Abstract
The yeast strain (Y18) was isolated from a soil sample collected from Fildes Peninsula, Antarctica. The strain is a psychrophilic yeast with optimum and maximum growth temperatures of 10 °C and 18 °C, respectively. Teliospores were formed after 7 d on malt agar, when the germination of teliospores was observed. Both inositol and D-glucuronate were assimilated. Positive results of the DBB (diazonium blue B) color reaction, urease test, and starch formation were observed. The major CoQ is Q8. All results indicated that Y18 belongs to the genes of Mrakia. The 18S rDNA sequence analyses showed that Y18 is closely related to Mrakia frigida. DNA-DNA relatedness study, and some biochemistry characteristics indicated that Y18 represents a new species for which Mrakia psychrophila sp. nov. is proposed.
Keywords: Mrakia psychrophila, Psychrophilic yeast, 18S rDNA sequence, Teliospores
INTRODUCTION
Psychrophiles are more likely to be found in permanently cold environments such as polar region, marine environment, and deep water (Sabri et al., 2001; D′Amico et al., 2006). In these environments, psychrophilic and psychrotrophic microorganisms are believed to play key roles in the biodegradation of organic matter and the cycling of essential nutrients (Welander, 2005; Lambo and Patel, 2006). Psychrophilic yeasts are very important due to their physiological adaptation and contributions to soil fertility, and they also have potential application in biotechnology. Ruberto et al.(2005) isolated some Rhodococcus strains having the ability to degrade hydrocarbon at low temperature. Cold active enzymes from psychrophilic yeasts can be applied to the food industry, for clarification of fruit juice and other fields at low temperature. Scorzetti et al.(2000) isolated a Cryptococcus adeliensis sp. nov. from Terre Adelie, Antarctica, which produced a cold-active xylanase. Nakagawa et al.(2004) isolated some psychrophilic yeast strains of Cryptococcus cylindricus and Mrakia frigida from soil of Abashiri. The isolated strains can grow on pectin as the only carbon at below 5 °C, and showed the activities of several cold-active pectinolytic enzymes. Cold active lipase A and lipase B from Candida antarctica have been expressed at Candida Antarctica and Escherichia coli, respectively, for the biotechnological application (Pfeffer et al., 2006; Liu et al., 2006). Kourkoutas et al.(2002) also reported the psychrophilic yeast can be used in low temperature fermentation.
Leucosporidium was first introduced by Fell et al.(1969) as a heterobasidiomycetous yeast genus. Three types of CoQ system in this genus were found. The Q10 system in L. capsuligenan and L. antaucticum, the Q9 system in L. scottii, and the Q8 system in L. frigidum, L. gelidum, L. nivale and L. stokesiin. The species with Q8 system was later classified as the Mrakia species by Yamada and Komagata (1987). Diaz and Fell (2000) studied the internal transcribed spacer (ITS) and intergenic spacer (IGS) regions of rDNA from four psychrophilic Mrakia species. Babeva et al.(2002) reported a new species of Mrakia curviuscula from forest substrates collected in the central part of European Russia. There are 5 species in the Mrakia genus, with most of them being psychrophiles or psychrotrophs, and all of them isolated from low temperature environments. Recently, López-Archilla et al.(2004) isolated some Mrakia species from Tinto River. Margesin et al.(2005) studied the cold-active pectate lyases from psychrophilic Mrakia frigida. We report here the isolation and characteristics of a psychrophilic yeast strain Y18, isolated from soil samples near the Great Wall Station of China in Fildes Peninsula, Antarctica.
MATERIALS AND METHODS
Yeast isolation
The soil samples were collected from Fildes Peninsula, Antarctica. Aliquots of the samples were suspended in malt extract and incubated on a rotary shaker at 0~5 °C for 3 d. Then the suspensions were diluted and spread on malt agar plates. After being incubated at 10 °C for 7 d, the colonies that appeared on the plates were replicated; one set was incubated at 10 °C and the other at 20 °C. The colonies that grew at 10 °C but did not at 20 °C were collected.
