Abstract
The correlation of serum arylesterase (PON1) activity on phenylacetate determined by an integrated method to classical biochemical indexes of liver damage was investigated for the use of PON1 activity to evaluate liver damage. PON1 reaction curve as absorbance at 270 nm for 0.20 mmol/L phenylacetate hydrolysis was analyzed by the integrated method to determine maximal PON1 reaction rate. Classical biochemical indexes of liver damage were determined routinely. The 95% confidence threshold of PON1 activity in sera from healthy individuals was 2.12 mkat/L [(4.73±1.31) mkat/L, n=105]. PON1 activity in clinical sera was closely correlated to serum albumin, total protein and the ratio of albumin to globulins, but was weakly correlated to both direct and total bilirubin in serum. There were no correlations of PON1 activity to γ-glutamyltransferase, alkaline phosphatase, alanine aminotransferase and aspartate aminotransferase. Among 127 clinical sera with PON1 activity>2.12 mkat/L, there were 92% healthy individuals examined by albumin, 90% healthy individuals examined by total protein, 88% healthy individuals examined by total bilirubin, 86% healthy individuals examined by direct bilirubin and 64% healthy individuals examined by the ratio of albumin to globulins, respectively. In each group of healthy individuals judged by classical biochemical indexes of close correlation to PON1 activity, percentage of healthy individuals examined by PON1 activity was always >80%. These results suggested PON1 activity on phenylacetate estimated by the integrated method was also suitable for the evaluation of liver damage.
Keywords: Arylesterase, Albumin, Alanine aminotransferase, Liver damage, Ratio of albumin to globulins, Integrated method, Bilirubin
INTRODUCTION
Human serum arylesterase (arylesterase, EC 3.1.1.2, also known as paraoxonase1, PON1) originates from liver with two subtypes. PON1 activity in serum was associated with plasma lipid peroxidation and cardiovascular diseases (Junge and Klees, 1984; Mackness et al., 2004; Berkowitz et al., 2004; Harel et al., 2004; James, 2006). PON1 activity was suggested to be used as an additional test of liver damage and an indicator of the sensitivity of individual to neurotoxins such as organophosphate pesticides (Ferre et al., 2002; Charlton-Menys et al., 2006). Paraoxon is in common use for the assay of PON1 activity for clinical investigation (Ferre et al., 2002; Charlton-Menys et al., 2006). However, both the toxicity of paraoxon and its pollution to medical laboratory are great concerns. Moreover, it is the protein content of PON1 that is useful for laboratory diagnosis of liver damage, but two subtypes of PON1 hydrolyze paraoxon at different catalytic capacity, which suggests unreliable indexing of PON1 protein content by PON1 activity on paraoxon (Nakanishi et al., 2003). Therefore, the use of paraoxon to measure PON1 activity is not desirable for laboratory diagnosis of liver damage.
On the other hand, PON1 subtypes catalyze the hydrolysis of phenylacetate with comparable potency, and nontoxicity of phenylacetate confers on it easiness to operate (Liao et al., 2001; Nakanishi et al., 2003). It was found that serum PON1 activity accounted for more than 95% of the enzyme activity in serum for catalyzing phenylacetate hydrolysis (Liao et al., 2001). Therefore, phenylacetate may be an alternative substrate for measuring PON1 activity for liver damage test. However, PON1 activity in serum from healthy individual is so high on phenylacetate that the dilution of serum before assay is necessary, which unavoidably reduces the efficiency of routine analysis (Junge and Klees, 1984).
Recently, a new integrated method by nonlinearly fitting the integrated rate equation with the predictor variable of reaction time to enzyme reaction curve was established for the estimation of maximal product concentration (or constant background as its equivalent), maximal reaction rate and Michaelis-Menten constant of enzyme (Liao et al., 2001; 2003a; 2003b; 2004a; 2004b; 2005a; 2005b; 2006; 2007; Zhao et al., 2006a; 2006b). This new integrated method had much higher upper limit of linear response for enzyme activity assay at lower substrate concentration and was applicable to routine assay of PON1 activity on phenylacetate without dilution of serum (Liao et al., 2001; 2004a). Herein, the correlation of PON1 activity on phenylacetate estimated by this integrated method to classical biochemical indexes of liver damage test was investigated to evaluate the feasibility of PON1 activity on phenylacetate for the evaluation of liver damage.
