Abstract
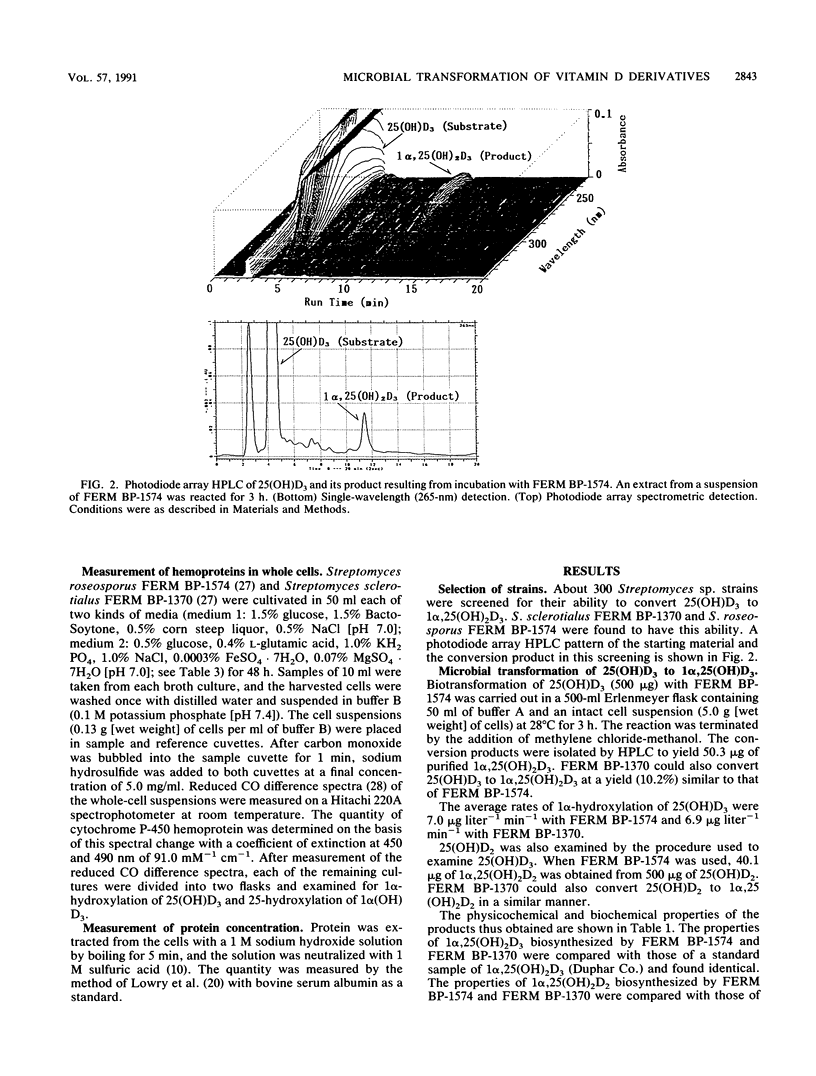
To enzymatically synthesize vitamin D derivatives, we screened about 300 Streptomyces sp. strains. Streptomyces sclerotialus FERM BP-1370 and Streptomyces roseoporus FERM BP-1574 were found to have the ability to convert 25-hydroxyvitamin D3 and 1 alpha-hydroxyvitamin D3, respectively, to 1 alpha, 25-dihydroxyvitamin D3. The average rates of 1 alpha hydroxylation of 25-hydroxyvitamin D3 were 6.9 micrograms liter-1 min-1 with FERM BP-1370 and 7.0 micrograms liter-1 min-1 with FERM BP-1574. The specific cytochrome P-450 inhibitors carbon monoxide, SKF-525-A, and metyrapone inhibited the hydroxylation of 1 alpha- and 25-hydroxyvitamin D3 to 1 alpha, 25-dihydroxyvitamin D3 by FERM BP-1370 and FERM BP-1574. The cytochromes P-450 of these strains were detected by reduced CO difference spectra in the whole-cell suspensions. The appearance of cytochrome P-450 suggests that the cytochromes P-450 of FERM BP-1370 and FERM BP-1574 carry out the hydroxylation of 25- and 1 alpha-hydroxyvitamin D3 to 1 alpha, 25-dihydroxyvitamin D3.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkhem I., Holmberg I., Oftebro H., Pedersen J. I. Properties of a reconstituted vitamin D3 25-hydroxylase from rat liver mitochondria. J Biol Chem. 1980 Jun 10;255(11):5244–5249. [PubMed] [Google Scholar]
- Brooks M. H., Bell N. H., Love L., Stern P. H., Orfei E., Queener S. F., Hamstra A. J., DeLuca H. F. Vitamin-D-dependent rickets type II. Resistance of target organs to 1,25-dihydroxyvitamin D. N Engl J Med. 1978 May 4;298(18):996–999. doi: 10.1056/NEJM197805042981804. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Gibson D. T. Metabolism of naphthalene by cell extracts of Cunninghamella elegans. Arch Biochem Biophys. 1978 Feb;186(1):121–127. doi: 10.1016/0003-9861(78)90471-x. [DOI] [PubMed] [Google Scholar]
- Chandler J. S., Pike J. W., Hagan L. A., Haussler M. R. A sensitive radioreceptor assay for 1 alpha, 25-dihydroxyvitamin D in biological fluids. Methods Enzymol. 1980;67:522–528. doi: 10.1016/s0076-6879(80)67065-7. [DOI] [PubMed] [Google Scholar]
- Clements M. R., Johnson L., Fraser D. R. A new mechanism for induced vitamin D deficiency in calcium deprivation. Nature. 1987 Jan 1;325(6099):62–65. doi: 10.1038/325062a0. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- DeLuca H. F., Schnoes H. K. Vitamin D: recent advances. Annu Rev Biochem. 1983;52:411–439. doi: 10.1146/annurev.bi.52.070183.002211. [DOI] [PubMed] [Google Scholar]