Morphological, physiological and biochemical characterization
The morphological and physiological properties of the isolated strain were examined by using the methods of van der Walt and Yarrow (1984). The studies of the life cycles were based on the methods of Fell et al.(1969). The range of growth temperatures and optimum temperature were determined by the method of van Uden (1984). All tests were performed at 10 °C unless otherwise indicated. Ubiquinones were extracted and purified by using the method of Yamada and Kondo (1973). The major ubiquinone was identified by high performance liquid chromatography (HPLC) as described by Nakase and Suzuki (1988).
18S rDNA sequencing and phylogenetic analysis
The 18S rDNA region of strain Y18 was amplified by polymerase chain reaction (PCR) as described by Takashima and Nakase (1996) using a pair of primers: P1 5′-TACCTGGTTGATCCTGCCAG-3′ and P2 5′-TAATGTCCTTCCGCAGGTCAC-3′. The PCR products were purified, ligated with pGEM-T vector, and then transformed into E. coli JM109. The recombinant plasmid containing the 18S rDNA was isolated and the whole sequence of the 18S rDNA fragment was determined. The 18S rDNA sequence of the strain Y18 is deposited in GenBank with the accession number being AJ223490. The 18S rDNA sequence of Y18 was aligned with that of other concerned yeast species by using Clustal W program version 1.7 (Thompson et al., 1994). The phylogenetic tree was constructed with Treecon for Windows program version 1.2 (van de Peer and de Wachter, 1994) using Neighbor-Joining analysis. Bootstrap sampling was performed based on 100 replicates. The following references of 18S rDNA sequences were obtained from the nucleotide sequence databases (DDBJ, EMBL and GenBank): Filobasidiella neoformans (M55625), Tremella globipora (U00976), Tremella moriformis (U90977), Trichosporon cutaneum (X60182), Bullera crocea (D31648), Tremella foliacea (L22262), Filobasidium floriforme (D13469), Cryptococcus albidus (D31655), Mrakia psychrophila Y18 (AJ223490), Mrakia frigida (D12802), Phaffia rhodozyma (D31656), Leucosporidium lari-marini (D12805), Cystofilobasidium capitatum (D12801), Erythrobasidium hasegawianum (D12803), Rhodosporidium toruloides (D12806), Rhodotorula glutinis (X69853), Leucosporidium scottii (X53499), Sporobolomyces roseus (X60181), Sporidiobolus johnsonii (L22261).
DNA base composition and DNA-DNA relatedness
DNA was isolated and purified by the methods of Zhu et al.(1993). DNA base composition was determined by the method of Ruan et al.(1990). For the DNA-DNA relatedness test, DNA probes were labeled with [α-32P]dCTP following the instruction of the nick translation kit (Boehringer Mannheim, Germany). Unlabelled DNA was dotted on the nitrocellulose membrane with the concentration of 25 μg/cm2. DNA hybridization was carried out according to the method of Molecular Cloning, a Laboratory Manual (2nd Ed.,1989).
