Abstract
Background: Recent autopsy study showed a high incidence of cerebrovascular lesions in Alzheimer’s disease (AD). To assess the impact of cerebrovascular pathology in AD, we used diffusion tensor imaging (DTI) to study AD patients with and without cerebrovascular lesions. Materials and Methods: Conventional and DTI scans were obtained from 10 patients with probable AD, 10 AD/V patients (probable AD with cerebrovascular lesions) and ten normal controls. Mean diffusivity (D) and fractional anisotropy (FA) values of some structures involved with AD pathology were measured. Results: D value was higher in AD patients than in controls in hippocampus and the cingulate gyrus. In AD/V patients, increased D value was found in the same structures and also in the thalamus and basal ganglia compared to controls. There was a significant difference of D value between AD and AD/V patients. FA value reduced in the white matter of left inferior temporal gyrus and in the bilateral middle cingulate gyrus in patients with AD/V compared with controls. The MMSE (mini-mental state examination) score significantly correlated with FA value in the right hippocampus (r=0.639, P<0.019), in the right anterior cingulate gyrus (r=0.587, P<0.035) and in left parahippocampal gyrus (r=0.559, P<0.047). Conclusion: Cerebrovascular pathology had stronger impact on the D value than the AD pathology alone did. Elevated D value in thalamic and basal ganglia may contribute to cognitive decline in AD/V patients. Reduced FA values in AD/V patients may indicate that cerebrovascular pathology induced more severe white matter damage than the AD pathology alone did.
Keywords: Diffusion tensor imaging (DTI), Alzheimer’s disease (AD), Cerebrovascular, Magnetic resonance imaging (MRI)
INTRODUCTION
Diffusion tensor imaging (DTI) is a magnetic resonance imaging (MRI) technique that can be used to characterize the orientational properties of the diffusion process of water molecules (Basser et al., 1994a). Application of this technique to the brain has been demonstrated to provide exceptional information on white matter architecture. Usually, the information is conveyed into two types of parameters: mean diffusivity (D) which is a measure of the average molecular motion independent of any tissue directionality and is affected by cellular size and integrity (Basser et al., 1994b; Pierpaoli et al., 1996), and the fractional anisotropy (FA), which is one of the most used measures of deviation from isotropy and reflects the degree of alignment of cellular structures within fiber tracts, as well as their structural integrity (Basser and Pierpaoli, 1996).
Alzheimer’s disease (AD), a progressive neurodegenerative disorder, is the most common cause of dementia in the elderly. Pathological changes originate in the medial temporal region, in the entorhinal cortex and hippocampus, subsequently spreading over the entire limbic cortex and then into neocortical association areas (Braak and Braak, 1998). Prior studies showed that there were higher D values in the hippocampi, the temporal stem, the cingulum, the corpus callosum, the centrum semiovale, and the frontal, temperal, occipital and parietal white matter of AD patients than in controls (Kantarci et al., 2001; Fellgiebel et al., 2004; Bozzali et al., 2002; Naggara et al., 2006; Xie et al., 2006; Muller et al., 2005). There were lower FA values in the hippocampus, in the corpus callosum, in the cingulum, in the white matter of the frontal, temporal and parietal lobes of AD patients than in controls (Fellgiebel et al., 2004; Bozzali et al., 2002; Naggara et al., 2006; Xie et al., 2006; Takahashi et al., 2002). Cerebrovascular lesions in AD were not assessed in these prior studies. Recent autopsy studies showed high incidence of cerebrovascular lesions in AD. One study of 730 consecutive cases of AD autopsy reported cerebrovascular pathology in 48% of those AD cases. In particular, minor to moderate cerebrovascular lesions (lacunes, amyloid angiopathy with or without lacunes) were observed in 31.6% of cases and major cerebrovascular lesions (old and recent infarcts and hemorrhages) in 16.7% of cases (Jellinger and Mitter-Ferstl, 2003). A population-based longitudinal study on people over 75 also led to the same conclusion (Agüero-Torres et al., 2006).
In order to clarify whether the elevated D values and reduced FA values were associated with the cerebrovascular pathology in AD, we investigated 20 probable AD (ten AD patients without cerebrovascular lesions and ten AD patients with cerebrovascular lesions) and ten normal controls by using DTI. We focused on the regions involved with the AD pathology (white matter of inferior temporal gyrus; anterior, middle and posterior cingulate gyrus; hippocampus, parahippocampal gyrus and amygdale; lentiform nucleus; caudate nucleus; anterior thalamus and dorsal medial of the thalamus). The D and FA values of those regions were measured.