- Gray R. W., Omdahl J. L., Ghazarian J. G., DeLuca H. F. 25-Hydroxycholecalciferol-1-hydroxylase. Subcellular location and properties. J Biol Chem. 1972 Dec 10;247(23):7528–7532. [PubMed] [Google Scholar]
- Hansson R., Holmberg I., Wikvall K. 25-Hydroxylation vitamin D3 and side chain hydroxylations of 5 beta-cholestane-3 alpha, 7 alpha, 12 alpha-triol by purified rabbit and rat liver microsomal cytochromes P-450. J Biol Chem. 1981 May 10;256(9):4345–4349. [PubMed] [Google Scholar]
- Helmer B., Schnoes H. K., DeLuca H. F. 1H nuclear magnetic resonance studies of the conformations of vitamin D compounds in various solvents. Arch Biochem Biophys. 1985 Sep;241(2):608–615. doi: 10.1016/0003-9861(85)90587-9. [DOI] [PubMed] [Google Scholar]
- Hiwatashi A., Ichikawa Y. Purification and organ-specific properties of cholecalciferol 25-hydroxylase system: cytochrome P-450D25-linked mixed function oxidase system. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1443–1449. doi: 10.1016/s0006-291x(80)80027-1. [DOI] [PubMed] [Google Scholar]
- Hiwatashi A., Nishii Y., Ichikawa Y. Purification of cytochrome P-450D1 alpha (25-hydroxyvitamin D3-1 alpha-hydroxylase) of bovine kidney mitochondria. Biochem Biophys Res Commun. 1982 Mar 15;105(1):320–327. doi: 10.1016/s0006-291x(82)80047-8. [DOI] [PubMed] [Google Scholar]
- Ikekawa N. Structures and biological activities of vitamin D metabolites and their analogs. Med Res Rev. 1987 Jul-Sep;7(3):333–366. doi: 10.1002/med.2610070304. [DOI] [PubMed] [Google Scholar]
- Kametani T., Furuyama H. Synthesis of vitamin D3 and related compounds. Med Res Rev. 1987 Apr-Jun;7(2):147–171. doi: 10.1002/med.2610070202. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leibman K. C. Effects of metyrapone on liver microsomal drug oxidations. Mol Pharmacol. 1969 Jan;5(1):1–9. [PubMed] [Google Scholar]
- Lu M. C., Wung W. E., Shih L. B., Callejas S., Gearien J. E., Thompson E. B. Molecular modification of anticholinergics as probes for muscarinic receptors. 1. Amino esters of alpha-substituted phenylacetic acid and related analogues. J Med Chem. 1987 Feb;30(2):273–278. doi: 10.1021/jm00385a008. [DOI] [PubMed] [Google Scholar]
- Lund J., DeLuca H. F. Biologically active metabolite of vitamin D3 from bone, liver, and blood serum. J Lipid Res. 1966 Nov;7(6):739–744. [PubMed] [Google Scholar]
