containing 0.5 μCi of [γ-32P]ATP with or without 200 μM 6-TG. The reaction was carried out for 10 min at 37°C and then stopped by boiling with sample buffer for 5 min. Reaction products were separated on 4–20% SDS/polyacrylamide gels and analyzed by autoradiography.
Immunofluorescence.
PC12 or cortical cells were treated for 10 min in 2% paraformaldehyde, rinsed with PBS, and exposed to primary antibodies overnight at 4°C in PBS containing 0.05% Tween 20 (PBST) plus 1% BSA and 2% normal goat serum. Cells were washed three times with PBST, incubated for 2 h at room temperature with Alexa Fluor 488- or Alexa Fluor 594-conjugated secondary antibodies (Molecular Probes/Invitrogen, Eugene, OR), and rinsed in PBS.
Plasmids.
The short hairpin DNA sequences were synthesized by IDT (Coralville, IA), cloned into BS/U6 (34), and subcloned into PEGFP-C1 (Clontech, Mountain View, CA). Mst3a and Mst3b were expressed in pcDNA3.1 (Invitrogen, Carlsbad, CA).
Mutagenesis and 5′ RACE.
Kinase-dead Mst3b was made by using a QuikChange mutagenesis kit (Stratagene, La Jolla, CA). The 5′ ends of the genes encoding rat Mst3a and Mst3b were obtained by 5′ RACE of rat brain cDNA (Invitrogen).
Transfection of PC12 Cells.
PC12 cells were grown on poly-l-lysine in four-well plates (Nunc/Nalge, Rochester, NY) in DMEM plus 5% FCS (HyClone, Logan, UT), 10% heat-inactivated horse serum (Sigma), 2 mM glutamine (Sigma), and penicillin/streptomycin (Sigma). Cells were transfected by using Geneshuttle 20 (QBiogene, Irvine, CA). To obtain stable transfectants, the media was replaced 2 d later with complete media plus 500 ng/ml G418 (Research Products, Mount Prospect, IL). Cultures were maintained in G418 for 2 weeks. Single colonies from each of the four wells were picked and plated in separate wells and maintained in complete media plus G418.
Primary Neuronal Cultures.
Neuronal cultures were prepared from embryonic day 15 Sprague–Dawley rat fetuses as described previously (35). Serum-containing media was replaced with 2× medium E (36) plus 20 μM forskolin and sodium bicarbonate (2 g/liter), and the cells were incubated in 5% CO2 at 37°C. After 4 h, neurons were transfected by calcium phosphate precipitation.
Quantitative Analysis of Neurite Outgrowth.
PC12 cultures were evaluated after 2 d of treatment by an observer blind to the experimental conditions in each well. Neurite outgrowth was assessed in 150 consecutive cells per well under inverted-phase or UV illumination (×200 magnification). Unless noted otherwise, neurite outgrowth is operationally defined as the fraction of cells with neurites ≥2 cell diameters in length averaged over four replicate wells. All results are presented ±SEM and are representative of at least three independent experiments. In the embryonic cortical cultures, neurons represent 90% of the cells present, as verified by glial fibrillary acidic protein and neurofilament labeling. After 3 d, cells were fixed, stained for Tau-1 and Map2; Tau-1 staining is seen in the distal part of the axon. Tau-1-positive (axonal) and Map2-positive/Tau-1-negative (dendritic) outgrowth was quantified by examining at least 50 consecutive cells.
Acknowledgments
We thank Gong Du and Sara Vasquez for technical assistance; Dr. Guo-fu Hu (Harvard Medical School) for help in isolating Mst3b; the Children's Hospital Developmental Disabilities Research Core for use of its histology and genetics facilities; and Drs. Michael Greenberg, Lloyd Greene, Azad Bonni, Paul Rosenberg, and Thomas Schwartz for critically reviewing the manuscript. This work was supported by National Institutes of Health Grant EY 05690 (to L.I.B.); Boston Life Sciences; and a Howard Hughes Medical Institute Medical Student Research Fellowship (to J.E.O.).
Abbreviations
- 6-TG
6-thioguanine
- IP
immunoprecipitation
- NGF
nerve growth factor
- shRNA
small hairpin RNA.
Footnotes
Conflict of interest statement: L.I.B. is a paid consultant to Boston Life Sciences, which also provides partial funding to the laboratory.
This article is a PNAS direct submission.