MATERIALS AND METHODS
Subject
All subjects gave their informed consent prior to their inclusion in this study. We studied 20 patients with probable AD. The patients were divided into two groups: pure AD (AD without cerebrovascular lesions) and AD/V (AD with cerebrovascular lesions). The pure AD group included 6 women and 4 men (mean age 71 years). The AD/V group included 5 women and 5 men (mean age 74 years). The National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) criteria was used for the diagnosis of clinically probable AD. Cases that satisfied criteria for probable AD and have multiple infarcts or lacunes observed on T2 and/or T2 FLAIR (fluid-attenuated inversion recovery) sequence MRI were classified as AD/V. Patients who have no multiple infarcts or lacunes observed were classified as pure AD. Ten healthy controls (6 women, 4 men; mean age 70 years) were recruited. The age difference between controls and patients was not statistically significant. The controls had no complaints of cognitive problems and no evidence of cognitive deficits on formal testing. Major systemic, psychiatric, and other neurological illnesses were carefully investigated and excluded in all subjects. Sudden onset and vascular risk factors were carefully investigated and excluded in the AD/V cases. MRI was normal in all control subjects except aging atrophy of the brain.
Neuropsychological tests
Mini-mental state examination (MMSE) was used to assess cognitive function for each patient. Three AD and three AD/V patients were severely demented (MMSE≤10), five AD and five AD/V patients were moderately demented (MMSE=11~20), and two AD and two AD/V patients was mildly demented (MMSE≥21). All controls were of the normal degree (MMSE=28~30).
MRI acquisition
MRI was obtained by using a 1.5-T Vision MRI system (GE Signa Twinspeed, USA). A standard (8HRBRAIN) head coil was used. Prior to DTI, all subjects underwent T2 FLAIR sequence: repetition time (TR) 8802 ms, echo time (TE) 120 ms, matrix 512×512, field of view (FOV) 240 mm, 5 mm thickness, and 1 mm gap. DTI data were acquired by using a single-shot, diffusion weighted echo-planar imaging sequence (TR=8000 ms, TE=80 ms). The imaging matrix was 128×128, with a field of view of 246 mm×246 mm. Transverse sections of 3-mm thickness were acquired parallel to the anterior commissure-posterior commissure line. Coronal sections were angulated, perpendicular to the anterior commissure-posterior commissure line. Scan sections covered the entire hemisphere and brainstem without gaps. Diffusion weighting was encoded along 25 independent orientations, and the b value was 1000 s/mm2. The acquisition time per dataset was approximately 9 min.
