Abstract
Diabetes results from the absolute or relative deficiency of insulin-producing β cells. The prospect that non-β pancreatic cells could be harnessed to become β cells has led to interest in understanding the plasticity of pancreatic cells. Recent studies, however, have shown that adult β cells are largely derived from preexisting β cells. In this issue of the JCI, Desai et al. show that acinar cells, the major cell type in the pancreas, do not contribute to new β cells formed during pancreatic regeneration (see the related article beginning on page 971). These studies suggest that the fate of adult pancreatic cell lineages is immutable. However, also in this issue of the JCI, Collombat et al. demonstrate that inducing a single transcription factor named Arx in adult β cells causes these cells to undergo massive transdifferentiation into α and pancreatic polypeptide endocrine cells (see the related article beginning on page 961). This finding points to an unexpected plasticity of postnatal pancreatic endocrine cells.
Two experimental strategies have emerged in the past years to restore β cell mass in type 1 diabetes. One is to activate a regenerative response in vivo, the other to generate new β cells in vitro. Both approaches require an understanding of how new β cells are formed.
For many years, the conventional wisdom has been that adult β cells have limited, if any, capacity to regenerate. This conception was based in part on the fact that transient treatment with β cell toxins like streptozotocin or alloxan leads to the destruction of β cells and permanent diabetes mellitus. However, healthy β cells clearly grow in response to increased demand, for example, in obesity or pregnancy (1, 2). Furthermore, studies have demonstrated that β cells undergo robust regeneration after diverse forms of injury, including subtotal pancreatectomy, pancreatic duct ligation, or EGF and gastrin treatment following alloxan-induced diabetes (reviewed in ref. 3). While these models do not necessarily reflect the pathogenesis of human diabetes, they show that β cells possess the natural ability to regenerate.
One of the most important challenges in this field is to determine the cellular origin of newly formed β cells during regeneration. An attractive idea is that regeneration recapitulates embryonic development. It is now known that pancreatic and duodenal homeobox 1–positive (Pdx1+) precursor cells of the early embryonic pancreas give rise to all epithelial lineages of the adult pancreas, suggesting the existence of a common pancreatic precursor cell (4). It is also known that β cells arise from ductal structures during embryonic development (5, 6). On the other hand, it has been shown that after diverse forms of β cell injury in adults, clusters of β cells appear in the vicinity of pancreatic ducts, while Pdx1 levels in duct cells often increase (3, 7). This was often taken to indicate that β cells in such circumstances are derived from pancreatic duct cells, as occurs during embryogenesis. Other candidate origins of regenerating β cells have been proposed, most notably acinar cells, as discussed below. However, proving (or disproving) the contribution of any potential source of new β cells requires some form of cell lineage tracing.
A Cre/loxP-based lineage tracing study recently provided a breakthrough in the field, showing that throughout the lifetime of an adult mouse, new β cells are largely generated from the replication of β cells (8). Similarly, following partial pancreatectomy, most β cells were also derived from preexisting β cells (8). As discussed elsewhere (3), this study could not rule out the possibility that other pancreatic cells give rise to β cells after more robust regeneration responses (or even in small amounts in the tested conditions), but it has clearly set the standard for future studies addressing the origin of new β cells.
Acinar cells as a source of new β cells?
In this issue of the JCI, Desai et al. (9) tested the contribution of acinar cells to new β cells formed during pancreatic regeneration. As the most abundant cell of the pancreas, acinar cells could provide a useful source of insulin-producing endocrine cells. In vitro, adding EGF and leukemia inhibitory factor (LIF) to exocrine cells promotes the formation of new β cells at a rate that cannot be accounted for by proliferation of contaminating β cells (10). Similarly, EGF plus nicotinamide causes cultured acinar cells, genetically labeled with amylase promoter–driven Cre recombinase, to differentiate into insulin-producing cells (11). Furthermore, there have been indirect indications that acinar to β cell differentiation may occur in vivo (12). Desai, Stoffers, and colleagues have now created a transgenic mouse model in which the elastase promoter drives the expression of a hormone-inducible form of Cre selectively in pancreatic acinar cells (9). Upon treatment of adult mice with hormone, Cre activity caused the indelible activation of the β-galactosidase reporter gene only in acinar cells. The authors then induced β cell regeneration by various methods (pancreatectomy and exendin-4 treatment, duct ligation, or pancreatitis). Subsequent examination of β cells revealed that none expressed the reporter gene, indicating that acinar cells do not give rise to new β cells during regeneration. As the authors carefully point out, this does not disprove that acinar cells can be harnessed to produce insulin using in vitro strategies, as previously reported (10, 11). In fact, the apparent conflict between the in vivo and in vitro models is not entirely surprising, because the maintenance of differentiated cellular states is not an entirely cell-autonomous process. Placing cells outside of their natural niche and allowing exposure to different intercellular signals can have profound consequences on cellular plasticity and differentiation. In summary, the potential for acinar cells to serve as a source of autologous insulin-producing cells remains to be fully examined, although the study by Desai et al. (9) provides a clear message that acinar to β cell transdifferentiation is not a prominent mechanism of β cell regeneration in vivo.