MATERIALS AND METHODS
Chemicals and materials
Phenylacetate was from Aldrich (USA). Other chemicals were analytical reagents. During routine physical examination, 48 sera from apparently-healthy individuals were collected in the First Affiliated Hospital of Chongqing Medical University. Another 57 sera confirmed to be healthy by all classical biochemical indexes of liver damage test were collected in Liangping County Renmin Hospital. These two parts were combined as the sera pool of healthy individuals. Other clinical sera were randomly collected from Liangping County Renmin Hospital.
Monitor of PON1 reaction and estimation of its activity
Tris-HCl buffer at pH 7.4 (50.0 mmol/L plus 2.0 mmol/L CaCl2) was preheated to (25.0±0.5) °C. Reaction mixture was composed of 2.0 ml buffer, 20 μl aqueous substrate solution to final 0.20 mmol/L, undiluted serum 5.0 μl (Liao et al., 2001; 2004a). Reaction was initiated by the addition of substrate, and absorbance at 270 nm was monitored after 40 s lag at 10 s intervals within 5.0 min. Reaction data was formatted to enable being read-in by the program written in Visual Basic 6.0 for the analysis of enzyme reaction curve (Liao, 2002; Liao et al., 2005b; 2006; Zhao et al., 2006a; 2006b). Maximal reaction rate of PON1 was estimated by fitting the integrated Michaelis-Menten rate equation with the predictor variable of reaction time to PON1’s reaction curve with K m fixed at 1.0 mmol/L (Liao et al., 2001; 2004a). Results were the mean of duplicated assays with variation coefficient below 10%. Maximal reaction rate capable for formation of one mole product per second was defined as one kat.
Determination of classical biochemical indexes of liver damage test and examination of their agreement with PON1 activity
Results were given as mean±SD. Threshold of PON1 activity in sera pool of healthy individuals was established at 95% confidence limit to identify the healthy individuals from the pool of clinical sera. Classical biochemical indexes for liver damage test were determined on Hitachi 7020 Autoanalyzer with commercial reagent kits (Shanghai Kehua Bioengineering Co. Ltd., China) following established protocol. The correlations of PON1 activity in these clinical sera to classical biochemical indexes of liver damage test were analyzed with SAS 8e. Among healthy individuals judged by PON1 activity in clinical sera, the percentage of healthy individuals was examined by classical biochemical indexes that showed close correlation to PON1 activity (thresholds for the healthy individuals were albumin >30 g/L, total protein >60 g/L, the ratio of albumin to globulins (A/G) >1.2, total bilirubin <22.0 μmol/L, direct bilirubin <7.5 μmol/L, respectively). Similarly, the percentage of healthy individuals was reexamined by PON1 activity in each group of healthy individuals screened from the same pool of clinical sera by classical biochemical indexes to be closely correlated to PON1 activity.