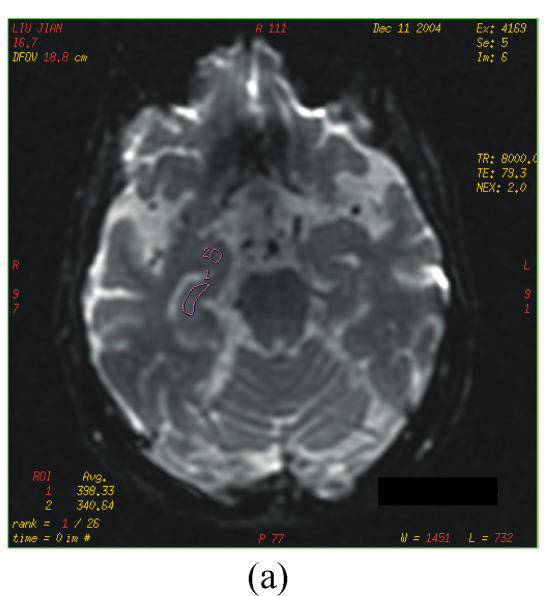
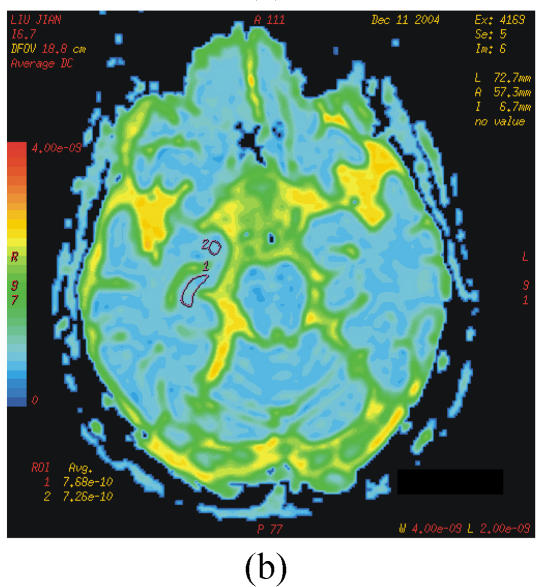
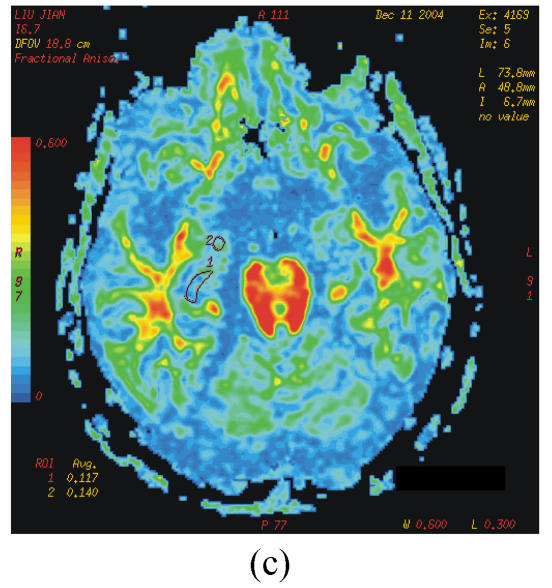
The DTI datasets were transferred to a workstation and processed by using functool LX software. D and FA were measured in different region which were carefully selected on the dual echo scans to avoid partial volume averaging from the CSF (cerebrospinal fluid). Regions of interest (ROIs) were range 20~60 mm2. The selected areas appeared normal on T2 image and avoided vascular lesions. The following areas were measured bilaterally: the white matter of inferior temporal gyrus; anterior, middle and posterior cingulate gyrus; hippocampus, parahippocampal gyrus and amygdale; lentiform nucleus; caudate nucleus; anterior thalamus and dorsal medial thalamus (Figs.1a~1c).
Fig. 1.
b=0 s/mm2 image from the echo planar imaging (EPI) sequence for DTI (a), apparent diffusion coefficient (ADC) (b) and FA images (c)
ROIs for measurement of D and FA indices: 1=hippocampus; 2=amygdale
Statistical analysis
Non-parametric Mann-Whitney U tests were used to compare D and FA values measured from patients and healthy controls. The correlations between D values, FA values and the MMSE scores were investigated using Spearman Rank Correlation Coefficient.
RESULTS
D were significantly higher in the bilateral hippocampus and right posterior cingulate gyrus from pure AD patients than in the corresponding regions from healthy controls (Table 1). D were also significantly higher in the left hippocampus, in the bilateral dorsal medial thalamus and anterior thalamus, as well as in the right anterior and middle cingulate gyrus, in the right caudate nucleus, in the bilateral lentiform nucleus from patients with AD/V than in the corresponding regions from healthy controls (Table 1). D values in pure AD patients differ significantly from those in AD/V in the bilateral dorsal medial thalamus and anterior thalamus, as well as in the right anterior and middle cingulate gyrus, in the right caudate nucleus, in the right lentiform nucleus (Table 1). FA were significantly lower in the white matter of left inferior temporal gyrus and in the bilateral middle cingulate gyrus from patients with AD/V than in the corresponding regions from healthy controls (Table 2). There were no significant differences in FA between pure AD patients and controls (Table 2). The MMSE score significantly correlated with FA value in the right hippocampus (r=0.639, P<0.019), in the right anterior cingulate gyrus (r=0.587, P<0.035) and left parahippocampal gyrus (r=0.559, P<0.047).
Table 1.
