Abstract
Idiopathic pulmonary arterial hypertension (IPAH) is a rare disease of unknown etiology. The exact pathogenesis of pulmonary arterial hypertension is still not well known. In the past decades, many protein molecules have been found to be involved in the development of IPAH. With proteomic techniques, profiling of human plasma proteome becomes more feasible in searching for disease-related markers. In present study, we showed the protein expression profiles of the serum of IPAH and healthy controls after depleting a few high-abundant proteins in serum. Thirteen spots had changed significantly in IPAH compared with healthy controls and were identified by LC-MS/MS. Alpha-1-antitrypsin and vitronectin were down-regulated in IPAH and may be valuable candidates for further explorations of their roles in the development of IPAH.
Keywords: Idiopathic pulmonary arterial hypertension, Two-dimensional gel electrophoresis, LC-MS/MS
INTRODUCTION
Idiopathic pulmonary arterial hypertension (IPAH), formerly known as primary pulmonary hypertension, is a rare disorder of unknown etiology occurring more often in women than in men. Pulmonary vasoconstriction, vascular remodelling, and thrombosis in situ are thought to play important roles in the pathogenesis of PAH (Humbert et al., 2004). Over the past 2 decades, many protein molecules have been found to be involved in the development of PAH. Mutations in the bone morphogenic protein receptor-II (BMPR-II) have been shown to play a major role in familial PAH (Lane et al., 2000; Deng et al., 2000; Machado et al., 2001). Decreased expression of endothelial NO synthase is also shown in PAH cases (Giaid and Saleh, 1995), which may result in pulmonary vasoconstriction and endothelium dysfunction. All these studies suggested that alterations of protein might play important roles in the pathogenesis of PAH. So, understanding the protein expression profiles may provide a window to assess the pathological consequences occurring in IPAH.
Two-dimensional gel electrophoresis (2-DE) in combination with mass spectrometry (MS) is a high-throughput method in identifying differences of protein expression, which has been widely used in cardiovascular fields (Arrell et al., 2006; Bezstarosti et al., 2006; Gallego-Delgado et al., 2006; Li et al., 2006; Teixeira et al., 2006). Plasma proteins are useful targets for diagnostic, prognostic, and therapeutic development. With proteomic techniques, profiling of human plasma proteome becomes more feasible in searching for disease-related markers (Anderson and Anderson, 2002). It was reported that plasma level of complement 4a des Arg was significantly higher in IPAH patients compared with normal controls by surface enhanced laser desorption/ionization-time of flight-mass spectrometry (SELDI-TOF-MS) analysis of plasma samples of IPAH patients (Abdul-Salam et al., 2006). However, the proteomic analysis of plasma and serum samples represents an extreme challenge due to the presence of a few highly abundant proteins such as albumin and immunoglobulin which tend to mask those of lower abundance and thus prevent their detection and identification in proteomics studies. Hence, depletion of major proteins is one potential strategy for enhancing detection sensitivity in serum or plasma (Echan et al., 2005). Multi-dimensional separation techniques combining liquid chromatography with gel electrophoresis have been applied for plasma proteomics (Pieper et al., 2003; Okano et al., 2006). Here we reported the protein changes in the serum of IPAH patients by using 2-DE in combination with MS after depleting a few high-abundant proteins in serum.
MATERIALS AND METHODS
Materials
Immobilized pH gradient (IPG) strips (240 mm, pH 3~10 non-linear), IPG buffer (pH 3~10 linear), cover fluid, glycerol, agarose, albumin and IgG removal kit and silver staining kit were purchased from GE healthcare (London, UK). Urea, CHAPS (3-[(3-cholamidopropyl) dimethylammonio]-1-pro-panesulfenate), DTT (dithiothreitol), iodoacetamide, trypsin, ACN (acetonitrile) and TFA (trifluoroacetic acid) were obtained from Sigma (St. Louis, MO, USA). Acrylamide, bis-acrylamide, glycine, tris and SDS (sodium dodecyl sulfate) were purchased from Amresco (Solon, OH, USA). Other chemicals were domestic products (analytical grade).
