Abstract
Objective: To investigate the effect of berbamine on human hepatoma cell line SMMC7721. Methods: The effects of 24 h and 48 h incubation with different concentrations (0~64 μg/ml) of the berbamine on SMMC7721 cells were evaluated using 3-4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide (MTT) assay. Hoechst 33258 staining was conducted to distinguish the apoptotic cell, and the appearance of sub-G1 stage was determined by PI (propidium iodide) staining, the percentage of apoptotic cell was determined by flow cytometry following annexin V/PI staining. Flow cytometry was performed to analyze the cell cycle distribution and the mitochondrial membrane potential (∆ψ m); the expression of activated caspase3 and caspase9 was analyzed by Western-blot. Results: The proliferation of SMMC7721 was decreased after treatment with berbamine in a dose- and time-dependent manner. Berbamine could induce apoptosis in SMMC7721 cells and could cause cell cycle arrest in G0/G1 phase, to induce loss of mitochondrial membrane potential (∆ψ m) and activate caspase3 and caspase9. Berbamine-induced apoptosis could be blocked by the broad caspase inhibitor z-VAD-fmk. Conclusion: Berbamine exerts antiproliferative effects on human hepatocellular carcinoma SMMC7721 cells. The anticancer activity of berbamine could be attributed partly to its inhibition of cell proliferation and induction of apoptosis in cancer cells through loss in mitochondrial transmembrane potential and caspase activation.
Keywords: Berbamine, Apoptosis, Mitochondrial membrane potential, Caspase, Hepatoma
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most frequently occurring cancer worldwide and the third most common cause of cancer-related death (Parkin et al., 2001). HCC affects more than 500 000 people globally with 110 000 deaths annually. More than one-half of them are Chinese (Hall and Wild, 2003). The prognosis for patients with hepatoma is poor because there is no effective treatment of metastatic disease. Effective chemotherapeutic agents for this disease have not been developed.
Natural products including plants, microorganisms and marines provide rich resources for anticancer drug discovery. There are many anticancer plants or herbal formulations, which provide evidence for the identification of new anticancer compounds and receive increasing scientific attention recently.
Berbamine (bisbenzylisoquinoline) is a natural compound from the plant Berberis amurensis used in Chinese traditional medicine. The formula of berbamine is presented in Fig.1, and its molecular weight is 753.80. Both animal and clinical studies showed that berbamine could stimulate normal hematopoiesis of cancer patients undergoing chemotherapy or radiotherapy and has been used to protect tumor patients from cytotoxic effects of chemotherapeutic agents on bone marrow (Yang et al., 1982; Liu and Zhou, 1996; Ge et al., 1998). Some studies showed that berbamine has anti-inflammatory, anti-arrhythmic effects and antineoplastic activity (Zhu and Sui, 1986; Xu et al., 2004; Küpeli et al., 2002). In previous studies, we found that berbamine could induce apoptosis of Bcr/Abl-positive K562 leukemia cells by suppressing the expression of Bcr/Abl fusion gene and activation of caspase3, and that berbamine could also reverse multidrug resistance by reducing P-glycoprotein expression (Wu et al., 2005; Xu et al., 2006; Dong et al., 2004; Sun et al., 2006). However, its effect on other kinds of cancer and the mechanism are poorly understood. In this study, we evaluated the anti-tumor effect of berbamine on human hepatoma cell line SMMC7721 in vitro.
Fig. 1.