A case of pancreatic endocrine cell alchemy
While lineage tracing studies of adult acinar and β cells suggest that in vivo postnatal pancreatic cell fates are irreversibly fixed, another study from Collombat and colleagues in this issue of the JCI provides a remarkable example of cellular plasticity in the adult pancreas (13).
One of the most interesting aspects of the study by Collombat et al. (13) is that it exploits the paradigm of the transcriptional regulatory mechanisms of the developing pancreas to manipulate adult cell plasticity (14, 15) (Figure 1). It is currently known that the transcription factor neurogenin 3 (Ngn3) orchestrates the regulatory network that gives rise to pancreatic endocrine cells (16). Subsequent regulatory checkpoints refine the specification of endocrine cell subtypes (17–20). For example, it was previously shown that the ablation of the gene encoding the transcription factor paired box gene 4 (Pax4) causes a complete block of embryonic β cell formation and a concomitant increase in the number of glucagon-producing α cells (18). In contrast, deletion of the gene encoding the transcription factor aristaless-related homeobox (Arx) causes the opposite phenotype: no α cells are formed, but β cell generation increases (17). This led to the hypothesis that Arx and Pax4 are mutually exclusive regulators that specify major pancreatic endocrine cell subtypes (Figure 1) (17). However, because these studies were based on loss of function, they could not prove that the factors are indeed sufficient to generate such effects. For this reason Collombat et al. (13) chose to force the expression of the pro–α cell factor Arx in endodermal precursors or endocrine progenitors of the embryonic pancreas. This prevented the formation of β cells during pancreatic development and increased the number of α cells. Intriguingly, Arx misexpression also caused an increase in the number of pancreatic polypeptide (PP) cells. This was unexpected, because Pax4 KO embryos, in which Arx expression prevails in endocrine progenitor cells, do not exhibit increased PP cell formation (17). This finding may reflect that Arx specifies PP cell formation only in cells previously exposed to Pax4 (Figure 1). In summary, these experiments clearly showed that Arx is sufficient for suppressing the β cell fate. It is also sufficient for specifying α cells, although this effect is not as selective as anticipated from the KO studies.
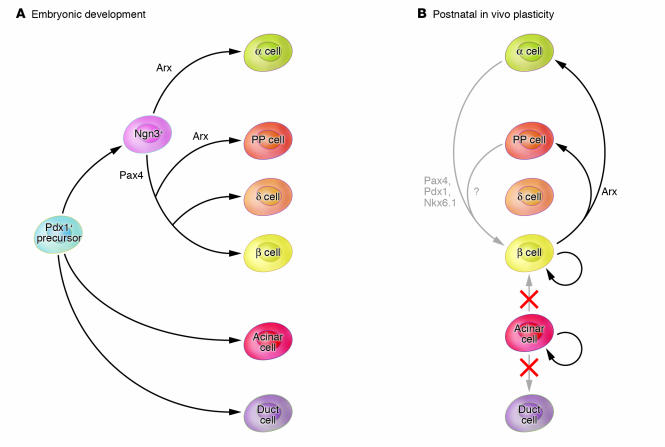
Figure 1. Lineage relationships and plasticity of the embryonic and adult pancreas.