IPGphor isoelectric focusing (IEF) system, DALTsix system, ImageScanner and ImageMaster 2D Elite software were purchased from GE healthcare (London, UK). SpeedVac and LTQ linear ion trap mass spectrometer were obtained from Thermo (Thermo Finnigan, San Jose, CA). Lyophilizer was purchased from Virtis (Gardiner, NY, USA).
Serum sample preparation
The study protocol was approved by the ethics review board of the First Affiliated Hospital, School of Medicine, Zhejiang University, China. Written informed consent was obtained from all participants. Twenty patients with IPAH (according to the diagnostic criteria of the National Institutes of Health Registry on IPAH) and 20 healthy volunteers as controls were included in the study. All patients were in functional class II to III (New York Heart Association functional class). Venous blood was collected and centrifuged at 2000×g for 10 min. The supernatant sera were obtained and stored at −80 °C.
One millilitre serum was obtained from each patient and healthy volunteer. The serum of patients and control group was mixed respectively. The serum albumin and IgG were removed using the albumin and IgG removal kit (GE healthcare). The protein concentrations were determined by a Bradford assay.
2-DE analysis
Isoelectric focusing (IEF) was performed using 240 mm IPG strips with IPGphor system. Two hundred micrograms protein sample containing lysis buffer was diluted with rehydration solution [8 mol/L urea, 2% (w/v) CHAPS, 0.5% (v/v) IPG buffer pH 3~10 linear, 0.002% (w/v) bromophenol blue] to 450 μl and then loaded on the strip holder. Six gels were run at once, three gels were IPAH samples, while three controls. The IPG gels were rehydrated for 12 h under 30 V at 20 °C. IEF was performed using the following parameters: 500 V for 1 h, 1000 V for 1 h, 8000 V for 8 h and 20 min. After IEF, the strips were equilibrated for 15 min in SDS equilibration buffer [6 mol/L urea, 2% (w/v) SDS, 50 mmol/L Tris-HCl pH 8.8, 30% (v/v) glycerol, 0.002% (w/v) bromophenol blue] containing 100 mmol/L DTT and then in SDS equilibration buffer containing 250 mmol/L iodoacetamide. When the equilibration was finished, the strips were loaded onto a vertical 12.5% (w/v) SDS-PAGE and sealed with 0.5% (w/v) agarose. The vertical electrophoresis was performed at 10 °C with a condition of 5 W/gel for 30 min and then 15 W/gel until the bromophenol blue dye reached the bottom of the gel.
Silver staining and imaging of the 2-D gels
Silver staining was performed according to the protocal of Plusone Silver-staining kit from GE healthcare with some modifications. The gels were first fixed over night with 40% (v/v) ethanol and 10% (v/v) acetic acid, and then incubated in the sensitizing solution [30% (v/v) ethanol, 0.2% (w/v) sodium thi-osulphate and 6.8% (w/v) sodium acetate] for 30 min. After washing three times with milli-Q water for 15 min each, the gels were stained in 0.25% (w/v) silver nitrate solution for 20 min. Development was performed in 2.5% (w/v) sodium carbonate with 0.04% (v/v) formaldehyde for 5 min, stop solution [1.46% (w/v) EDTA] was used to terminate the development. The stained gels were scanned in an ImageScanner and analyzed with ImageMaster 2D Elite software. The protein spots were detected, background subtracted and matched. The protein spots with significant changes were selected for further identification.
In-gel digestion
The selected protein spots from all gels were excised and placed in eppendorf tubes. After washed twice in milli-Q water, the gels were destained in a 1:1 mixture of 30 mmol/L potassium ferricyanide and 100 mmol/L sodium thiosulfate. The gels were then washed twice in milli-Q water, dehydrated with ACN and dried in a SpeedVac for 20 min. The gels were digested overnight with 10 µl 20 ng/µl sequencing grade trypsin at 37 °C. The peptide fragments were extracted with 30 µl solution containing 50% (v/v) ACN, 5% (v/v) TFA. The solutions containing peptide fragments were dried in a lyophilizer and reconstituted by adding 10 µl 0.1% (v/v) formic acid.