The structural formula of berbamine
MATERIALS AND METHODS
Chemicals and reagents
Fetal calf serum (FCS), RPMI 1640 and antibiotic-antimycotic (100×) were purchased from Gibocal BRL (Gaithersburg, MD, USA). A stock solution of berbamine (1 mg/ml) in saline was prepared, from which a series of working dilutions were made. Berbamine, dimethyl sulfoxide (DMSO), 3-4,5-dimethyl-thiazol-2-yl-2,5-diphenyltetrazolium bromide (MTT), tryspin-EDTA, Hoechst 33258, Rhodamine123, PI (propidium iodide) and ribonuclease (RNase) were purchased from Sigma Chemical Co. (St Louis, USA). Annexin V-FLUOS staining kit was purchased from Immunotec (Marseille, France). PE-caspase3, mouse IgG-PE, cytoperm/cytofix kit were purchased from BD PharMingen (San Diego, CA, USA). Caspase inhibitor (z-VAD-fmk) was purchased from Biovision (Mountain View, CA, USA). Caspase3 and caspase9 antibody were purchased from Cell Signaling (Beverly, MA, USA).
Cell lines and culture
Hepatoma cell line SMMC7721 was obtained from Cancer Institute of Zhejiang University and cultured in RPMI 1640 medium supplemented with 10% FCS and antibiotics in a humidified atmosphere of 5% CO2 at 37 °C.
Cell growth inhibition assay
Cells (5000 well−1) were plated in 96-well plates in 200 µl of the complete RPMI 1640 medium. After cells were permitted to adhere for 24 h, then freshed medium containing different dose of berbamine from 0~64 µg/ml. After 24 h or 48 h, IC 50 value was calculated by the MTT method. Cells were incubated at 37 °C in 50 µl of MTT (5 mg/ml) for 4 h. After the medium and MTT were removed, 200 µl of DMSO were added to each well, and then placed on a plate shaker for 5 min at room temperature. Absorbance at 570 nm of the mixture was measured using a microplate ELISA reader. Cell survival rate was calculated as the percentage of MTT inhibition as follows: percentage of survival=(mean experimental absorbance/mean control absorbance)×100%.
Staining of apoptotic cells with Hoechst 33258
After drug treatment, cells were washed with 0.1 mol/L PBS (pH 7.2) and resuspended in the same buffer. One hundred microlitres of cell suspension (1×106 ml−1) was incubated with 1 µl of Hoechst 33258 (1 mg/ml in ddH2O) for 10 min. Apoptotic cells were evaluated by fluorescence microscopy.
Flow cytometric analysis of cell cycle status and apoptosis
The flow cytometric evaluation of the cell cycle status and apoptosis was performed according to a method described previously (Dong et al., 2004). In brief, 2×106 cells were treated with berbamine (0~50 µg/ml), and then washed twice with PBS. The cells were fixed overnight with cold 70% ethanol, following staining with PI solution containing 50 µg/ml PI and 10 µg/ml RNase. After incubation at room temperature for 60 min, cells were analyzed by flow cytometry (FACSCalibur; Becton Dickinson, USA) using Cell Quest software. The percentage of cells in the apoptotic sub-G1 and G1, S-phase, and G2-M phase were calculated using Modfit software.
Annexin V/propidium iodide (PI) staining assay
Apoptosis was assessed by measuring membrane redistribution of phosphatidilserine using an annexin V-FITC apoptosis detection kit (Koopman et al., 1994). According to the manufacturer’s protocol, after drug treatments, cells were collected and washed twice with PBS, followed by being resuspended in 500 µl of staining solution containing FITC-conjugated annexin V antibody (5 µl) and propidium iodide (PI, 5 µl of 250 µg/ml stock solution). After incubation on ice for 30 min, cells were analyzed by flow cytometry. Basal apoptosis and necrosis were identically determined on untreated cells. The percentage of cells undergoing apoptosis was determined by three independent experiments.
Flow cytometry assay for mitochondrial membrane potential (∆ψ m)
After drug treatment, cells were collected and washed twice with PBS, then incubated in the presence of Rhodamine123 (final concentration of 2.5 µg/ml) for 10 min in the dark. Cells were washed with 0.1 mol/L PBS and resuspended in the same buffer. The mitochondrial membrane potential (∆ψ m) was analyzed by flow cytometry.
Flow cytometry assay for activated caspase3
After drug treatment, cells were collected and treated by cytoperm/cytofix kit according to the manufacturer’s protocol, and then labelled with caspase3 McAb-PE. Mouse IgG-PE was used as isotype control. The cells were analyzed by flow cytometer.