RESULTS
Phenotypical characteristics and generic assignment
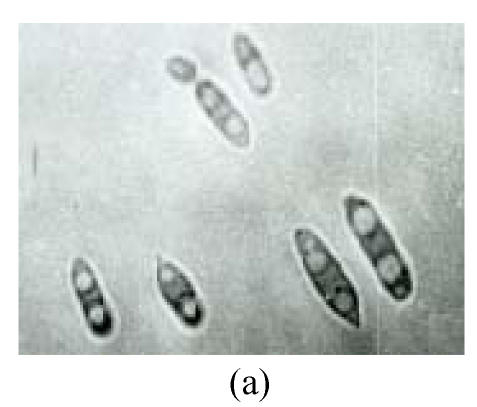
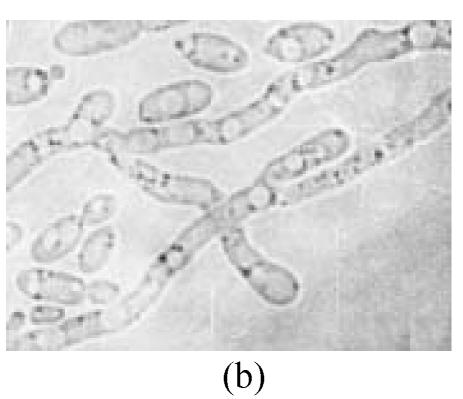
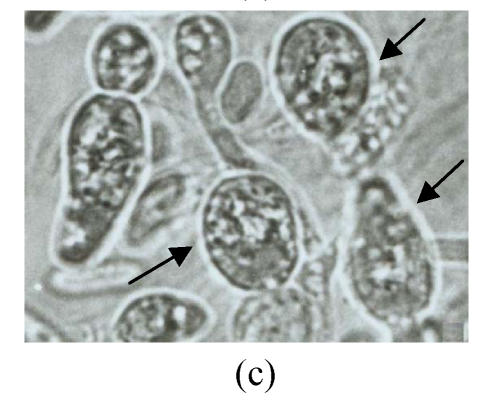
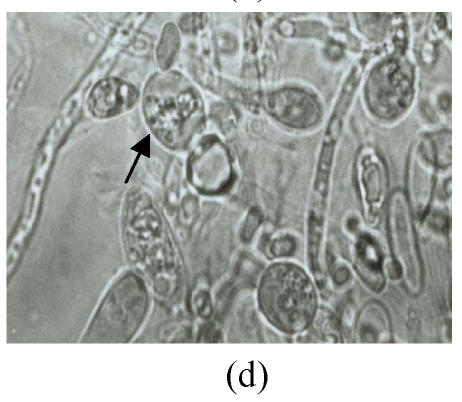
Twelve psychrophilic yeast strains were isolated from soil samples of Fildes Peninsula, Antarctica. One strain designated as Y18 was selected for further studies for its low optimum temperature. Y18 is a psychrophilic yeast that can grow at 0 °C and at optimum and maximum growth temperatures of 10 °C and 18 °C, respectively. The DBB (diazonium blue B) color reaction, urease test, and starch formation were positive. The major ubiquinone system was Q8. The comparison of biochemical features between Y18 and other strains of Mrakia are shown in Table 1. After one week on malt agar at 10 °C, hypha without clamp connection were produced. Teliospores formed after one week growth on malt agar. Pieces of agar with teliospores were dissected from the plate and placed in sterile distilled water and stored at 10 °C for 2 to 10 weeks. The teliospores were then streaked on agar plates (2%) and incubated at 10 °C. One-celled promycelium with terminal sporidia were observed after one week (Fig.1).
Table 1.
DNA-DNA relatedness among Y18 and phylogenetically closely related species
| Species | Y18 | M. frigida | M. gelida | M. nivalis | M. stokesii | M. curviuscula |
| mol% G+C | 59 | 54.1 | 56.1 | ND | ND | 49.3~50 |
| Y18 homology (%) with | 100 | 30 | 24 | 22 | ND | ND |
Fig. 1.
