Abstract
IFN-γ has long been recognized as a signature proinflammatory cytokine that plays a central role in inflammation and autoimmune disease. There is now emerging evidence indicating that IFN-γ possesses unexpected properties as a master regulator of immune responses and inflammation. In this issue of the JCI, Guillonneau et al. show that indefinite allograft survival induced by CD40Ig treatment is mediated by CD8+CD45RClow T cells through the production of IFN-γ (see the related article beginning on page 1096), supporting the emerging view that IFN-γ is critical in the self-regulation of inflammation. These contradictory roles of IFN-γ, perhaps best understood by the principle of yin and yang, represent one of nature’s paradoxes, whereby the same cytokine functions as an inducer as well as a regulator for inflammation. Understanding this complex process of IFN-γ signaling is essential, as it has therapeutic implications.
Zhuangzi (1020–1078 AD), a Chinese philosopher of Taoism, said, “Material force moves and flows in all directions and in all manners. Its two elements (yin and yang) interact and unite to establish he (harmony), so it gives rise to the concrete. Thus the multiplicity of things is produced. In their ceaseless successions the two elements of yin and yang constitute the great principles of the universe” (1).
T cell responses to antigens are designated Th1 responses (e.g., production of IFN-γ, IL-2, IL-12) or Th2 responses (e.g., production of IL-4, IL-5, IL-13) based upon the production of cytokines (2). A newly identified subset of T cells, Th17 cells, has recently been added to this classification of T cells critical to inflammation (3–5). According to this rather simplistic classification, many autoimmune inflammatory conditions, such as MS, RA, and their respective animal models — EAE and collagen-induced arthritis (CIA) — can be considered predominantly of the Th1 type. These Th1 autoimmune pathologies are characteristically associated with the overproduction of IFN-γ by autoreactive or other inflammatory T cells activated either systemically or at the site of inflammation.
Paradoxical roles of IFN-γ in relation to its known proinflammatory properties
Once inflammation is initiated, IFN-γ is produced and subsequently acts through various molecules and pathways of the immune system to intensify the inflammatory process. There is an overwhelming body of literature extensively documenting the proinflammatory nature of IFN-γ, which has led to the mainstream opinion that IFN-γ is a prime proinflammatory cytokine in inflammation and autoimmune disease. However, emerging evidence from animal models of autoimmune disease (e.g., EAE and CIA) as well as analysis of human autoimmune disease is beginning to indicate that our current thinking regarding the role of IFN-γ as an exclusively proinflammatory cytokine does not accurately reflect the complex properties of IFN-γ. Early indications of the paradoxical roles of IFN-γ come from models of autoimmune disease as demonstrated either by administration or blocking of IFN-γ in these models or by attempts to induce autoimmune inflammation in IFNG-knockout mice (6). For example, there was increased susceptibility and severity of EAE and CIA in susceptible mouse strains genetically deficient in IFN-γ or the IFN-γ receptor (7–9). Furthermore, knockout of the gene encoding IFN-γ renders mouse strains genetically less susceptible to exhibit full-blown disease and is accompanied by markedly increased T cell responses and high titers of specific antibodies (10). However, these important contradictions were never satisfactorily explained and were often considered exceptions to the role of IFN-γ as a Th1 cytokine.