Western-blot
Cell lysates were prepared and quantified according to established methods. To each well of a 10% SDS-polyacrylamide gel, 30 µg total protein was applied, electrophoresed, and transferred to PVDF membrane. Membranes were blocked using Tris-buffered saline with 5% nonfat milk. Blots were then probed with the primary antibody mouse anti-caspase3 or caspase9, in blocking buffer, and subsequently by a secondary anti-mouse IgG antibody. Detection was done using chemiluminescence kit (Pierce, Rockford, USA). The expression of actin was used as a control.
Statistical analysis
Data obtained represented mean values of at least three different experiments and were expressed as the mean±SD. Statistical analysis was determined by Student’s t-test. P<0.05 was considered statistically significant.
RESULTS
Berbamine inhibits the growth of SMMC7721
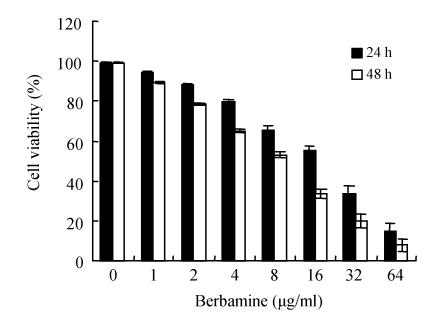
To determine whether berbamine has growth-inhibitory effects on hepatoma SMMC7721 cells, cells were exposed to different concentrations of berbamine (0~64 µg/ml) for 24 h and 48 h. The cell growth was evaluated by MTT assay. As presented in Fig.2, berbamine treatment resulted in inhibition of the growth of SMMC7721. Results demonstrated that the SMMC7721 cell viability was inhibited by berbamine at 24 h in a dose-dependent manner, the IC 50 value was (22.8±1.3) µg/ml. Similarly, a dose-dependent inhibition of SMMC7721 cell growth mediated by berbamine was observed at 48 h with an IC 50 value of (10.9±0.5) µg/ml (Fig.2).
Fig. 2.
Effects of berbamine on the growth of SMMC7721 cells for 24 h or 48 h. The activity was compared to the control well of the same cells and the results were presented as mean±SD for triplicate
Berbamine induces apoptosis of SMMC7721
To define whether berbamine-mediated growth-inhibitory activity is associated with apoptosis, we used Hoechst 33258 to investigate the changes in the cells’ nuclei. Normal cells showed homogeneous staining of their nuclei. In contrast, when cells were treated with berbamine (25 µg/ml for 24 h), apoptotic cells showed irregular staining of their nuclei as a result of chromatin condensation and nuclear fragmentation; some cells exhibited typical apoptotic bleb phenomenon (Fig.3).
Fig. 3.
Fluorescence photomicrographs of cells stained with Hoechst 33258 (400×). (a) Untreated SMMC7721 cells showing diffusely stained intact nuclei; (b) After SMMC7721 cells treated with berbamine 25 µg/ml for 24 h, apoptotic cells showed the condensed chromatin
Simultaneous staining with annexin V-FLOUS and PI distinguished between intact cells, early apoptosis, late apoptosis and cell death. Assessment of SMMC7721 cells exposed to 25 µg/ml berbamine for different lengths of time (0, 12, 24 and 48 h) showed that 2%, 13%, 52%, 68% cells respectively were undergoing apoptosis. This indicated that berbamine can induce apoptosis in SMMC7721 cells in a time-dependent manner (Fig.4).
Fig. 4.