RESULTS
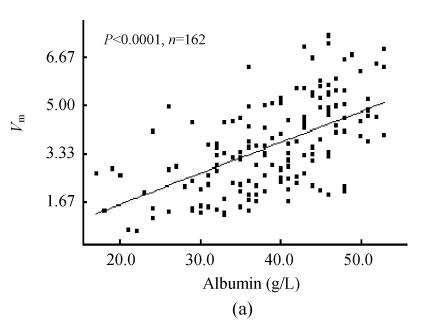
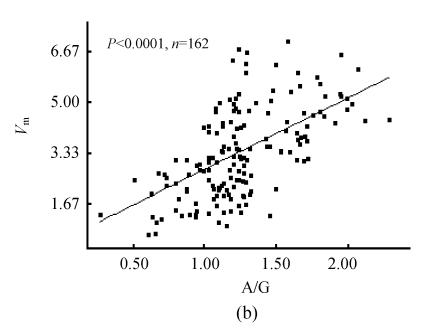
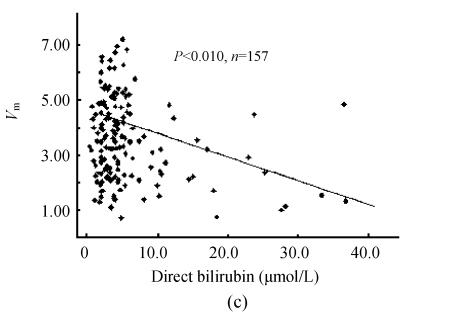
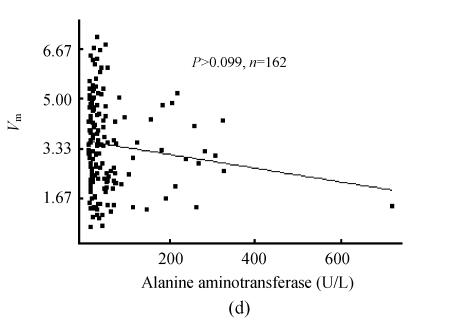
The 95% confidence lower limit of PON1 activity in all sera was 0.70 mkat/L [(3.90±1.60) mkat/L, n=210]. In the sera pool of healthy individuals, the 95% confidence threshold of PON1 activity was 2.12 mkat/L [(4.73±1.31) mkat/L, n=105]. In the pool of clinical sera, PON1 activity showed close correlation to total protein content, albumin level and A/G (Figs.1a and 1b, n=162). There were six outliers of direct bilirubin level in the pool of clinical sera (>50 μmol/L). Whether these outliers were included or not for analysis, there was weak correlation of serum direct bilirubin to PON1 activity (Fig.1c). So was total bilirubin (data not given). There was wider range of the distribution of alanine aminotransferase activity with one outlier (>400 U/L). However, whether this outlier was included or not for analysis, there was no significant correlation of alanine aminotransferase activity to PON1 activity (Fig.1d). Wide distribution of the activities of γ-glutamyltransferase, aspartate aminotransferase and alkaline phosphatase still showed no significant correlations to PON1 activity.
Fig. 1.
Correlation of PON1 activity to common biochemical indexes of liver damage. (a) PON1 vs albumin; (b) PON1 vs A/G; (c) PON1 vs direct bilirubin; (d) PON1 vs alanine aminotransferase
Among healthy individuals judged by PON1 activity (n=127), percentage of healthy individuals was 92% examined by albumin, 90% examined by total protein, 88% examined by total bilirubin, 86% examined by direct bilirubin, respectively. However, the percentage of healthy individuals examined by A/G was only 64%. Examined by PON1 activity among healthy individuals based on classical biochemical indexes showing close correlation to PON1 activity, percentage of healthy individuals was 82% among those judged by albumin (n=143), 81% among those judged by total protein (n=140), 91% among those judged by A/G (n=89), 82% among those judged by total bilirubin (n=136), 85% among those judged by direct bilirubin (n=130), respectively.
DISCUSSION
PON1 was released into plasma after synthesis in liver, consequently the capacity of liver to synthesize proteins will affect serum PON1 content. Albumin, total protein and A/G were classical biochemical indexes of protein syntheses for liver damage test. It was found that serum PON1 activity on phenylacetate was closely correlated to these classical indexes of protein syntheses for liver damage test, and the examination of liver damage by PON1 activity showed higher cross-agreement to that by protein synthesis of liver. These properties indicated PON1 activity on phenylacetate was useful for laboratory diagnosis of liver capacity to synthesize proteins. Serum bilirubin is also a characteristic index of liver damage, but serum bilirubin levels do not always follow normal distribution. The weak correlation of PON1 activity to serum bilirubin levels may be due to this unusual distribution pattern of serum bilirubin levels. However, there was good cross-agreement between the judgment of liver damage by PON1 activity and serum bilirubin levels, which also supported the diagnostic role of PON1 on phenylacetate in liver damage test (Fig.1c). Albumin-related indexes were in common use for diagnosis of chronic liver damage, and serum bilirubin levels were related to both chronic and acute liver damage while γ-glutamyltransferase and aminotransferases were mainly related to acute liver damage. It was found that PON1 activity showed no correlation to serum alkaline phosphatase, aminotransferase and γ-glutamyltransferase activities while it showed correlations to both serum albumin and bilirubin levels. Therefore, PON1 activity on phenylacetate had the same importance in laboratory diagnosis of chronic liver damage as its activity on paraoxon (Ferre et al., 2002; Charlton-Menys et al., 2006).