LC-MS/MS Analysis
The peptide mixtures were separated by reverse phase high performance liquid chromatography (column C18, 15 cm×150 µm, CTI, CA) followed by LTQ linear ion trap mass spectrometer. The peptides were eluted using 0.1% (v/v) formic acid in water and 0.1% (v/v) formic acid in acetonitrile. The MS/MS was performed on a LTQ linear ion trap mass spectrometer. Spectra were collected from the mass analyzer using full scan mode over the m/z range 300~2000. Three maximal signal of each full scan were obtained. The acquired MS/MS spectra were searched against the IPI human database using TurboSEQUEST program (BioWorks 3.0 software, Thermo Electron Corporation, USA). Protein identification results were filtered with the Xcorr (one charge≥1.9, two charges≥2.2, three charges≥3.75) and DelCn (≥0.1).
Statistical analysis
The data were expressed as mean±SD. The Student’s t-test was used for statistical analysis between two groups. It was considered statistically significant if P<0.05.
RESULTS
Protein expressions in serum of IPAH and healthy controls
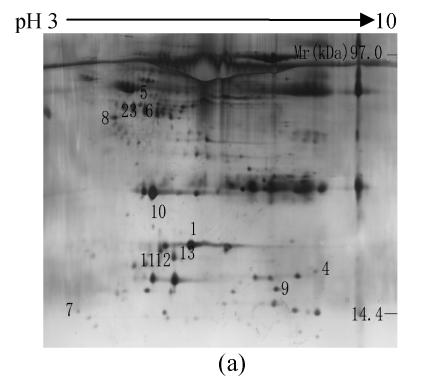
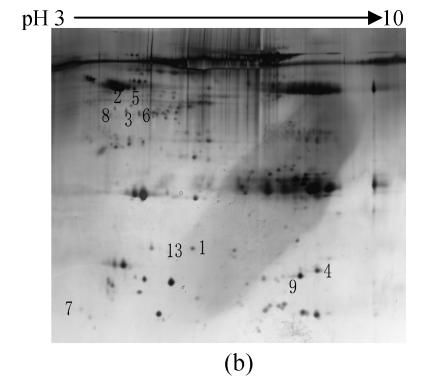
To investigate the effect of IPAH on protein expression of serum, the proteins from the serum of IPAH and healthy controls were isolated by 2-DE and silver staining. The 2-DE patterns were displayed in Fig.1. 220±58 and 212±54 spots were isolated in IPAH and controls, respectively. Compared with the control group, 13 spots had changed significantly in IPAH. One spot (spot 4) was up-regulated, nine spots were down-regulated and three spots were disappeared.
Fig. 1.
Representative 2-DE maps of serum proteins. Proteins were separated by 2-DE and visualized by silver staining. Two hundred micrograms of protein was loaded on 24 cm, non-linear IPG strips of pH 3~10 for first dimension electrophoresis, followed by vertical 12.5% SDS-PAGE for second dimension electrophoresis. (a) Healthy controls; (b) IPAH patients
Protein identification by LC-MS/MS
Protein spots with significant changes were excised and subjected to trypsin digestion, LC-MS/MS and data searching. The total 13 spots were identified (Table 1). Two proteins (haptoglobin and albumin protein) were expressed as multiple spots on the 2-D gels, suggesting that they were either charge isoforms or fragments of the same protein. A representative protein spot (spot 1) was showed in Fig.2. The spot was down-regulated in the 2-DE map of PAH compared with control group and identified as alpha-1-antitrypsin.
Table 1.