(a) Fluorescence-activated sorting analysis of annexin-V-FITC/PI for quantification of berbamine-induced apoptosis in SMMC7721 cells. 1: untreated, 2: treated for 8 h, 3: treated for 16 h, 4: treated for 24 h; (b) Time-dependent alteration of apoptosis induced by berbamine 25 µg/ml. Each value is the mean±SD of three determinations. * P<0.001 vs control
Berbamine induces cell cycle arrest in G0/G1 phase
To assess the effect of berbamine on cell cycle progression, SMMC7721 cells were harvested after 0, 12, 24 and 48 h exposure to 25 µg/ml berbamine and stained with PI for flow cytometry analysis and the results were shown in Fig.5. The sub-G1 population indicated apoptotic-associated chromatin degradation. As the treatment time increased, the percentage of cells in sub-G1 significantly increased from 0.62% (control), 1.31% (12 h), and 50.32% (24 h) to 65.12% (48 h). In the non-apoptotic population, there was no apparently significant change in the cell cycle distribution after 12 h exposure to berbamine. But after 24 h and 48 h exposure to berbamine, cells in G0/G1 phase increased from 61.52% (control) to 66.0% (24 h) and 75.09% (48 h), and the G2/M phase decreased from 14.17% (control) to 8.64% (24 h) and 2.37% (48 h) (Fig.5).
Fig. 5.
Alteration of cell cycle distribution induced by berbamine (25 µg/ml) for different time. Cells were fixed and stained with PI. The percentage of non-apoptotic cells within each cell cycle was determined by FCM. Compared to the control, berbamine could induce apoptosis (increasing sub-G1 population). (a) Untreated SMMC7721 cells; (b) SMMC7721 cells treated for 12 h; (c) SMMC7721 cells treated for 24 h; (d) SMMC7721 cells treated for 48 h
Mitochondrial membrane depolarizes in berbamine-treated cells
To determine involvement of mitochondrial mediated pathway in berbamine-induced apoptotic cell death, we measured changes in ∆ψ m. Berbamine treatment to SMMC7721 cells resulted in a rapid dissipation of ∆ψ m in a time-dependent manner (Fig.6). Flow cytometric results revealed high level of Rhodamine123 binding (92.5%) to the mitochondrial of untreated SMMC7721 cells. There is a decrease in the fluorescence (90.61%) after 12 h exposure to berbamine. However, a significant decrease in the fluorescence was observed starting from 24 h (34.29%) and 48 h (20.15%) after berbamine treatment.
Fig. 6.
Effect of berbamine on the changes in mitochondrial membrane potential. Loss in mitochondrial membrane potential was analyzed by FCM at a single cell level using Rhodamine123 as fluorescent probe. After berbamine treatment, cells were stained with Rhodamine123, analyzed on a flow cytometer and histogram display of Rhodamine123 (x-axis) vs counts (y-axis) has been shown in logarithmic fluorescence intensity. Data were representative of three similar experiments
(a) Untreated SMMC7721 cells; (b) SMMC7721 cells treated for 12 h; (c) SMMC7721 cells treated for 24 h; (d) SMMC7721 cells treated for 48 h
Berbamine induces activation of caspase3 and caspase9
To determine whether caspase3 plays a role in berbamine mediated apoptosis of SMMC7721 cells, we assessed the activated caspase3 protein level of the SMMC7721 cells before and after treatment with berbamine using flow cytometry (FCM). As shown in Fig.7a. After SMMC7721 cells were exposed to berbamine for different time (0, 12, 24, 48 h), the percentage of activated caspase3 increased from 1%, 3%, 14% to 28%. Western-blot result showed that pro-caspase3 decreased (Fig.7b). We also assessed the caspase9 level. As shown in Fig.7c, the cleaved caspase9 increased in a time-dependent manner. To further define the role of caspase in berbamine-induced apoptosis, cells were pretreated with broad-spectrum caspase inhibitor, z-VAD-fmk. Cells were treated with 100 µmol/L of z-VAD-fmk 2 h before berbamine treatment. Apoptosis was detected 24 h after treatment. As shown in Fig.7d, z-VAD-fmk attenuated berbamine-induced apoptosis did not inhibit berbamine-induced apoptosis completely. These findings suggest that the mechanism of SMMC7721 cell apoptosis induced by berbamine is involved in activation of caspase.