(A) All differentiated pancreatic epithelial lineages are derived from Pdx1+ precursor cells of the early embryonic pancreas. A subset of Pdx1+ precursors expressing Ngn3 become committed to the endocrine lineage. The transcription factors Pax4 and Arx further specify β versus α endocrine cells. (B) Given the shared developmental origin, several studies have addressed the possible interconversion of adult pancreatic lineages. For example, in vitro studies indicate that acinar cells can be differentiated into ductal or insulin-producing cells (10, 11). In this issue of the JCI, Desai et al. (9) have now demonstrated that during regeneration of the pancreas, acinar cells proliferate but do not give rise to β cells or duct cells. Together with an earlier study showing that adult β cells are largely derived from preexisting β cells (8), these findings suggest that in vivo, the plasticity of differentiated pancreatic cells is very limited. However, another study in this issue of the JCI (13) provides a clear example of pancreatic cell plasticity in vivo, showing that inducible misexpression of the transcription factor Arx in adult mature β cells causes transdifferentiation into α and PP endocrine cells. This illustrates how knowledge of developmental transcriptional mechanisms can be exploited to change cellular fates in the adult pancreas. It is conceivable, for example, that misexpression of pro–β cell specification factors such as Pax4 might be employed to generate β cells from α cells. Nkx6.1, NK6 transcription factor related, locus 1.
In the second part of the same study (13), Arx misexpression was induced in β cells. In one set of experiments this was done postnatally, long after β cells had developed. This resulted in the remarkable finding that within just a few days, normal, healthy β cells lost all traces of β cell markers and became either PP or α cells. Importantly, the authors employed genetic lineage tracing to demonstrate that there was a true transformation between endocrine cell subtypes, which does not occur under normal conditions (4).
The recapitulation of developmental transcriptional mechanisms to induce transdifferentiation as demonstrated by Collombat et al. (13) is very appealing, but it faces theoretical hurdles. One is that once terminal differentiation occurs, epigenetic mechanisms are thought to restrict fate changes. Another is that developmental gene networks are thought to operate more like complex systems than as simple lineal cascades, making it difficult to obtain clearly directed effects from the manipulation of a single factor. It is thus remarkable that a single transcription factor can entirely reprogram the fate of a healthy differentiated β cell (13). Previous studies had shown a similar β to α cell transition in INS-1 β cell lines after inhibition of Pdx1, although the mechanisms that control differentiation in tumor cell lines can be profoundly different, as exemplified by the fact that INS-1 sublines can coexpress both insulin and glucagon genes (21). The deletion of Pdx1 in insulin-producing cells in mice also causes β cell loss and replacement by α cells (22), but theoretically this could be because the loss of β cells can lead to increased α cell growth. The Collombat et al. study is substantial in that it employed lineage tracing to conclusively demonstrate transdifferentiation between distinct pancreatic cell types in vivo.
This unexpected discovery raises several important questions. First, the results suggest that the epigenome of adult pancreatic endocrine cells is permissive of reprogramming to alternate endocrine subtypes. If this is true, could misexpression of Pax4 in α cells analogously cause transdifferentiation of α cells into β cells? If not, what other DNA-binding proteins need to be induced or suppressed? Because α cells are not destroyed in type 1 diabetes, this would provide a conceptually simple approach to therapeutically increase β cell mass. A more challenging possibility is that even if acinar cells do not normally contribute to the β cell population after regeneration, in vivo transdifferentiation may be achieved using artificial strategies that mimic embryonic endocrine differentiation. This study also raises interesting mechanistic questions. Although β and α cells share several transcription factors, they differ in the content of other regulatory target genes. It is striking that even in a nonphysiological setting, a single transcription factor can simultaneously cause the inactivation of β cell–specific genes and activate α cell– and PP cell–specific genes. One possible explanation is that a major function of Arx in the adult transdifferentiation model is to suppress either one or a combination of β cell–specific transcription factors. PP and α cells in this context would largely reflect default programs for endocrine cells that do not express other endocrine subtype–specific regulators. Regardless of the exact mechanisms involved, the demonstration that there is no true “point of no return” in adult pancreatic endocrine cells — and the conceptual simplicity with which it was achieved — strongly hints that non–β pancreatic endocrine cells are a plausible future source of β cells.
Acknowledgments
The authors’ research is supported by the Juvenile Diabetes Research Foundation, the European Union Sixth Framework Programme, Instituto de Salud Carlos III, the European Foundation for the Study of Diabetes, and the Ministerio de Educación y Ciencia.