Identification of altered proteins in the serum of IPAH patients
| Spot No. | IPI accession Number | Protein name | Molecular weight | pI | Protein coverage (%) | Mean volume ratio (IPAH/healthy group) |
| 1 | IPI00553177.1 | Alpha-1-antitrypsin precursor | 46736.49 | 5.37 | 11.48 | 0.11* |
| 2 | IPI00431645.1 | Haptoglobin precursor | 31381.78 | 8.48 | 43.77 | 0.20∆ |
| 3 | IPI00218732.2 | Serum paraoxonase/arylesterase 1 | 39618.16 | 5.08 | 6.78 | 0.23∆ |
| 4 | IPI00022434.2 | Albumin protein | 71704.74 | 6.42 | 8.29 | 6.20* |
| 5 | IPI00479805.5 | Apolipoprotein A-IV precursor | 45372.16 | 5.28 | 42.93 | 0.30* |
| 6 | IPI00555812.4 | Vitamin D-binding protein precursor | 52963.76 | 5.40 | 11.60 | 0.21∆ |
| 7 | IPI00298971.1 | Vitronectin precursor | 54305.86 | 5.55 | 5.23 | 0.49* |
| 8 | IPI00166729.3 | Zinc-alpha-2-glycoprotein precursor | 33872.27 | 5.57 | 18.98 | 0.27* |
| 9 | IPI00164623.4 | Complement C3 precursor | 187306.25 | 6.02 | 2.58 | 0.06* |
| 10 | IPI00022434.2 | Albumin protein | 71704.74 | 6.42 | 14.19 | 0 |
| 11 | IPI00022434.2 | align="left"Albumin protein | 71704.74 | 6.42 | 11.80 | 0 |
| 12 | IPI00431645.1 | Haptoglobin protein | 31381.78 | 8.48 | 8.54 | 0 |
| 13 | IPI00303476.1 | ATP synthase beta chain, mitochondrial precursor | 56559.96 | 5.26 | 7.56 | 0.05∆ |
The fold differences between the IPAH group and the control group were represented by the mean volume ratio of IPAH/healthy group. Spot Nos. 10, 11 and 12 were disappeared in the serum of IPAH patients
P<0.01 vs healthy group
P<0.05 vs healthy group
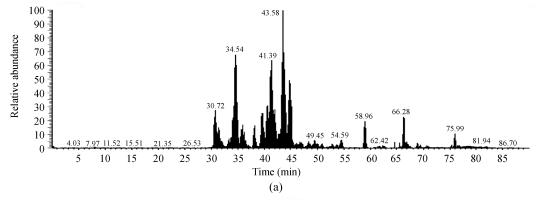
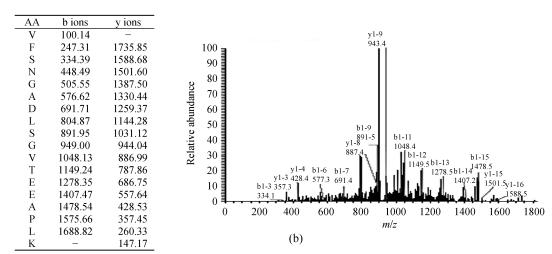
Fig. 2.
Representative protein spot identified with LC-MS/MS. (a) Base peak chromatogram of spot 1; (b) Fragmentation spectrum of the ion with m/z 918.74 from a tryptic peptide of spot 1. The identified sequence is VFSNGADLSGVTEEAPLK interpreted as y series and b series ions. The spot was identified as alpha-1-antitrypsin
DISCUSSION
To the best of our knowledge, this was the first study to report the protein changes in the serum of IPAH patients using 2-DE combined with MS. In this study we employed the proteomic techniques screening out some altered proteins in the serum of IPAH patients after fractionating the sample by depletion of a few high-abundant proteins. The expression levels of 13 protein spots, which were identified by LC-MS/MS, showed significant changes in IPAH patients compared with controls.
Alpha-1-antitrypsin, mainly produced in the liver, is a serine proteinase inhibitor. It reaches the lungs by diffusing from the circulation. Major functions of the protein are to inactivate neutrophil elastase and other proteases to maintain a protease-antiprotease balance (Lomas and Mahadeva, 2002), to protect connective tissue of the lung from degradation by elastase, and to prevent the destruction of pulmonary extracellular matrix. It is established that the deficiency of alpha-1-antitrypsin is associated with emphysema (Brantly et al., 1988). In present study, the level of alpha-1-antitrypsin was decreased in IPAH patients, which could tip the elastase-antielastase balance unfavourably towards accelerated lung breakdown and predispose to pulmonary vascular remodelling. It was reported that progression of pulmonary hypertension was associated with increased serine elastase activity. Moreover, administration of inhibitors of serine elastase to monocrotaline-exposed rats reduced changes of pulmonary hypertension (Cowan et al., 2000). In addition to elastase inhibition, alpha-1-antitrypsin could also prevent lung endothelial cell apoptosis by inhibiting caspase-3 activity (Petrache et al., 2006). The decrease of alpha-1-antitrypsin might induce to increased apoptosis of endothelial cell, which could result in proliferation of apoptosis-resistant endothelial cell (Teichert-Kuliszewska et al., 2006) and arteriolar occlusion (Zhao et al., 2005). The proliferating endothelial cells often form plexiform lesions and may also contribute to vascular wall thickening. Impairment of vascular and endothelial homeostasis was thought to play a major role in the initiation and development of PAH (Tuder et al., 2001; Napoli and Loscalzo, 2004).