Morphological feathers of yeast strain Y18 (×1000). (a) Vegetative cells, after cultivation for 3 d in malt extract at 10 °C; (b) Mycelia, after growing on malt agar for 7 d at 10 °C; (c) Teliospores (arrow) after one week on malt agar; (d) One-celled promycelium with terminal sporidia (arrow)
Phylogenetic analysis and DNA-DNA relatedness
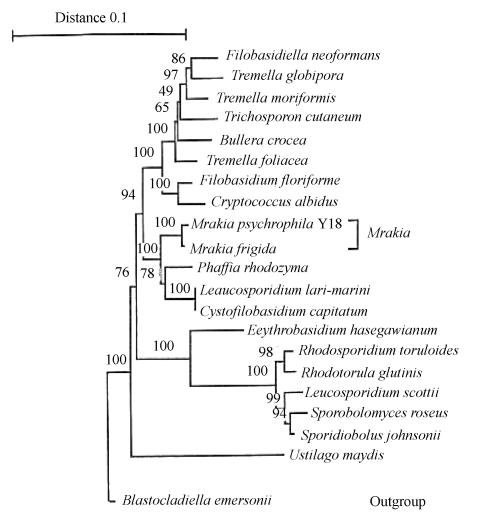
The complete 18S rDNA region of Y18 was sequenced and 1 806 bases were determined. Based on the alignment of the sequences compared, a phylogenetic tree was constructed using Neighbor-Joining method (Fig.2). The strain Y18 was clustered together with Mrakia frigida. Subsequently, DNA-DNA homology values of Y18 with other species of Mrakia were examined. The data (Table 1) indicated that Y18 represents a new species clearly distinct from its phylogenetically closely related species. With the generic assignment consideration based on phenotypic characters as discussed above, and the finding that the strain is clustered together with Mrakia frigida, a new name of Mrakia psychrophila is proposed for Y18 with the specific epithet referring to the psychrophilic property of the species.
Fig. 2.
The phylogenetic tree depicting the relationships of the strain Y18 with other basidiomycetous yeasts based on 18S rDNA sequences. The tree was constructed by the Neighbor-Joining method and the Kimura two parameter calculation models. The numerals represent the confidence level from 100 replicate bootstrap sampling. The scale bar indicates the 0.1 substitutions per nucleotide position
Latin diagnosis of Mrakia psychrophila sp. nov.
In extracto malti 10 °C post 3 dies cellulae cylindricae, singulae, binae, aut catenatae, (3.0~4.5) μm×(7.5~15) μm, opt. temp. 10 °C, max. temp. 18 °C, crescentes ad 0 °C, generatae per gemmantem polarem. Post 30 dies pelliculae non efformatae sed cum sedimento ponderoso. In agaro malti post 7 dies colonia laevis, cremea in colori, mucosa et mollis in textura. Hyphae e cellulis singulis, uninucleatae, sine septis nodosis. Teliosporae intercalares aut terminales, sphericae aut ovoideae, (5~7) μm×(4~8) μm, incrementum ad (7~8) μm×(9~14) μm, singulae aut brevicatenatae.
Fermentat Glucosum (lente), sucrosum (lente), cellobiosum (lente). Non fermentat galactosum, maltosum, melibiosum, lactosum, melezitosum, raffinosum, inulinum.
Assimilat galactosum, sorbosum (exigue), D-glucosaminum, D-ribosum (exigue), D-xylosum, L-arabinosum, D-arabinosum, rhamnosum, maltosum, trehalosum, α-methyl-D-glucosidum, salicinum, arbutinum, melibiosum, lactosum, raffinosum, melezitosum, inulinum, amylum solubile, glycerolum, ergthritolum, ribitolum, xylitolum, D-glucitolum, mannitolum, inositolum, D-glucono-1,5-lactonum, D-gluconatum, D-glucuronatum (exigue), lacticum, succinicum, citricum, methanolum (exigue), ethanolum, kalium nitricum, ethylaminum, L-lysinum, creatinum, creatininum. Non assimilat erythritolum, DL-lacticum, citricum, sodiinitrosum.
Crescit sine vitamines; amylum formatur; findendum arbutini absens; incrementum in 50% agaro glucosi extracti fermenti positivum; urea finduntur; Diazonium Blue B respondet; cum cycloheximido 0.01% crescit; G+C, 59.0 mol%; ubiquinonum majus, Q8.
Holotypus: AS 2.1971 (originaliter ut Y18) conseratur in collectionibus culturarum quas ‘China General Microbiological Culture Collection Center’, Instituti Microbiologici Academiae Sinicae, Beijing sustentat.