Regulatory roles for IFN-γ
These important issues are addressed in the report by Guillonneau et al. in this issue of the JCI, which shows that indefinite allograft survival in rats can be achieved by therapeutic intervention with CD40Ig, which interrupts the interaction of CD40 and CD40 ligand (CD40L) (11). The authors demonstrate that this regulatory effect is mediated by CD8+CD45RClow T cells induced by the treatment. More importantly, the resulting CD8+CD45RClow T cells act through the production of IFN-γ to induce indoleamine 2,3-dioxygenase (IDO) expression in graft endothelial cells, which accounts for the graft acceptance. The data support the conclusion that IFN-γ produced locally by CD8+CD45RClow T cells is directly responsible for the immune regulation and the treatment effect of CD40Ig through the suppressive mechanism involving IDO. The study adds to growing evidence of the regulatory role of IFN-γ in immune response and inflammation. The regulatory mechanism of IFN-γ seems to involve its interaction with the immune system at multiple levels or checkpoints. At the level of immune regulation, in a recent study to delineate the mechanism of the increased susceptibility of IFN-γ–knockout mice to EAE, the authors unexpectedly discovered that IFN-γ was required for the expression of Foxp3 and the peripheral conversion of CD4+ Tregs in the course of EAE (12). This process was markedly impaired in IFN-γ–deficient mice. At the level of T cell activation, IFN-γ appears to induce overexpression of negative costimulatory molecules in synovial inflammatory T cells and macrophages in RA (13). The negative, but not the positive, costimulatory molecules (i.e., programmed cell death 1 [PD-1] and PD-1 ligand 1 [PD-L1]) were found to be selectively upregulated through IFN-γ overproduced in the rheumatoid synovium as a result of local inflammation. Furthermore, similar to the observation described by Guillonneau and colleagues (11), IFN-γ appears to induce resistance of synovial inflammatory T cells to IDO-mediated deprivation of tryptophan in patients with RA (14). There are still other examples of the regulatory role of IFN-γ in inflammation, such as the role of IFN-γ in antagonizing the function of IL-17, a critical pathogenic cytokine in autoimmune conditions (3, 4, 15, 16). Such a regulatory effect would be considered highly counterintuitive for IFN-γ, a recognized primary proinflammatory cytokine. However, it can be appreciated that at the height of inflammation in the course of an autoimmune pathology, the immune system is mobilized to contain excess inflammation through a number of regulatory mechanisms. Although a few other cytokines have similar roles in inflammation (17), IFN-γ appears, by comparison, to act as a master regulator upstream of many inflammatory and regulatory pathways. This self-regulatory process, through the work of IFN-γ, is important for immune system homeostasis. As a result, most inflammatory processes are self limiting and rarely develop into pathological conditions.
Interpretation of the IFN-γ paradox by the principle of yin and yang
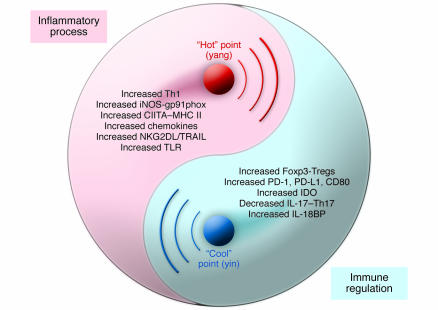
As illustrated in Figure 1, the paradoxical actions of IFN-γ appear to follow the principle of yin and yang, as do many of nature’s paradoxes. That is, the roles of IFN-γ only exist in a well-defined, integrated system in which the two elements of a proinflammatory process (yang) and an antiinflammatory/regulatory process (yin) interact to achieve and maintain balance. When inflammation reaches its culmination, high levels of IFN-γ turn the inflammatory process into an opposing one by activating the regulatory mechanisms that deliver negative feedback. As a renowned ancient Chinese philosopher of Confucianism, Shao Yong (1012–1077 AD), stated, “Yang cannot exist by itself; it can exist only when it is supported by yin. Hence yin is the foundation of yang. Similarly, yin cannot alone manifest itself; it can manifest itself only when accompanied by yang. Hence yang initiates the expression of yin. Yang controls the origination and enjoys the completion of a process while yin follows the effects produced by yang and completes the work of yang” (18). It is left to us to comprehend the underlying process that flips the switch, allowing two opposite processes to occur during inflammation, and to discover what determining factors and requirements are involved.
Figure 1. The paradoxical roles and interplay of IFN-γ in inflammation and autoimmune disease.