Vitronectin is a multifunctional glycoprotein in plasma, platelets, and extracellular matrices (Preissner and Seiffert, 1998). It is involved in coagulation, fibrinolysis, cell attachment and spreading (Zhuang et al., 1996). Impaired endothelial cell adhesion and spreading caused by vitronectin glycation may also result in endothelial dysfunction (Price and Loscalzo, 1999). In this study, the level of vitronectin is down-regulated in IPAH patients compared with controls, suggesting that it might be associated with pulmonary endothelial dysfunction in IPAH. In addition to promoting cell adhesion and migration, vitronectin contributes to hemostasis through regulation of blood coagulation and fibrinolysis (Mayasundari et al., 2004). In situ thrombosis is thought to have a role in IPAH development. The fate of formed thrombi is determined by the balance between coagulation system and fibrinolytic system. Huber et al.(1994) reported that there was a tendency towards coagulation with impaired fibrinolytic ability in IPAH. Fay et al.(1999) observed that vitronectin-deficient mice formed occlusive arterial thrombi at an accelerated rate compared with wild-type mice, suggesting that vitronectin played an antithrombotic role at sites of arterial injury. Reheman et al.(2005) identified the different roles of plasma vitronectin (inhibition) and platelet vitronectin (enhancement) in platelet aggregation. However, it was also reported that vitronectin supports thrombus formation and stability after vascular injury (Reheman et al., 2005). The decrease of plasma vitronectin in IPAH might create a condition favouring platelet aggregation and thrombus formation. In this study, vitronectin came from the serum. However, the concentration of vitronectin in plasma does not significantly differ from that in serum (Tomasini and Mosher, 1990).
The expression of some spots such as Serum paraoxonase/arylesterase 1 (spot 3), apolipoprotein A-IV precursor (spot 5), vitamin D-binding protein precursor (spot 6), zinc-alpha-2-glycoprotein precursor (spot 8), complement C3 precursor (spot 9) and ATP synthase beta chain (spot 13) had changed significantly in IPAH. However, the roles of these proteins in the development of PAH remain unclear and need further investigation.
Limitation
Firstly, although 13 proteins were identified in IPAH patients from this experiment, their function remained to be answered. Secondly, five spots (spot 2, spot 12 and spot 4, spot 10, spot 11) were identified as haptoglobin and albumin, suggesting that the high concentration proteins in serum, which could mask the presence of low abundant proteins, were not completely removed. Depletion of albumin and IgG was not enough for improving protein profiling capacities. To deplete multiple high-abundance proteins in the prefractionation step, more efficient methods may be needed such as multi-dimensional separation techniques combining liquid chromatography with gel electrophoresis. Thirdly, some low-abundance proteins released into plasma from diseased tissues may not be detected. Lastly, a limited series of protein changes could take place after plasma was allowed to clot, which might prevent some disease-related proteins from being detected and identified.
In conclusion, this study established a serum protein database for IPAH. Thirteen proteins were identified by LC-MS/MS. Alpha-1-antitrypsin and vitronectin may be considered as candidates for further investigation of pathophysiological mechanism, and as potentially therapeutic targets for IPAH.
Acknowledgments
The authors gratefully acknowledge the assistance of Min Zheng and Shuping Li in two-dimensional gel electrophoresis analysis.