In comparison to the amount of phenylacetate used by the classical initial rate method, only 5% substrate was needed by this integrated method to determine PON1 activity for such a higher upper limit of linear response with reasonable efficiency. Therefore, for routine assay of serum enzyme activity with hazardous substrate, this integrated method may be generally preferred to the classical initial rate method. It is PON1 protein that has a diagnostic role in liver damage test, which requires the same catalytic activity of PON1 subtypes on the substrate that is used to measure PON1 activity for the diagnosis of liver damage. PON1 subtypes show comparable catalytic activities to hydrolyze phenylacetate while different catalytic activities to hydrolyze paraoxon (Nakanishi et al., 2003). The activity of PON1 on phenylacetate still exhibits satisfactory specificity (Liao et al., 2001). Currently common autoanalyzers cannot measure absorbance at 270 nm, and the use of recording spectrophotometer can facilitate semi-automated analysis in PON1 activity on phenylacetate. The advance of autoanalyzers makes it feasible to measure absorbance at 270 nm for fully-automated analysis of PON1 activity on phenylacetate in future, but neither the toxicity of paraoxon nor its pollution to medical laboratory is solvable. Aside from comparable catalytic activities of PON1 subtypes on phenylacetate, the safety and easiness of operation with phenylacetate is a great advantage over paraoxon. Therefore, the use of phenylacetate to measure PON1 activity by the integrated method may be more desirable for the evaluation of liver damage by PON1 activity.
Footnotes
Project (No. 30200266) supported by the National Natural Science Foundation of China
References
- 1.Berkowitz GS, Wetmur JG, Birman-Deych E, Obel J, Lapinski RH, Godbold JH, Holzman IR, Wolff MS. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environ Health Perspect. 2004;112(3):388–391. doi: 10.1289/ehp.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charlton-Menys V, Liu Y, Durrington PN. Semiautomated method for the determination of serum paraoxonase activity using paraoxon as substrate. Clin Chem. 2006;52(3):453–457. doi: 10.1373/clinchem.2005.063412. [DOI] [PubMed] [Google Scholar]
- 3.Ferre N, Camps J, Prats E, Villela E, Paul A, Figuera L, Joven J. Serum paraoxonase activity: a new additional test for the improved evaluation of chronic liver damage. Clin Chem. 2002;48(2):261–268. [PubMed] [Google Scholar]
- 4.Harel M, Aharoni A, Gaidukov L, Brumshtein B, Khersonsky O, Meged R, Dvir H, Ravelli RB, McCarthy A, Toker L, et al. Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat Struct Mol Biol. 2004;11(5):412–419. doi: 10.1038/nsmb767. [DOI] [PubMed] [Google Scholar]
- 5.James RW. A long and winding road: defining the biological role and clinical importance of paraoxonases. Clin Chem Lab Med. 2006;44(9):1052–1059. doi: 10.1515/CCLM.2006.207. [DOI] [PubMed] [Google Scholar]
- 6.Junge W, Klees H. 1,2-Arylesterase. Methods Enzym Anal. 1984;4:8–14. [Google Scholar]
- 7.Liao F. The Software for Kinetic Analysis of Enzyme Reaction Process. 31-2002-software-875. Chongqing Copyright Bureau, China. 2002
- 8.Liao F, Liu WL, Zhou QX, Zeng ZC, Zuo YP. Assay of serum arylesterase activity by fitting reaction curve to an integrated rate equation. Clin Chim Acta. 2001;314(1/2):67–76. doi: 10.1016/S0009-8981(01)00631-3. [DOI] [PubMed] [Google Scholar]