- Madhok T. C., DeLuca H. F. Characteristics of the rat liver microsomal enzyme system converting cholecalciferol into 25-hydroxycholecalciferol. Evidence for the participation of cytochrome p-450. Biochem J. 1979 Dec 15;184(3):491–499. doi: 10.1042/bj1840491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon J. P., Hamilton J. G., Nauss-Karol C., Karol R. J., Ashley C. J., Matuszewski D. S., Tratnyek C. A., Bryce G. F., Miller O. N. An improved competitive protein binding assay for 1,25-dihydroxyvitamin D. Arch Biochem Biophys. 1980 Apr 15;201(1):277–285. doi: 10.1016/0003-9861(80)90512-3. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Peterson J. A., Ullrich V., Hildebrandt A. G. Methyrapone interaction with Pseudomonas putida cytochrome P-405. Arch Biochem Biophys. 1971 Aug;145(2):531–542. doi: 10.1016/s0003-9861(71)80013-9. [DOI] [PubMed] [Google Scholar]
- Pike J. W., Haussler M. R. Characteristics and purification of the intstinal receptor for 1,25-dihydroxyvitamin D. Methods Enzymol. 1980;67:508–522. doi: 10.1016/s0076-6879(80)67064-5. [DOI] [PubMed] [Google Scholar]
- Rosazza J. P., Smith R. V. Microbial models for drug metabolism. Adv Appl Microbiol. 1979;25:169–208. doi: 10.1016/s0065-2164(08)70150-3. [DOI] [PubMed] [Google Scholar]
- Seino Y., Tanaka H., Yamaoka K., Yabuuchi H. Circulating 1 alpha,25-dihydroxyvitamin D levels after a single dose of 1 alpha,25-dihydroxyvitamin D3 or 1 alpha-hydroxyvitamin D3 in normal men. Bone Miner. 1987 Sep;2(6):479–485. [PubMed] [Google Scholar]
- Seino Y., Yamaoka K., Ishida M., Yabuuchi H., Ichikawa M., Ishige H., Yoshino H., Avioli L. V. Biochemical characterization of 1,25(OH)2D3 receptors in chick embryonal duodenal cytosol. Calcif Tissue Int. 1982 May;34(3):265–269. doi: 10.1007/BF02411248. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., DeLuca H. F., Ikekawa N. High-pressure liquid chromatography of vitamin D metabolites and analogs. Methods Enzymol. 1980;67:370–385. doi: 10.1016/s0076-6879(80)67046-3. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Sicinski R. R., DeLuca H. F., Sai H., Ikekawa N. Unique rearrangement of ergocalciferol side chain in vitro: production of a biologically highly active homologue of 1,25-dihydroxyvitamin D3. Biochemistry. 1986 Sep 23;25(19):5512–5518. doi: 10.1021/bi00367a025. [DOI] [PubMed] [Google Scholar]