Footnotes
Project (No. A-007) supported by the Key Medicine Foundation of Zhejiang Province, China
References
- 1.Abdul-Salam VB, Paul GA, Ali JO, Gibbs SR, Rahman D, Taylor GW, Wilkins MR, Edwards RJ. Identification of plasma protein biomarkers associated with idiopathic pulmonary arterial hypertension. Proteomics. 2006;6(7):2286–2294. doi: 10.1002/pmic.200500510. [DOI] [PubMed] [Google Scholar]
- 2.Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1(11):845–867. doi: 10.1074/mcp.R200007-MCP200. [DOI] [PubMed] [Google Scholar]
- 3.Arrell DK, Elliott ST, Kane LA, Guo Y, Ko YH, Pedersen PL, Robinson J, Murata M, Murphy AM, Marban E, et al. Proteomic analysis of pharmacological preconditioning: novel protein targets converge to mitochondrial metabolism pathways. Circ Res. 2006;99(7):706–714. doi: 10.1161/01.RES.0000243995.74395.f8. [DOI] [PubMed] [Google Scholar]
- 4.Bezstarosti K, Das S, Lamers JM, Das DK. Differential proteomic profiling to study the mechanism of cardiac pharmacological preconditioning by resveratrol. J Cell Mol Med. 2006;10(4):896–907. doi: 10.1111/j.1582-4934.2006.tb00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Brantly M, Nukiwa T, Crystal RG. Molecular basis of alpha-1-antitrypsin deficiency. Am J Med. 1988;84(6A):13–31. doi: 10.1016/0002-9343(88)90154-4. [DOI] [PubMed] [Google Scholar]
- 6.Cowan KN, Heilbut A, Humpl T, Lam C, Ito S, Rabinovitch M. Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat Med. 2000;6(6):698–702. doi: 10.1038/76282. [DOI] [PubMed] [Google Scholar]
- 7.Deng ZM, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67(3):737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Echan LA, Tang HY, Ali-Khan N, Lee K, Speicher DW. Depletion of multiple high-abundance proteins improves protein profiling capacities of human serum and plasma. Proteomics. 2005;5(13):3292–3303. doi: 10.1002/pmic.200401228. [DOI] [PubMed] [Google Scholar]
- 9.Fay WP, Parker AC, Ansari MN, Zheng XX, Ginsburg D. Vitronectin inhibits the thrombotic response to arterial injury in mice. Blood. 1999;93(6):1825–1830. [PubMed] [Google Scholar]
- 10.Gallego-Delgado J, Lazaro A, Osende JI, Esteban V, Barderas MG, Gomez-Guerrero C, Vega R, Vivanco F, Egido J. Proteomic analysis of early left ventricular hypertrophy secondary to hypertension: modulation by antihypertensive therapies. J Am Soc Nephrol. 2006;17(12 Suppl. 3):S159–164. doi: 10.1681/ASN.2006080937. [DOI] [PubMed] [Google Scholar]
- 11.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333(4):214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 12.Huber K, Beckmann R, Frank H, Kneussl M, Mlczoch J, Binder BR. Fibrinogen, t-PA, and PAI-1 plasma levels in patients with pulmonary hypertension. Am J Respir Crit Care Med. 1994;150(4):929–933. doi: 10.1164/ajrccm.150.4.7921465. [DOI] [PubMed] [Google Scholar]
- 13.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary artery hypertension. N Engl J Med. 2004;351(14):1425–1436. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 14.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension: the international PPH consortium. Nat Genet. 2000;26(1):81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Xiao YB, Gao YQ, Yang TD. Comparative proteomics analysis of differentially expressed phosphoproteins in adult rat ventricular myocytes subjected to diazoxide preconditioning. Drug Metabol Drug Interact. 2006;21(3-4):245–258. doi: 10.1515/dmdi.2006.21.3-4.245. [DOI] [PubMed] [Google Scholar]