Fig. 7.
(a) Berbamine induced apoptosis through activating caspase3. The activation of caspase3 in SMMC7721 cells was assessed by flow cytometry; (b) Western-blot showed pro-caspase3 protein level change. Cells were treated with berbamine (25 µg/ml) for indicated times; (c) Berbamine induced apoptosis through activating caspase9. The activation of caspase9 in SMMC7721 cells was assessed by Western-blot. Cells were treated with berbamine (25 µg/ml) for indicated times; (d) Effects of caspase inhibitor, z-VAD-fmk, on berbamine-induced apoptosis detected by annexin V-staining. Apoptotic cells were determined 24 h after treatment with berbamine (25 µg/ml) in the presence (+) or absence (−) of z-VAD-fmk. Each value is the mean±SD of three determinations
DISCUSSION
Berbamine is a natural small molecular compound from plant Berberis amurensis, it has been used widely, because of its immune protection function and activities, such as stimulating normal hematopoiesis, being anti-inflammatory, anti-arrhythmic, etc. Although some studies showed berbamine had anti-leukemia activity (Wu et al., 2005; Xu et al., 2006), it was still not applied in clinic cancer treatment, as its effect on other kinds of cancer and the mechanism are poorly understood.
The present study found that berbamine had antiproliferative effect on human hepatocellular carcinoma SMMC7721 cells in dose-dependent manner. Berbamine can induce apoptosis, loss in mitochondrial transmembrane potential (MTP) and active caspase3 in SMMC7721 cells.
Apoptosis is a genetic program that allows control of cellular homeostasis. Disruption of apoptosis can contribute to a number of diseases, including cancer (Thompson, 1995; Schmitt and Lowe, 1999). Activation of apoptotic pathway is a key mechanism by which cytotoxic drugs kill cancer cells. Black tea, tanshinone IIA and other natural products inhibit tumor growth (Bhattacharyya et al., 2005: Wang et al., 2005; Hsu et al., 2005). Nucleus condensation and apoptotic bodies appearance are universal characteristics of cells undergoing apoptosis. Furthermore, apoptotic cells showed a decreased DNA content below the G0/G1 level of that defined as ‘sub-G1’ peak. Annexin V-FITC/PI labelling analysis showed the externalization of phosphatidylserine (PS). Our results indicated that berbamine could induce apoptosis in time-dependent manner. It suggests that the growth inhibition of berbamine for SMMC7721 cells is caused by apoptosis.
Disturbance of the cancer cell cycle is one of therapeutic targets for development of new anticancer drugs (Carnero, 2002), accumulated evidence has shown that cell cycle arrest might result in apoptosis due to the existence of cell cycle checkpoint and feedback control (Pietenpol and Stewart, 2002). But several evidences have suggested that some anticancer drug induced apoptosis may occur via a signaling pathway independent of cell cycle arrest (Hsu et al., 2005). The result of cell cycle analysis evaluated by flow cytometry showed that berbamine could induce cell cycle arrest in G0/G1 phase. It suggests that apoptosis induced by berbamine is related to cell cycle arrest.
Activation of the family of caspases was known as a crucial mechanism for induction of death signals to apoptosis. Caspases participate in a cascade that is triggered in response to proapoptotic signals and culminates in cleavage of a set of proteins, resulting in disassembly of the cell (Enari et al., 1998; Thornberry and Lazebnik, 1998). Caspase3 is the primary activator of apoptotic DNA fragmentation (Wolf et al., 1999). Our results showed that berbamine can cause activated caspase3 and caspase9 in time-dependent manner. Elevation of caspase3 and caspase9 by berbamine was initiated after 12 h of berbamine exposure and maintained to 48 h. Our other results also showed that berbamine-induced apoptosis on SMMC7721 cells could be inhibited after broad caspase inhibitor z-VAD-fmk pretreatment. It indicates that berbamine induced apoptosis is mediated by caspase-dependent pathway.