Mean (SD) D values of the selected areas from pure AD, AD/V patients and controls
|
D (m2·s−1×10−10) |
P values |
|||||
| Pure AD | AD/V | Co | AD vs Co | AD/V vs Co | AD vs AD/V | |
| Left hippocampus | 8.46 (0.88) | 9.15 (0.66) | 7.76 (0.33) | 0.027 | 0.005 | 0.079 |
| Right hippocampus | 8.55 (0.89) | 8.71 (1.15) | 7.94 (0.91) | 0.046 | 0.106 | 0.661 |
| Left dorsal medial thalamus | 7.06 (0.38) | 8.46 (1.07) | 6.87 (0.24) | 0.563 | 0.007 | 0.008 |
| Right dorsal medial thalamus | 7.01 (0.49) | 8.01 (0.65) | 6.99 (0.24) | 0.954 | 0.019 | 0.019 |
| Left anterior thalamus | 7.05 (0.32) | 8.71 (1.49) | 7.13 (0.29) | 0.372 | 0.014 | 0.027 |
| Right anterior thalamus | 7.04 (0.27) | 7.88 (0.65) | 6.98 (0.35) | 0.808 | 0.023 | 0.014 |
| Right anterior cingulated gyrus | 7.52 (0.41) | 8.21 (0.55) | 7.41 (0.25) | 0.529 | 0.008 | 0.048 |
| Right middle cingulated gyrus | 6.66 (0.27) | 7.23 (0.44) | 6.63 (0.30) | 0.793 | 0.040 | 0.023 |
| Right posterior cingulated gyrus | 7.25 (0.79) | 7.13 (0.54) | 6.66 (0.24) | 0.046 | 0.188 | 0.884 |
| Right audate nucleus | 7.00 (0.57) | 9.69 (2.39) | 7.01 (0.09) | 0.862 | 0.004 | 0.008 |
| Left lenticular nucleus | 6.52 (0.28) | 7.85 (0.93) | 6.47 (0.24) | 0.487 | 0.042 | 0.057 |
| Right lenticular nucleus | 6.75 (0.67) | 8.12 (1.00) | 6.46 (0.36) | 0.325 | 0.008 | 0.027 |
Note: Mann-Whitney U tests for non-parametric data; Co: Controls
Table 2.
Mean (SD) FA values of the selected areas from pure AD, AD/V patients and controls
|
FA |
P values |
|||||
| Pure AD | AD/V | Co | AD vs Co | AD/V vs Co | AD vs AD/V | |
| Left middle cingulated gyrus | 0.505 (0.09) | 0.400 (0.06) | 0.515 (0.08) | 0.753 | 0.010 | 0.057 |
| Right middle cingulated gyrus | 0.472 (0.06) | 0.395 (0.11) | 0.515 (0.06) | 0.189 | 0.013 | 0.143 |
| Left inferior temporal gyrus | 0.280 (0.06) | 0.245 (0.02) | 0.286 (0.04) | 0.600 | 0.048 | 0.188 |
Note: Mann-Whitney U tests for non-parametric data; Co: Controls
In AD/V patients, infarcts and lacunes were detected in the cortical and/or subcortical area of frontal lobe, temperal lobe and parietal lobe, in the basic ganglia, in the centrum semiovale and in the external capsule, and also white matter hyperintensity were detected on T2 FLAIR sequence MRI.
DISCUSSION
Several studies showed that D value of the hippocampus and posterior cingulate gyrus were significantly higher in patients with AD than in normal control subjects (Kantarci et al., 2001; Fellgiebel et al., 2004; Rose et al., 2000). Our findings are in agreement with those in prior studies. It was postulated that this increase in D value reflects loss of neural cell, decreased fiber density, including the disruption and loss of axonal membranes or myelin (Kantarci et al., 2001).
In the prior DTI studies on AD patients, impact of cerebrovascular pathology was not discussed. Our data showed D value differs not only between patients with AD/V and controls, but also between AD group and AD/V group. The different D value of these two groups is not caused by the severity of the disease because the patients in AD and AD/V groups are chosen in proportion according to the severity of their cognitive dysfunction.