- 16.Lomas DA, Mahadeva R. Alpha 1-antitrypsin polymerization and the serpinopathies: pathobiology and prospects for therapy. J Clin Invest. 2002;110(11):1585–1590. doi: 10.1172/JCI200216782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machado RD, Pauciulo MW, Thomson JR, Lane KB, Morgan NV, Wheeler L, Phillips JA, Newman J, Williams D, Galie N, et al. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet. 2001;68(1):92–102. doi: 10.1086/316947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayasundari A, Whittemore NA, Serpersu EH, Peterson CB. The solution structure of the N-terminal domain of human vitronectin. J Biol Chem. 2004;279(28):29359–29366. doi: 10.1074/jbc.M401279200. [DOI] [PubMed] [Google Scholar]
- 19.Napoli C, Loscalzo J. Nitric oxide and other novel therapies for pulmonary hypertension. J Cardiovasc Pharmacol Ther. 2004;9(1):1–8. doi: 10.1177/107424840400900i101. [DOI] [PubMed] [Google Scholar]
- 20.Okano T, Kondo T, Kakisaka T, Fujii K, Yamada M, Kato H, Nishimura T, Gemma A, Kudoh S, Hirohashi S. Plasma proteomics of lung cancer by a linkage of multi-dimensional liquid chromatography and two-dimensional difference gel electrophoresis. Proteomics. 2006;6(13):3938–3948. doi: 10.1002/pmic.200500883. [DOI] [PubMed] [Google Scholar]
- 21.Petrache I, Fijalkowska I, Medler TR, Skirball J, Cruz P, Zhen L, Petrache HI, Flotte TR, Tuder RM. Alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169(4):1155–1166. doi: 10.2353/ajpath.2006.060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pieper R, Gatlin CL, Makusky AJ, Russo PS, Schatz CR, Miller SS, Su Q, McGrath AM, Estock MA, Parmar PP, et al. The human serum proteome: display of nearly 3700 chromatographically separated protein spots on two-dimensional electrophoresis gels and identification of 325 distinct proteins. Proteomics. 2003;3(7):1345–1364. doi: 10.1002/pmic.200300449. [DOI] [PubMed] [Google Scholar]
- 23.Preissner KT, Seiffert D. Role of vitronectin and its receptors in haemostasis and vascular remodeling. Thromb Res. 1998;89(1):1–21. doi: 10.1016/S0049-3848(97)00298-3. [DOI] [PubMed] [Google Scholar]
- 24.Price DT, Loscalzo J. Cellular adhesion molecules and atherogenesis. Am J Med. 1999;107(1):85–97. doi: 10.1016/S0002-9343(99)00153-9. [DOI] [PubMed] [Google Scholar]
- 25.Reheman A, Gross P, Yang H, Chen P, Allen D, Leytin V, Freedman J, Ni H. Vitronectin stabilizes thrombi and vessel occlusion but plays a dual role in platelet aggregation. J Thromb Haemost. 2005;3(5):875–883. doi: 10.1111/j.1538-7836.2005.01217.x. [DOI] [PubMed] [Google Scholar]
- 26.Teichert-Kuliszewska K, Kutryk MJB, Kuliszewski MA, Karoubi G, Courtman DW, Zucco L, Granton J, Stewart DJ. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival. Circ Res. 2006;98(2):209–217. doi: 10.1161/01.RES.0000200180.01710.e6. [DOI] [PubMed] [Google Scholar]
- 27.Teixeira PC, Iwai LK, Kuramoto AC, Honorato R, Fiorelli A, Stolf N, Kalil J, Cunha-Neto E. Proteomic inventory of myocardial proteins from patients with chronic Chagas’ cardiomyopathy. Braz J Med Biol Res. 2006;39(12):1549–1562. doi: 10.1590/S0100-879X2006001200005. [DOI] [PubMed] [Google Scholar]
- 28.Tomasini BR, Mosher DF. Vitronectin. Prog Hemostas Thromb. 1990;10(2):269–305. [PubMed] [Google Scholar]
- 29.Tuder RM, Cool CD, Yeager M, Taraseviciene-Stewart L, Bull TM, Voelkel NE. The pathobiology of pulmonary hypertension. Endothelium. Clin Chest Med. 2001;22(3):405–418. doi: 10.1016/S0272-5231(05)70280-X. [DOI] [PubMed] [Google Scholar]
- 30.Zhao YD, Courtman DW, Deng YP, Kugathasan L, Zhang QW, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells. Efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96(4):442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- 31.Zhuang P, Blackburn MN, Peterson CB. Characterization of the denaturation and renaturation of human plasma vitronectin: I. Biophysical characterization of protein unfolding and multimerization. J Biol Chem. 1996;271(24):14323–14332. doi: 10.1074/jbc.271.24.14323. [DOI] [PubMed] [Google Scholar]