Description of Mrakia psychrophila sp. nov.
Growth in malt extract: After 3 d at 10 °C, the cells were cylindrical, (3~4.5) μm×(7.5~15) μm, (Fig.1a), reproducing by polar budding. After one month there was no formation of pellicle but thick sediment. Growth on malt agar: after one week, the colony was smooth, cream-colored, mucousy and soft in texture. The colony border was fringed with mycelia. Mycelia without clamp connections were present (Fig.1b), and the telispores were formed terminally on the mycelia (Fig.1c). Germination of teliospores was observed and one-celled promycelium with terminal sporidia was observed (Fig.1d). The fermentation and assimilation of carbon and nitrogen source are shown in Table 2.
Table 2.
Comparison of biochemical feature between Y18 and other strains of Mrakia
| Characteristic | Reaction of strains |
|||||
| Y18 | M. frigida | M. gelida | M. nivalis | M. stokisii | M. curviuscula | |
| Isolated site | Antarctic soil | Antarctic soil | Antarctic soil | Antarctic soil | Antarctic soil | Forest of Russia |
| Maximum temperature (°C) | 18 | 19 | 20 | 20 | 20 | 25 |
| Fermentation of | ||||||
| Sucrose | + | D | D | D | V | nd |
| Cellobiose | D | − | − | − | − | nd |
| Assimilation of | ||||||
| Sorbose | + | V | + | V | V | − |
| D-Ribose | + | − | V | V | V | s |
| D-Arabinose | + | D | V | D | − | V |
| Maltose | + | − | + | − | + | + |
| Methyl | + | − | V | − | − | nd |
| Arbutin | − | + | + | + | + | nd |
| Melibiose | + | − | + | V | − | − |
| Raffinose | + | + | + | + | + | − |
| Melezitose | + | − | + | − | + | + |
| Inulin | + | − | − | − | − | − |
| Starch | + | − | + | − | + | V |
| Glycerol | + | − | V | V | V | − |
| myo-Inositol | + | D | V | V | V | − |
| DL-Lactate | − | V | V | D | V | s |
| Citrate | − | + | V | + | + | + |
| Methanol | + | − | − | − | − | nd |
| NaNO2 | − | + | + | + | + | nd |
| Ethylamine | + | − | − | − | − | nd |
| L-Lysine | + | V | + | − | + | nd |
| Creatine | + | − | − | − | − | nd |
| Creatinine | + | − | − | − | − | nd |
| Others | ||||||
| Without vitamin | + | − | − | − | − | − |
| With 50% glucose (w/v) | + | − | − | − | − | nd |
Note: “−”, “+”, “D”, “s”, “V”, “nd” stand for “negative”, “positive”, “delayed positive”, “slow growth”, “variable growth”, and “not determined”, respectively
Maximum growth temperature: 18 °C; growth in vitamin-free medium: positive; starch formation: positive; splitting of arbutin: negative; growth on 50% (w/w) glucose yeast extract agar: positive; urease: positive; DBB reaction: positive; growth in the presence of 0.01% cycloheximide: positive; G+C content of the nuclear DNA: 59.0 mol%; major ubiquinone: Q8.
The type strain Y18 was deposited in the China General Microbiological Culture Collection Center, Institute of Microbiology, Chinese Academy of Sciences, Beijing, with the strain number AS 2.1971.
DISCUSSION
The results of morphology, biochemistry and physiology studies of Y18 support that Y18 belongs to the genus of Mrakia. The phylogenic tree showed Y18 classified into one group with Mrakia frigida, and the 18S rDNA sequence analysis also showed high homology (98.93%) between Y18 and Mrakia frigida. There are some distinct differences between Y18 and other species of Mrakia as shown in Table 2. Y18’s growth in 50% glucose-yeast extract agar, in presence of 0.01% cycloheximide, and without external vitamins were observed. Cellobioses, and sucrose were fermented by Y18. The optimum and maximum temperatures were low compared with other Mrakia species. DNA-DNA relatedness (Table 1) also supported that Y18 and M. frigida are not of the same species. In conclusion, there were quite a few differences between Y18 and other known Mrakia species in biochemical, physiological, and molecular characteristics. So we describe Y18 as a new species: Mrakia psychrophila. The type strain is designated Y18, and is deposited in the Institute of Microbiology, Academia Sinica, Beijing, China.