The significant elevated D values in AD/V patients might be due to the synergistic impact of AD pathology and cerebrovascular pathology. Autopsy study showed AD with vascular lesions cases had less severe AD pathology than those with AD alone (Riekse et al., 2004). Recent studies showed lower density of plaques and tangles in brains with cerebrovascular lesions (Jellinger, 2002). Thus in AD/V patients elevated D would be due to other changes than those associated to AD pathology. D value from AD/V patients is significantly higher than that from pure AD patients, which may indicate cerebrovascular pathology had stronger impact on D value than AD pathology alone did. Primary infarct and lacunes could demonstrate large elevated D values and reduced FA values that would confound the results, so we avoided these areas when we measure D value and FA value. Nevertheless infarct in AD/V could cause anterograde degeneration of the fibre pathways. Neuropathological studies indicated lacunes and demyelination often co-exist (95% of cases with lacunes had white matter demyelination) (Gold et al., 2005). The disruption of myelin and axons might be expected to increase the mean diffusivity of water molecules. In addition, the microvascular change might also contribute to higher D values. Microvascular changes with decreased density and structural abnormalities causing regional metabolic and blood-brain barrier dysfunctions with ensuing neuronal damage would lead to increased diffusion of water molecules.
Morphological substrates of cognitive decline associated with cerebrovascular disease are still strongly debated. In the cardiovascular health study, 23% of individuals older than age 65 had MRI evidence of lacunes; however, 89% were essentially clinically silent from a cognitive point of view. However, neuropathological studies indicated that small macroinfarcts and even microscopic ischemic lesions can lead to dementia. In a recent autopsy study of 72 cases, the researchers found thalamic and basal ganglia but not deep white matter lacunes are independent predictors of cognitive decline. They suggested the importance of location in defining the cognitive impact of lacunes (Gold et al., 2005). In the present data, the elevated D value in the thalamic and basal ganglia in AD/V patients may contribute to dementia progress.
Reduced FA value found in the white matter of left inferior temporal gyrus, in the bilateral middle cingulate gyrus from patients with AD/V indicated disturbed integrity of white matter tracts in this region. This alteration might be due to the breakdown of the myelin sheath and disintegration of axonal microfilaments caused by Wallerian degeneration. In pure AD patients FA did not differ from that in controls. Prior studies showed decreased FA in various regions of the brain in AD patients (Fellgiebel et al., 2004; Bozzali et al., 2002; Naggara et al., 2006; Xie et al., 2006; Takahashi et al., 2002), but the regions detected with decreased FA were different in different studies. There are some reasons for the discrepancy between findings in the prior studies and our study. One reason is that we did not measure D and FA in some structures such as the corpus callosum and the frontal and parietal white matter. Second, the small group size of our data and prior studies might cause different results. Third, the figures in some of those studies showed multiple infarcts, lacunar, as well as white matter hyperintensity and even the selected areas measured for D values and FA value showed hyperintensity on T2 image (Fellgiebel et al., 2004; Bozzali et al., 2002; Rose et al., 2000). We speculate that the results of these reports might reflect mixing changes due to cerebrovascular and pure AD pathology. The cerebrovascular damage might cause the differences, because cerebrovascular lesions are usually sporadic and asymmetric. Our results may suggest that AD with cerebrovascular pathology have more severe white matter damage. The correlations found between the MMSE score and FA value in the right hippocampus, in the left parahippocampal gyrus and in the right anterior cingulate gyrus suggested that damage of these structures contribute to the cognitive impairment of AD. These findings are consistent with prior observations (Petrella et al., 2003).
In conclusion, cerebrovascular pathology had stronger impact on the D value than the AD pathology alone did. Elevated D value in thalamic and basal ganglia may contribute to cognitive decline in AD/V patients. Reduced FA values in AD/V patients may indicate that cerebrovascular pathology induced more severe white matter damage than the AD pathology alone did. Damage of the right hippocampus, the left parahippocampal gyrus and the right anterior cingulate gyrus may contribute to the cognitive impairment of AD patients.
This conclusion is limited by the small number of enrolled patients and controls. Future studies of largescale sample have to be done to examine the relationship of DTI changes to pathology of AD.
Acknowledgments
This work complies with the current laws of the People’s Republic of China. The work has got the approval of the Ethics Committee.
