Abstract
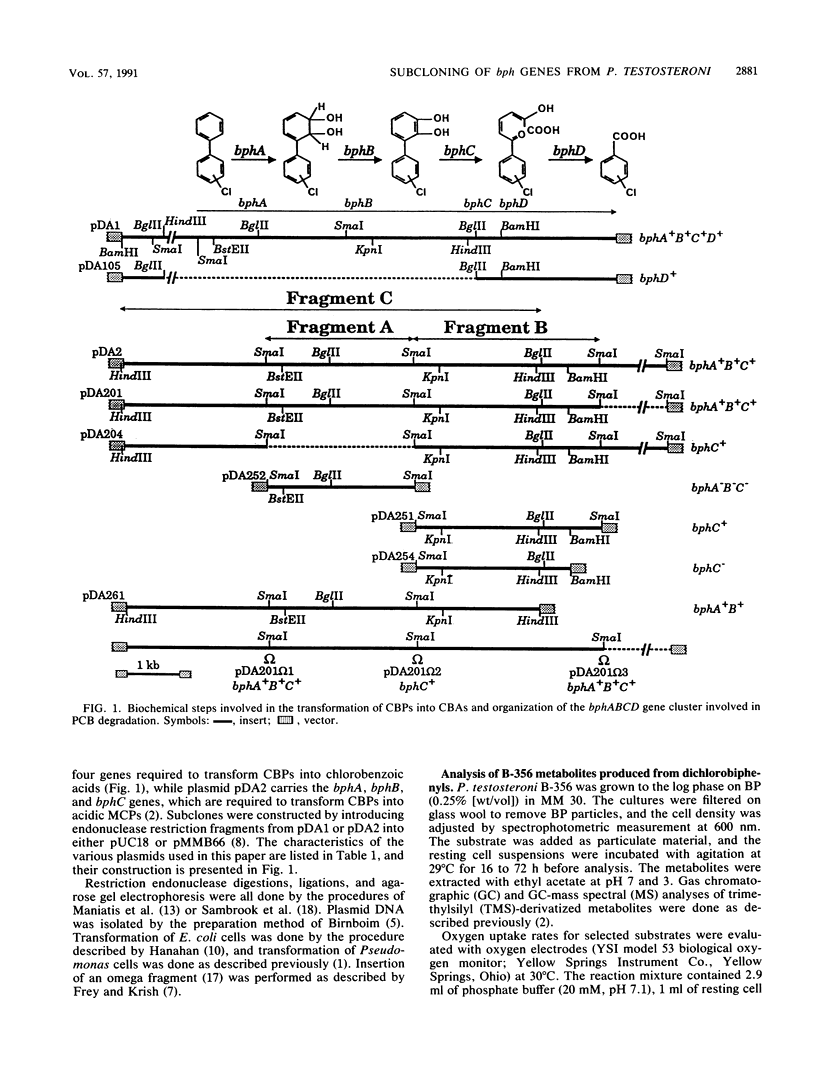
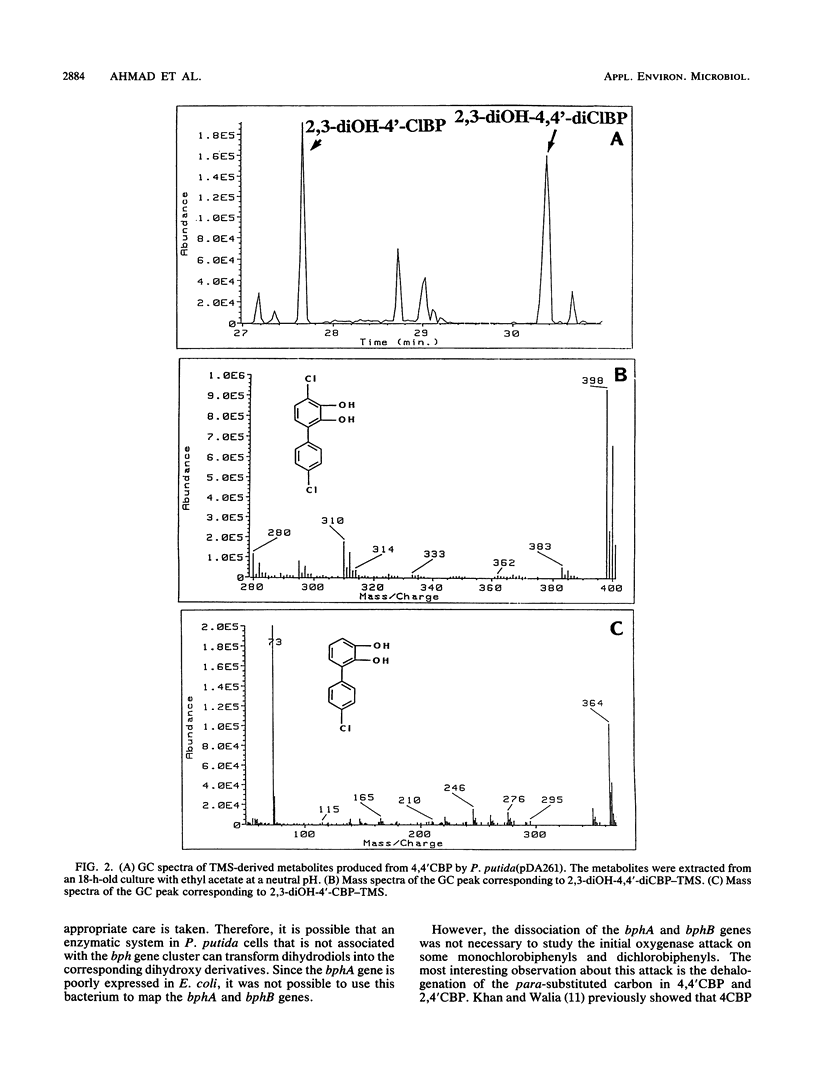
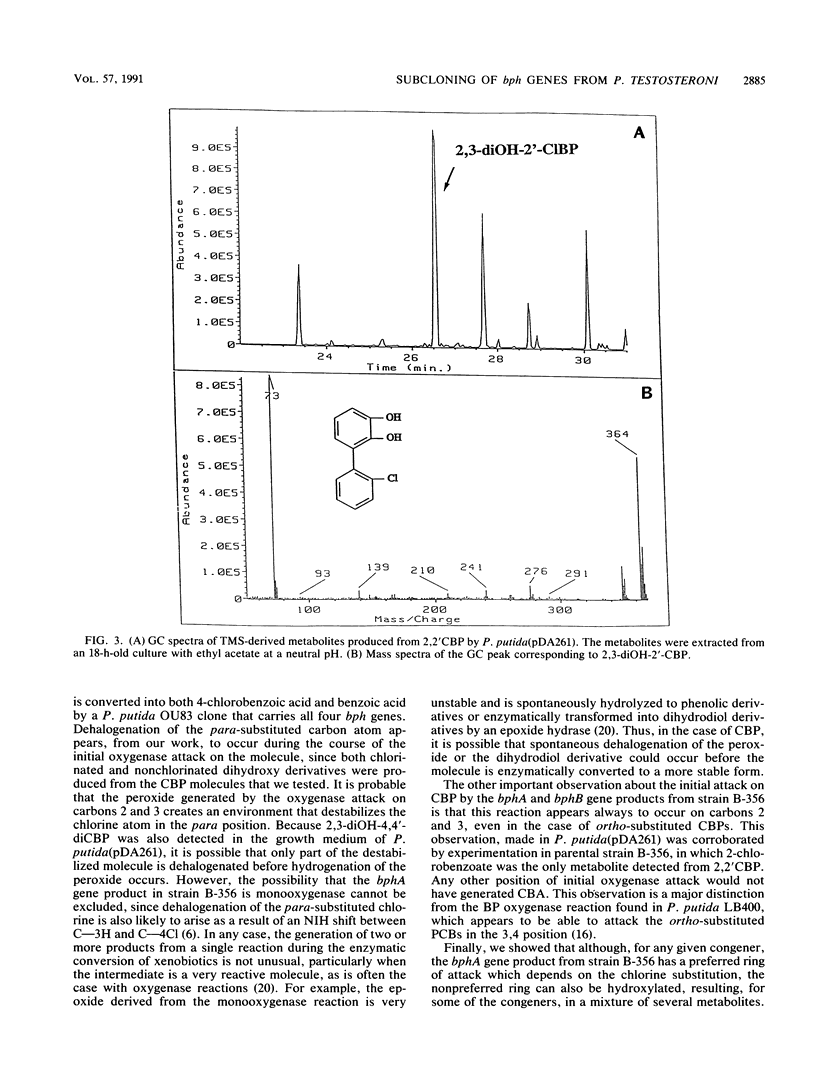
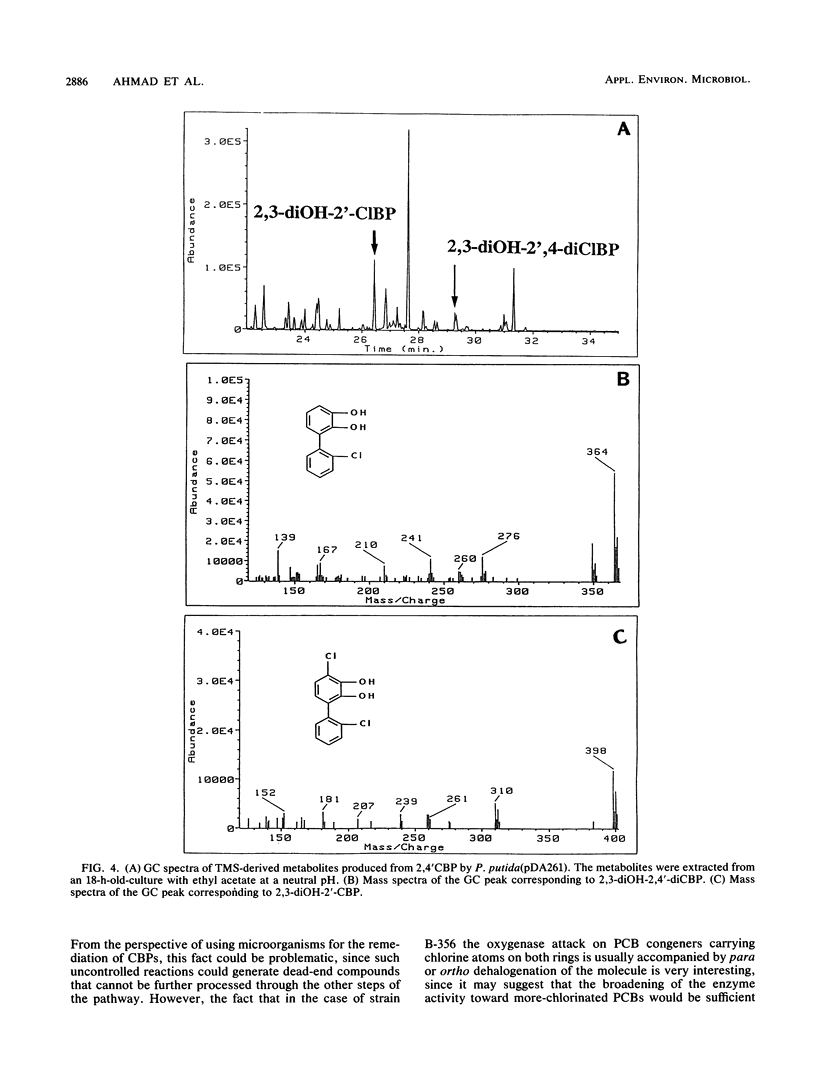
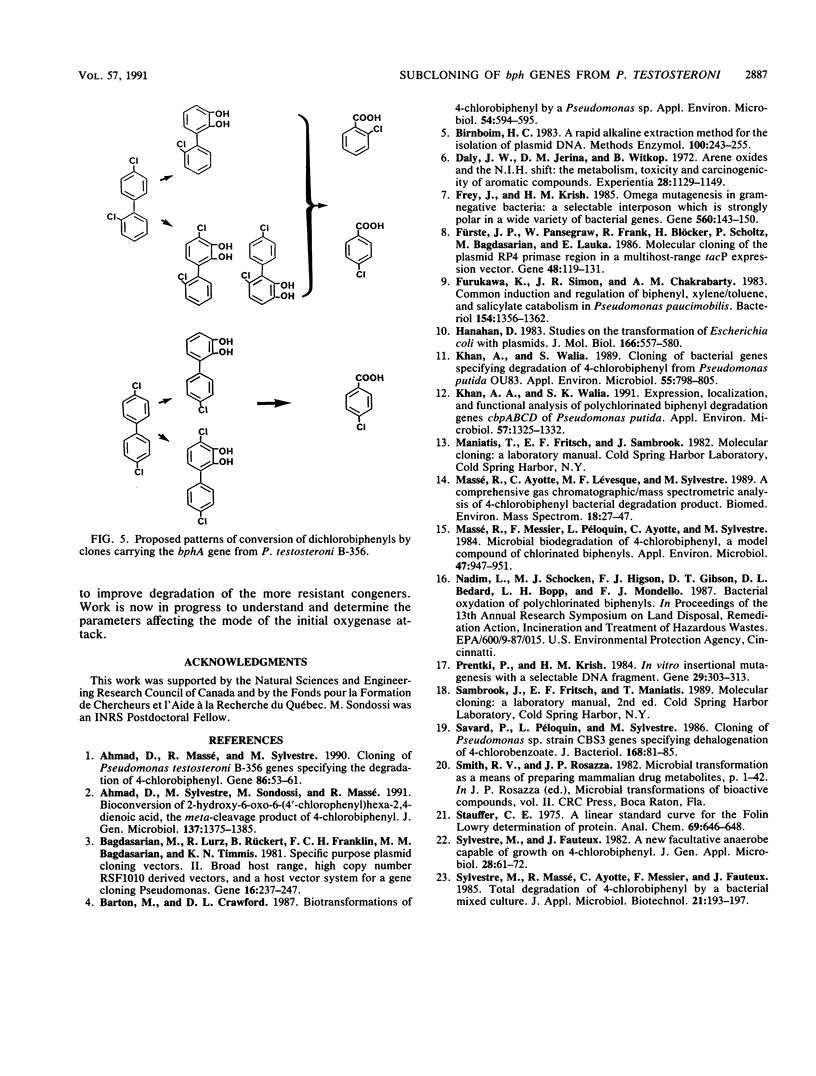
The bphA, -B, -C, and -D genes from Pseudomonas testosteroni B-356 were mapped to a 5.5-kb DNA fragment of cloned plasmids pDA1 and pDA2 by use of deletion and insertion mutants of these plasmids. The expression of each of these genes was evaluated in Escherichia coli and in Pseudomonas putida, and it was found that the bphC and bphD genes are well expressed in both E. coli and P. putida cells while the bphA and bphB genes are very poorly expressed in E. coli, even when placed downstream of a tac promotor. P. putida clones carrying the bphA gene were used to study the metabolites produced from 4,4'-dichlorobiphenyl, 2,2'-dichlorobiphenyl, and 2,4'-dichlorobiphenyl. It was shown that dehalogenation of 4-Cl and 2-Cl occurs in the course of the initial oxygenase attack on these molecules, which always occurs on carbons 2 and 3, independently of the positions