Acknowledgments
We thank Prof. Zheng Ruyong, Institute of Microbiology, Chinese Academy of Sciences, for her advice and correction of Latin description on the manuscript.
Footnotes
Project (No. 30170001) supported by the National Natural Science Foundation of China
References
- 1.Babeva IP, Lisichkina GA, Reshetova IS, Danilevich VN. Mrakia curviuscula sp. nov.: a new species of psychrophilic yeasts from forest substrates. Mikrobiologiia. 2002;71(4):526–532. [PubMed] [Google Scholar]
- 2.D′Amico S, Collins T, Marx JC, Feller G, Gerday C. Psychrophilic microorganisms: challenges for life. EMBO Rep. 2006;7(4):385–389. doi: 10.1038/sj.embor.7400662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz MR, Fell JW. Molecular analyses of the IGS & ITS regions of rDNA of the psychrophilic yeasts in the genus Mrakia . Antonie van Leeuwenhoek. 2000;77(1):7–12. doi: 10.1023/A:1002048008295. [DOI] [PubMed] [Google Scholar]
- 4.Fell JW, Statzell-Tallman AC, Hunter IL, Phaff HJ. Leucosporidium gen. n., the heterobasidiomycetous stage of several yeasts of the genus Candida . Antonie van Leeuwenhoek. 1969;35(1):433–462. doi: 10.1007/BF02219163. [DOI] [PubMed] [Google Scholar]
- 5.Kourkoutas Y, Koutinas A, Kanellaki M, Banat I, Marchant R. Continuous wine fermentation using a psychrophilic yeast immobilized on apple cuts at different temperatures. Food Microbiol. 2002;19(2-3):127–134. doi: 10.1006/fmic.2001.0468. [DOI] [Google Scholar]
- 6.Lambo A, Patel T. Cometabolic degradation of polychlorinated biphenyls at low temperature by psychrotolerant bacterium Hydrogenophaga sp. IA3-A. Curr Microbiol. 2006;53(1):48–52. doi: 10.1007/s00284-005-0194-8. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Schmid R, Rusnak M. Functional expression of Candida antarctica lipase B in the Escherichia coli cytoplasm—a screening system for a frequently used biocatalyst. Appl Microbiol Biotechnol. 2006;72(5):1024–1032. doi: 10.1007/s00253-006-0369-7. [DOI] [PubMed] [Google Scholar]
- 8.López-Archilla A, Gonzalez A, Terron M, Amils R. Ecological study of the fungal populations of the acidic Tinto River in southwestern Spain. Can J Microbiol. 2004;50(11):923–934. doi: 10.1139/w04-089. [DOI] [PubMed] [Google Scholar]
- 9.Margesin R, Fauster V, Fonteyne PA. Characterization of cold-active pectate lyases from psychrophilic Mrakia frigida . Lett Appl Microbiol. 2005;40(6):453–459. doi: 10.1111/j.1472-765X.2005.01704.x. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa T, Nagaoka T, Taniguchi S, Miyaji T, Tomizuka N. Isolation and characterization of psychrophilic yeasts producing cold-adapted pectinolytic enzymes. Lett Appl Microbiol. 2004;38(5):383–387. doi: 10.1111/j.1472-765X.2004.01503.x. [DOI] [PubMed] [Google Scholar]
- 11.Nakase T, Suzuki M. Sporobolomyces yuccicola, a new species of ballistosporous yeast equipped with ubiquinone-9. Antonie van Leeuwenhoek. 1988;54(1):47–55. doi: 10.1007/BF00393957. [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer J, Richter S, Nieveler J, Hansen CE, Rhlid R, Schmid R, Rusnak M. High yield expression of lipase A from Candida antarctica in the methylotrophic yeast Pichia pastoris and its purification and characterization. Appl Microbiol Biotechnol. 2006;72(5):931–938. doi: 10.1007/s00253-006-0400-z. [DOI] [PubMed] [Google Scholar]