During apoptosis divergent cellular stresses like DNA damage, etc. also converge on mitochondria. Recent reports provided evidence that MTP has a key role in controlling apoptotic responses in cells (Marchetti et al., 1996). Decrease in MTP is the early change in the mitochondria-mediated apoptosis. Loss of MTP changes mitochondrial permeability that triggers opening of the permeability transition (PT) pore (Green and Reed, 1998), which has been implicated as a critical stage in apoptosis in isolated mitochondria and several cellular models (Pallis et al., 2001). PT pore opening allows release of factors, e.g., cytochrome c, that initiate the final and degradative phase of apoptosis, such as caspase3, 6, and 9, which in turn cleave a number of cellular proteins, facilitating the final destruction of the cell (Hirsch et al., 1997; Bossy-Wetzel and Green, 1999). Our studies indicated that treatment with berbamine could dramatically induce loss of ∆ψ m, in SMMC7721 cells. These data suggest berbamine-induced apoptosis also by loss in MTP.
In summary, the potential anticancer activity of berbamine against human tumor hepatocellular carcinoma was investigated. Berbamine exhibited a strong inhibitory effect on the proliferation of SMMC7721 cells in vitro. The anticancer activity of berbamine could be attributed in part to its inhibition of proliferation and apoptosis induction of cancer cells through causing cell cycle arrest in G0/G1 phase, loss in MTP and caspase activation. The anti-tumor effects reported here are valuable for further investigation.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30400521), the Science and Technology Department of Zhejiang Province (Nos. 2004D31026 and 2002D3007) and the Education Department of Zhejiang Province (No. 20060427), China
References
- 1.Bhattacharyya A, Lahiry L, Mandal D, Sa G, Das T. Black tea induces tumor cell apoptosis by Bax translocation, loss in mitochondrial transmembrane potential, cytochrome c release and caspase activation. Int J Cancer. 2005;117(2):308–315. doi: 10.1002/ijc.21075. [DOI] [PubMed] [Google Scholar]
- 2.Bossy-Wetzel E, Green DR. Caspases induce cytochrome c release from mitochondria by activating cytosolic factors. J Biol Chem. 1999;274(25):17484–17490. doi: 10.1074/jbc.274.25.17484. [DOI] [PubMed] [Google Scholar]
- 3.Carnero A. Targeting the cell cycle for cancer therapy. Br J Cancer. 2002;87(2):129–133. doi: 10.1038/sj.bjc.6600458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong QH, Zheng S, Xu RZ, Lu QH, He LM. Study on effect of berbamine on multidrug resistance leukemia K562/Adr cells. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24(9):820–822. (in Chinese) [PubMed] [Google Scholar]
- 5.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata SA. A caspase-activated DNase that degrades DNA during apoptosis and its inhibitor ICAD. Nature. 1998;391(6662):43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 6.Ge MZ, Liu X, Zhang Y. An experiment on immune protection function of berbamine in mice injury by irradiation. Immunol J. 1998;14:238–240. (in Chinese) [Google Scholar]
- 7.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281(5381):1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 8.Hall AJ, Wild CP. Liver cancer in low and middle income countries. BMJ. 2003;326(7397):994–995. doi: 10.1136/bmj.326.7397.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch T, Marchetti P, Susin SA, Dallaporta B, Zamzami N, Marzo I, Geuskens M, Kroemer G. The apoptosis-necrosis paradox. Apoptogenic proteases activated after mitochondrial permeability transition determine the model of cell death. Oncogene. 1997;15(13):1573–1581. doi: 10.1038/sj.onc.1201324. [DOI] [PubMed] [Google Scholar]