References
- 1.Agüero-Torres H, Kivipelto M, von Strauss E. Rethinking the dementia diagnoses in a population-based study: what is Alzheimer’s disease and what is vascular dementia? A study from the Kungsholmen Project. Dement Geriatr Cogn Disord. 2006;22(3):244–249. doi: 10.1159/000094973. [DOI] [PubMed] [Google Scholar]
- 2.Basser PJ, Pierpaoli C. Microstructural features measured using diffusion tensor imaging. J Magn Reson B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 3.Basser PJ, Mattiello J, Le Bihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basser PJ, Mattiello J, Le Bihan D. Estimation of the effective self-diffusion tensor from the NMR spin-echo. J Magn Reson B. 1994;103(3):247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 5.Bozzali M, Falini A, Franceschi M, Cercignani M, Zuffi M, Scotti G, Comi G, Filippi M. White matter damage in Alzheimer’s disease assessed in vivo using diffusion tensor magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2002;72(6):742–746. doi: 10.1136/jnnp.72.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer’s disease. J Neural Transm Suppl. 1998;53:127–140. doi: 10.1007/978-3-7091-6467-9_11. [DOI] [PubMed] [Google Scholar]
- 7.Fellgiebel A, Wille P, Müller MJ, Winterer G, Scheurich A, Vucurevic G, Schmidt LG, Stoeter P. Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: a diffusion tensor imaging study. Dement Geriatr Cogn Disord. 2004;18(1):101–108. doi: 10.1159/000077817. [DOI] [PubMed] [Google Scholar]
- 8.Gold G, Kovari E, Herrmann FR, Canuto A, Hof PR, Michel JP, Bouras C, Giannakopoulos P. Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke. 2005;36(6):1184–1188. doi: 10.1161/01.STR.0000166052.89772.b5. [DOI] [PubMed] [Google Scholar]
- 9.Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109(5-6):813–836. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- 10.Jellinger KA, Mitter-Ferstl E. The impact of cerebrovascular lesions in Alzheimer disease—a comparative autopsy study. J Neurol. 2003;250(9):1050–1055. doi: 10.1007/s00415-003-0142-0. [DOI] [PubMed] [Google Scholar]
- 11.Kantarci K, Jack CRJr, Xu YC, Campeau NG, O′Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, et al. Mild cognitive impairment and Alzheimer disease: regional diffusivity of water. Radiology. 2001;219(1):101–107. doi: 10.1148/radiology.219.1.r01ap14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller MJ, Greverus D, Dellani PR, Weibrich C, Wille PR, Scheurich A, Stoeter P, Fellgiebel A. Functional implications of hippocampal volume and diffusivity in mild cognitive impairment. Neuroimage. 2005;28(4):1033–1042. doi: 10.1016/j.neuroimage.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 13.Naggara O, Oppenheim C, Rieu D, Raoux N, Rodrigo S, Dalla Barba G, Meder JF. Diffusion tensor imaging in early Alzheimer’s disease. Psychiatry Res. 2006;146(3):243–249. doi: 10.1016/j.pscychresns.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Petrella JR, Coleman RE, Doraiswamy PM. Neuroimaging and early diagnosis of Alzheimer disease: a look to the future. Radiology. 2003;226(2):315–336. doi: 10.1148/radiol.2262011600. [DOI] [PubMed] [Google Scholar]
- 15.Pierpaoli C, Jezzard P, Basser PJ, Blarnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 16.Riekse RG, Leverenz JB, McCormick W, Bowen JD, Teri L, Nochlin D, Simpson K, Eugenio C, Larson EB, Tsuang D. Effect of vascular lesions on cognition in Alzheimer’s disease: a community-based study. J Am Geriatr Soc. 2004;52(9):1442–1448. doi: 10.1111/j.1532-5415.2004.52405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose SE, Chen F, Chalk JB, Zelaya FO, Strugnell WE, Benson M, Semple J, Doddrell DM. Loss of connectivity in Alzheimer’s disease: an evaluation of white matter tract integrity with colour coded MR diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2000;69(4):528–530. doi: 10.1136/jnnp.69.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi S, Yonezawa H, Takahashi J, Kudo M, Inoue T, Tohgi H. Selective reduction of diffusion anisotropy in white matter of Alzheimer disease brains measured by 3.0 Tesla magnetic resonance imaging. Neurosci Lett. 2002;332(1):45–48. doi: 10.1016/S0304-3940(02)00914-X. [DOI] [PubMed] [Google Scholar]
- 19.Xie S, Xiao JX, Gong GL, Zang YF, Wang YH, Wu HK, Jiang XX. Voxel-based detection of white matter abnormalities in mild Alzheimer disease. Neurology. 2006;66(12):1845–1849. doi: 10.1212/01.wnl.0000219625.77625.aa. [DOI] [PubMed] [Google Scholar]