Footnotes
Nonstandard abbreviations used: Arx, aristaless-related homeobox; LIF, leukemia inhibitory factor; Ngn3, neurogenin 3; Pax4, paired box gene 4; Pdx1, pancreatic and duodenal homeobox 1; PP, pancreatic polypeptide.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:859–862 (2007). doi:10.1172/JCI31749.
See the related article beginning on page 961.
References
- 1.Lingohr M.K., Buettner R., Rhodes C.J. Pancreatic beta-cell growth and survival--a role in obesity-linked type 2 diabetes? Trends Mol. Med. 2002;8:375–384. doi: 10.1016/s1471-4914(02)02377-8. [DOI] [PubMed] [Google Scholar]
- 2.Bonner-Weir S. Life and death of the pancreatic beta cells. Trends Endocrinol. Metab. 2000;11:375–378. doi: 10.1016/s1043-2760(00)00305-2. [DOI] [PubMed] [Google Scholar]
- 3.Bouwens L., Rooman I. Regulation of pancreatic beta-cell mass. Physiol. Rev. 2005;85:1255–1270. doi: 10.1152/physrev.00025.2004. [DOI] [PubMed] [Google Scholar]
- 4.Herrera P.L. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 5.Pictet R.L., Clark W.R., Williams R.H., Rutter W.J. An ultrastructural analysis of the developing embryonic pancreas. Dev. Biol. 1972;29:436–467. doi: 10.1016/0012-1606(72)90083-8. [DOI] [PubMed] [Google Scholar]
- 6.Maestro M.A., et al. Hnf6 and Tcf2 (MODY5) are linked in a gene network operating in a precursor cell domain of the embryonic pancreas. Hum. Mol. Genet. 2003;12:3307–3314. doi: 10.1093/hmg/ddg355. [DOI] [PubMed] [Google Scholar]
- 7.Bonner-Weir S., Baxter L.A., Schuppin G.T., Smith F.E. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 1993;42:1715–1720. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- 8.Dor Y., Brown J., Martinez O.I., Melton D.A. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 9.Desai B.M., et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet β cell, regeneration. J. Clin. Invest. . 2007;117:971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baeyens L., et al. In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia. 2005;48:49–57. doi: 10.1007/s00125-004-1606-1. [DOI] [PubMed] [Google Scholar]
- 11.Minami K., et al. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc. Natl. Acad. Sci. U. S. A. 2005;102:15116–15121. doi: 10.1073/pnas.0507567102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lardon J., Huyens N., Rooman I., Bouwens L. Exocrine cell transdifferentiation in dexamethasone-treated rat pancreas. Virchows Arch. 2004;444:61–65. doi: 10.1007/s00428-003-0930-z. [DOI] [PubMed] [Google Scholar]
- 13.Collombat P., et al. 2007Embryonic endocrine pancreas and mature β cells acquire α and PP cell phenotypes upon Arx misexpression . J. Clin. Invest. 117961–970. 10.1172/JCI29115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murtaugh L.C. Pancreas and beta-cell development: from the actual to the possible. Development. 2007;134:427–438. doi: 10.1242/dev.02770. [DOI] [PubMed] [Google Scholar]
- 15.Servitja J.M., Ferrer J. Transcriptional networks controlling pancreatic development and beta cell function. Diabetologia. 2004;47:597–613. doi: 10.1007/s00125-004-1368-9. [DOI] [PubMed] [Google Scholar]
- 16.Gradwohl G., Dierich A., LeMeur M., Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collombat P., et al. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17:2591–2603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sosa-Pineda B., Chowdhury K., Torres M., Oliver G., Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 19.Prado C.L., Pugh-Bernard A.E., Elghazi L., Sosa-Pineda B., Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2924–2929. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sander M., et al. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- 21.Wang H., et al. Pdx1 level defines pancreatic gene expression pattern and cell lineage differentiation. J. Biol. Chem. 2001;276:25279–25286. doi: 10.1074/jbc.M101233200. [DOI] [PubMed] [Google Scholar]
- 22.Ahlgren U., Jonsson J., Jonsson L., Simu K., Edlund H. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–1768.. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]