of the chlorine atoms. Our data also suggest that in the case of polychlorobiphenyl congeners carrying chlorine atoms on both rings, it appears that, depending on the chlorine positions, dioxygenation will occur predominantly on one ring over the other. However, attack of the more resistant ring is not excluded, resulting in multiple conversion pathways.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad D., Massé R., Sylvestre M. Cloning and expression of genes involved in 4-chlorobiphenyl transformation by Pseudomonas testosteroni: homology to polychlorobiphenyl-degrading genes in other bacteria. Gene. 1990 Jan 31;86(1):53–61. doi: 10.1016/0378-1119(90)90113-6. [DOI] [PubMed] [Google Scholar]
- Ahmad D., Sylvestre M., Sondossi M., Massé R. Bioconversion of 2-hydroxy-6-oxo-6-(4'-chlorophenyl)hexa-2,4-dienoic acid, the meta-cleavage product of 4-chlorobiphenyl. J Gen Microbiol. 1991 Jun;137(6):1375–1385. doi: 10.1099/00221287-137-6-1375. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Barton M. R., Crawford R. L. Novel biotransformations of 4-chlorobiphenyl by a Pseudomonas sp. Appl Environ Microbiol. 1988 Feb;54(2):594–595. doi: 10.1128/aem.54.2.594-595.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Jerina D. M., Witkop B. Arene oxides and the NIH shift: the metabolism, toxicity and carcinogenicity of aromatic compounds. Experientia. 1972 Oct 15;28(10):1129–1149. doi: 10.1007/BF01946135. [DOI] [PubMed] [Google Scholar]
- Frey J., Krisch H. M. Omega mutagenesis in gram-negative bacteria: a selectable interposon which is strongly polar in a wide range of bacterial species. Gene. 1985;36(1-2):143–150. doi: 10.1016/0378-1119(85)90078-2. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Simon J. R., Chakrabarty A. M. Common induction and regulation of biphenyl, xylene/toluene, and salicylate catabolism in Pseudomonas paucimobilis. J Bacteriol. 1983 Jun;154(3):1356–1362. doi: 10.1128/jb.154.3.1356-1362.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Khan A. A., Walia S. K. Expression, localization, and functional analysis of polychlorinated biphenyl degradation genes cbpABCD of Pseudomonas putida. Appl Environ Microbiol. 1991 May;57(5):1325–1332. doi: 10.1128/aem.57.5.1325-1332.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Walia S. Cloning of bacterial genes specifying degradation of 4-chlorobiphenyl from Pseudomonas putida OU83. Appl Environ Microbiol. 1989 Apr;55(4):798–805. doi: 10.1128/aem.55.4.798-805.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé R., Messier F., Péloquin L., Ayotte C., Sylvestre M. Microbial biodegradation of 4-chlorobiphenyl, a model compound of chlorinated biphenyls. Appl Environ Microbiol. 1984 May;47(5):947–951. doi: 10.1128/aem.47.5.947-951.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Savard P., Péloquin L., Sylvestre M. Cloning of Pseudomonas sp. strain CBS3 genes specifying dehalogenation of 4-chlorobenzoate. J Bacteriol. 1986 Oct;168(1):81–85. doi: 10.1128/jb.168.1.81-85.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer C. E. A linear standard curve for the Folin Lowry determination of protein. Anal Biochem. 1975 Dec;69(2):646–648. doi: 10.1016/0003-2697(75)90172-4. [DOI] [PubMed] [Google Scholar]