- 13.Ruan JS, Liu ZH, Li H, et al. The Development and Application of Actinomyces. Beijing: Science Press; 1990. pp. 202–206. [Google Scholar]
- 14.Ruberto L, Vazquez S, Lobalbo A, Maccormack WP. Psychrotolerant hydrocarbon-degrading Rhodococcus strains isolated from polluted Antarctic soils. Antarctic Sci. 2005;17(1):47–56. doi: 10.1017/S0954102005002415. [DOI] [Google Scholar]
- 15.Sabri A, Bare G, Jacques P. Influence of moderate temperatures on myristoyl-CoA metabolism and acyl-CoA thioesterase activity in the psychrophilic antarctic yeast Rhodotorula aurantiaca . J Biol Chem. 2001;276(16):12691–12696. doi: 10.1074/jbc.M100155200. [DOI] [PubMed] [Google Scholar]
- 16.Scorzetti G, Petrescu L, Yarrow D, Fell JW. Cryptococcus adeliensis sp. nov., a xylanase producing basidiomycetous yeast from Antarctica. Antonie van Leeuwenhoek. 2000;77(2):153–157. doi: 10.1023/A:1002124504936. [DOI] [PubMed] [Google Scholar]
- 17.Takashima M, Nakase T. A phylogenetic study of the genus Tilletiopsis, Tilletiaria anomala and related taxa based on the small subunit ribosomal DNA sequences. J Gen Appl Microbiol. 1996;42:421–429. [Google Scholar]
- 18.Thompson JD, Higgins DG, Gibson TJ. Clustal W: improving the sensitivity of progressive multiple sequence alignments through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van de Peer Y, de Wachter R. Treecon for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Applic Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- 20.van der Walt JP, Yarrow D. Methods for the Isolation, Maintenance, Classification and Identification of Yeasts. In: Kreger-van Rij NJW, editor. The Yeasts: A Taxonomic Study. 3rd Ed. Amsterdam: Elsevier Sci. Publ; 1984. pp. 45–105. [Google Scholar]
- 21.van Uden N. Temperature profiles of yeast. Adv Microb Physiol. 1984;25:195–251. doi: 10.1016/s0065-2911(08)60293-3. [DOI] [PubMed] [Google Scholar]
- 22.Welander U. Microbial degradation of organic pollutants in soil in a cold climate. Soil Sediment Contam. 2005;14(3):281–291. doi: 10.1080/15320380590928339. [DOI] [Google Scholar]
- 23.Yamada Y, Kondo K. Coenzyme Q system in the classification of the yeast genera Rhodotorula and Cryptococcus, and the yeast-like genera Sporobolomyces and Rhodosporidium . J Gen Appl Microbiol. 1973;19:59–77. [Google Scholar]
- 24.Yamada Y, Komagata K. Mrakia gen. nov., a heterobasidiomycetous yeast genus for the Q8-equipped, self-sporulating organisms which produce a unicellular metabasidium, formerly classified in the genus Leucosporidium . J Gen Appl Microbiol. 1987;33:455–457. [Google Scholar]
- 25.Zhu H, Qu F, Zhu LH. Isolation of genomic DNAs from plants, fungi and bacteria using benzyl chloride. Nucleic Acids Res. 1993;21(22):5279–5280. doi: 10.1093/nar/21.22.5279. [DOI] [PMC free article] [PubMed] [Google Scholar]