- 10.Hsu YL, Kuo YC, Kuo PL, Ng LT, Kuo YH, Lin CC. Apoptotic effects of extract from Antrodia camphorate fruiting bodies in human hepatocellular carcinoma cell lines. Cancer Lett. 2005;221(1):77–89. doi: 10.1016/j.canlet.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Koopman G, Reutelingsperger CMP, Kuijten GAM, Keehnen RMJ, Pals ST, van Oers MTJ. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84(5):1415–1420. [PubMed] [Google Scholar]
- 12.Küpeli E, Kosar M, Yasilada E, Husnu K, Baser C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002;72(6):645–657. doi: 10.1016/S0024-3205(02)02200-2. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Zhou ZR. The effect of berbamine on the immunoregulation of BALB/c mice. J Chin Med Univ. 1996;25:229–231. (in Chinese) [Google Scholar]
- 14.Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, Macho A, Haeffner A, Hirsch F, Geuskens M, Kroemer G. Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med. 1996;184(3):1155–1160. doi: 10.1084/jem.184.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pallis M, Grundy M, Turzanski J, Kofler R, Russell N. Mitochondrial membrane sensitivity to depolarization in acute myeloblastic leukemia is associated with spontaneous in vitro apoptosis, wild-type TP53, and vicinal thiol/disulfide status. Blood. 2001;98(2):405–413. doi: 10.1182/blood.V98.2.405. [DOI] [PubMed] [Google Scholar]
- 16.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: GLOBOCAN 2000. Int J Cancer. 2001;94(2):153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 17.Pietenpol JA, Stewart ZA. Cell cycle checkpoint signaling: cell cycle arrest versus apoptosis. Toxicology. 2002;181-182:475–481. doi: 10.1016/S0300-483X(02)00460-2. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt CA, Lowe SW. Apoptosis and therapy. J Pathol. 1999;187(1):127–137. doi: 10.1002/(SICI)1096-9896(199901)187:1<127::AID-PATH251>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 19.Sun JR, Zhang XH, He ZW, Gu Y, Yu YZ, Fang YM, Lu QH, Dong QH, Xu RZ. The mechanism of apoptosis of chronic myeloid leukemia cells induced by the novel p210 bcr/abl inhibitor berbamine. Zhonghua Yi Xue Za Zhi. 2006;86(32):2246–2251. (in Chinese) [PubMed] [Google Scholar]
- 20.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 21.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281(5381):1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 22.Wang XJ, Wei YQ, Yuan SL, Liu GJ, Lu YR, Zhang J, Wang WD. Potential anticancer activity of tanshinoe IIA against human breast cancer. Int J Cancer. 2005;116(5):799–807. doi: 10.1002/ijc.20880. [DOI] [PubMed] [Google Scholar]
- 23.Wolf BB, Schuler M, Echeverri F, Green DR. Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J Biol Chem. 1999;274(43):30651–30656. doi: 10.1074/jbc.274.43.30651. [DOI] [PubMed] [Google Scholar]
- 24.Wu D, Lin MF, Zhao XY. Effects of berbamine on K562 cells and its mechanisms in vitro and in vivo. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005;13(3):373–378. (in Chinese) [PubMed] [Google Scholar]
- 25.Xu CQ, Dong DL, Du ZM, Chen DW, Gong DM, Yang BF. Comparison of the anti-arrhythmic effects of matrine and berbamine with amiodarone and RP58866. Yao Xue Xue Bao. 2004;39(9):691–694. (in Chinese) [PubMed] [Google Scholar]
- 26.Xu RZ, Dong QH, Yu Y, Zhao X, Gan X, Wu D, Lu Q, Xu X, Yu XF. Berbamine: a novel inhibitor of bcr/abl fusion gene with potent anti-leukemia activity. Leuk Res. 2006;30(1):17–23. doi: 10.1016/j.leukres.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 27.Yang K, Zhao X, Xiao P, Liu G. Clinical trial of berbamine in 405 leukopenia patients. Yao Hsueh Tung Pao. 1982;17:21–22. (in Chinese) [Google Scholar]
- 28.Zhu XW, Sui WZ. Experimental study on the anti-tumor action of berbamine in mice. Zhong Xi Yi Jie He Za Zhi. 1986;6(10):611–613. (in Chinese) [PubMed] [Google Scholar]