- 9.Liao F, Tian KC, Yang X, Zhou QX, Zeng ZC, Zuo YP. Kinetic substrate quantification by fitting the enzyme reaction curve to the integrated Michaelis-Menten equation. Anal Bioanal Chem. 2003;375(6):756–762. doi: 10.1007/s00216-003-1829-x. [DOI] [PubMed] [Google Scholar]
- 10.Liao F, Li JC, Zeng ZC, Zuo YP. Measurement of mouse liver glutathione S-transferase activity by the integrated method. J Med Coll PLA. 2003;18(5):295–300. [Google Scholar]
- 11.Liao F, Wang JH, Zeng ZC, Kang GF, Zuo YP, Wang YM, Wang N, Yuan XM, Song Z, Yu TT, et al. Measurement of serum arylesterase activity by the integrated method. J Third Mill Med Univ. 2004;26(10):923–925. (in Chinese) [Google Scholar]
- 12.Liao F, Zhu XY, Wang YM, Zeng ZC, Zuo YP. Assay of uricase activity by the integrated method at substrate concentration higher than Michaelis-Menten constant. J Fourth Mill Med Univ. 2004;25(17):1551–1554. (in Chinese) [Google Scholar]
- 13.Liao F, Zhu XY, Wang YM, Zuo YP. The comparison of the estimation of enzyme kinetic parameters by fitting reaction curve to the integrated Michaelis-Menten rate equations of different predictor variables. J Biochem Biophys Methods. 2005;62(1):13–24. doi: 10.1016/j.jbbm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Liao F, Zhao YS, Zhao LN, Tao J, Zhu XY, Wang YM, Zuo YP. Kinetic method for enzymatic analysis by predicting background with uricase reaction as model. J Med Coll PLA. 2005;20(6):338–344. [Google Scholar]
- 15.Liao F, Zhao YS, Zhao LN, Tao J, Zhu XY, Liu L. The evaluation of a direct kinetic method for serum uric acid assay by predicting the background absorbance of uricase reaction solution with an integrated method. J Zhejiang Univ Sci B. 2006;7(6):497–502. doi: 10.1631/jzus.2006.B0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao F, Zhao LN, Zhao YS, Tao J, Zuo YP. Integrated rate equation considering product inhibition and its application to kinetic assay of serum ethanol. Anal Sci. 2007 doi: 10.2116/analsci.23.439. in press. [DOI] [PubMed] [Google Scholar]
- 17.Mackness MI, Durrington PN, Mackness B. The role of paraoxonase 1 activity in cardiovascular disease: potential for therapeutic intervention. Am J Cardiovasc Drugs. 2004;4(4):211–217. doi: 10.2165/00129784-200404040-00002. [DOI] [PubMed] [Google Scholar]
- 18.Nakanishi M, Takanami Y, Maruyama T, Murata M, Motohashi Y, Nakano S, Uchida K, Maruyama C, Kyotani S, Tsushima M. The ratio of serum paraoxonase/arylesterase activity using an improved assay for arylesterase activity to discriminate PON1R192 from PON1Q192 . J Atheroscler Thromb. 2003;10(6):337–342. doi: 10.5551/jat.10.337. [DOI] [PubMed] [Google Scholar]
- 19.Zhao YS, Zhao LN, Yang GQ. Characterization of an intracellular uricase from Bacillus fastidious ATCC 26904 and its application to serum uric acid assay by a patented kinetic method. Biotechnol Appl Biochem. 2006;45(2):75–80. doi: 10.1042/BA20060028. [DOI] [PubMed] [Google Scholar]
- 20.Zhao LN, Tao J, Zhao YS, Liao F. Quantification of reduced glutathione by analyzing glutathione-S-transferase reaction process taking into account of product inhibition. J Xi’an Jiaotong Univ Med Sci. 2006;27(3):300–303. (in Chinese) [Google Scholar]