References
- 1.Schwab ME. Science. 2002;295:1029–1031. doi: 10.1126/science.1067840. [DOI] [PubMed] [Google Scholar]
- 2.Lee DH, Strittmatter SM, Sah DW. Nat Rev Drug Discovery. 2003;2:872–878. doi: 10.1038/nrd1228. [DOI] [PubMed] [Google Scholar]
- 3.Fischer D, Petkova V, Thanos S, Benowitz LI. J Neurosci. 2004;24:8726–8740. doi: 10.1523/JNEUROSCI.2774-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 5.Silver J, Miller JH. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 6.Zurn A, Do K. Proc Natl Acad Sci USA. 1988;85:8301–8305. doi: 10.1073/pnas.85.21.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benowitz LI, Jing Y, Tabibiazar R, Jo SA, Petrausch B, Stuermer CA, Rosenberg PA, Irwin N. J Biol Chem. 1998;273:29626–29634. doi: 10.1074/jbc.273.45.29626. [DOI] [PubMed] [Google Scholar]
- 8.Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Proc Natl Acad Sci USA. 2002;99:9031–9036. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrausch B, Tabibiazar R, Roser T, Jing Y, Goldman D, Stuermer CA, Irwin N, Benowitz LI. J Neurosci. 2000;20:8031–8041. doi: 10.1523/JNEUROSCI.20-21-08031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benowitz LI, Goldberg DE, Madsen JR, Soni D, Irwin N. Proc Natl Acad Sci USA. 1999;96:13486–13490. doi: 10.1073/pnas.96.23.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volonte C, Rukenstein A, Loeb DM, Greene LA. J Cell Biol. 1989;109:2395–2403. doi: 10.1083/jcb.109.5.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene LA, Volonte C, Chalazonitis A. J Neurosci. 1990;10:1479–1485. doi: 10.1523/JNEUROSCI.10-05-01479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volonte C, Greene LA. J Biol Chem. 1992;267:21663–21670. [PubMed] [Google Scholar]
- 14.Batistatou A, Volonte C, Greene LA. Mol Biol Cell. 1992;3:363–371. doi: 10.1091/mbc.3.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schinkmann K, Blenis J. J Biol Chem. 1997;272:28695–28703. doi: 10.1074/jbc.272.45.28695. [DOI] [PubMed] [Google Scholar]
- 16.Herskowitz I. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- 17.Dan I, Watanabe NM, Kusumi A. Trends Cell Biol. 2001;11:220–230. doi: 10.1016/s0962-8924(01)01980-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhou TH, Ling K, Guo J, Zhou H, Wu YL, Jing Q, Ma L, Pei G. J Biol Chem. 2000;275:2513–2519. doi: 10.1074/jbc.275.4.2513. [DOI] [PubMed] [Google Scholar]
- 19.Braumann T, Jastorff B, Richter-Landsberg C. J Neurochem. 1986;47:912–919. doi: 10.1111/j.1471-4159.1986.tb00697.x. [DOI] [PubMed] [Google Scholar]
- 20.Benowitz LI, Routtenberg A. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 21.Quach TT, Duchemin AM, Rogemond V, Aguera M, Honnorat J, Belin MF, Kolattukudy PE. Mol Cell Neurosci. 2004;25:433–443. doi: 10.1016/j.mcn.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Shi SH, Jan LY, Jan YN. Cell. 2003;112:63–75. doi: 10.1016/s0092-8674(02)01249-7. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H, Guo W, Liang X, Rao Y. Cell. 2005;120:123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 25.Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Kanai Y, Cowan NJ, Hirokawa N. Nature. 1992;360:674–677. doi: 10.1038/360674a0. [DOI] [PubMed] [Google Scholar]
- 27.Kishi M, Pan YA, Crump JG, Sanes JR. Science. 2005;307:929–932. doi: 10.1126/science.1107403. [DOI] [PubMed] [Google Scholar]
- 28.Konishi Y, Stegmuller J, Matsuda T, Bonni S, Bonni A. Science. 2004;303:1026–1030. doi: 10.1126/science.1093712. [DOI] [PubMed] [Google Scholar]
- 29.Zukerberg LR, Patrick GN, Nikolic M, Humbert S, Wu CL, Lanier LM, Gertler FB, Vidal M, Van Etten RA, Tsai LH. Neuron. 2000;26:633–646. doi: 10.1016/s0896-6273(00)81200-3. [DOI] [PubMed] [Google Scholar]
- 30.Angelastro JM, Klimaschewski L, Tang S, Vitolo OV, Weissman TA, Donlin LT, Shelanski ML, Greene LA. Proc Natl Acad Sci USA. 2000;97:10424–10429. doi: 10.1073/pnas.97.19.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segal RA, Greenberg ME. Ann Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- 32.Kameshita I, Fujisawa H. Anal Biochem. 1989;183:139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- 33.Volonte C, Greene LA. J Neurochem. 1992;58:700–708. doi: 10.1111/j.1471-4159.1992.tb09774.x. [DOI] [PubMed] [Google Scholar]
- 34.Sui G, Soohoo C, Affar el B, Gay F, Shi Y, Forrester WC. Proc Natl Acad Sci USA. 2002;99:5515–5520. doi: 10.1073/pnas.082117599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dichter MA. Brain Res. 1978;149:279–293. doi: 10.1016/0006-8993(78)90476-6. [DOI] [PubMed] [Google Scholar]
- 36.Schwalb JM, Boulis NM, Gu MF, Winickoff J, Jackson PS, Irwin N, Benowitz LI. J Neurosci. 1995;15:5514–5525. doi: 10.1523/JNEUROSCI.15-08-05514.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]