These roles are best illustrated in the familiar diagram of yin and yang, the “Great Ultimate” [T’ai-chi] diagram. When an inflammatory process (yang) is initiated, IFN-γ is produced to promote inflammation through multiple genes of the immune system (some are indicated). As IFN-γ reaches its peak level (the “hot” point), inflammation intensifies (enlargement of yang area) and compresses its opposite. The dominant signal of IFN-γ then flows into the opposite area (yin) and activates a regulatory process through various genes and pathways to reach the “cool” point, resulting in shift of the dividing line toward the reduction of inflammation (enlargement of yin area, mutually compressing its opposite). CIITA, MHC class II–specific transactivator; IL-18BP, IL-18 binding protein; NKG2DL, NK group 2D ligand; TRAIL, TNF-related apoptosis–inducing ligand.
Therapeutic implications based on the complex roles of IFN-γ
A detailed understanding of the paradoxical roles of IFN-γ in inflammation has significant implications for delineating the underlying mechanisms and developing effective treatments for autoimmune pathologies. Why, in circumstances of autoimmune pathology, does IFN-γ produced at high levels nevertheless fail to regulate inflammation? One of the possibilities is that the regulatory mechanism activated by IFN-γ does not fail but rather is interrupted by factors abnormally produced in association with the given pathology. There is experimental evidence supporting such a possibility. For example, in one of the cases discussed above, persistent activation of T cells in rheumatoid synovium coexists with the overexpression of negative costimulatory molecules (e.g., PD-1). However, T cell function is interrupted by a soluble form of the same negative costimulatory molecule (soluble PD-1) overproduced in rheumatoid synovium (13). It appears that overproduction of soluble PD-1 is caused by an abnormal splicing variant associated with RA (13). More importantly, how does our understanding of the paradoxical roles of IFN-γ translate into our therapeutic strategy? In this context, it must be mentioned that an initial attempt to treat MS with IFN-γ led to early suspension of a clinical trial, as the treatment resulted in clinical worsening of the disease (19). This indicates that the therapeutic application of IFN-γ is a much more complex issue. Administration of exogenous IFN-γ at an artificially determined dosage and route is unlikely to mimic the complex in vivo situation required for IFN-γ to act as a regulator. Therefore, when compared with its general proinflammatory properties, a therapeutic window for use of IFN-γ as a regulatory cytokine, if it exists, is likely to be extremely narrow and clinically unpredictable. It is of interest to note that some of the currently approved immunomodulatory drugs for autoimmune disease may work partially through IFN-γ induction. One example is glatiramer acetate (GA), a treatment option for MS. GA is a mixture of random polymers composed of four amino acids frequently appearing in myelin basic protein, a putative autoantigen for MS. It stimulates T cells to produce both Th1 and Th2 cytokines, including IFN-γ. One of the possible regulatory mechanisms of GA may involve its ability to induce adaptive Tregs by inducing Foxp3 expression through IFN-γ production (20). By the same token, care must be taken in design of anti–IFN-γ therapy for the treatment of autoimmune disease, as such therapy may considerably interfere with the regulatory activity of endogenously produced IFN-γ. Much has yet to be learned about the molecular mechanism(s) required for IFN-γ to switch its roles at different stages of inflammation.
Footnotes
Nonstandard abbreviations used: CD40L, CD40 ligand; CIA, collagen-induced arthritis; GA, glatiramer acetate; IDO, indoleamine 2,3-dioxygenase; PD-1, programmed cell death 1; PD-L1, programmed cell death 1 ligand 1.
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:871–873 (2007). doi:10.1172/JCI31860.
See the related article beginning on page 1096.
References
- 1. Du, W.M. 1989. The continuity of being: Chinese visions of nature. InNature in Asian traditions of thought. J.B. Callicott and R. Ames, editors. State University Press of New York. Albany, New York, USA. 73–78. [Google Scholar]
- 2.Mosmann T.R., Cherwinski H., Bond M.W., Giedlin M.A., Coffman R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 3.Harrington L.E., et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 4.Park H., et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivanov I.I., et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. . Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Rosloniec E.F., Latham K., Guedez Y.B. Paradoxical roles of IFN-gamma in models of Th1-mediated autoimmunity. Arthritis Res. 2002;4:333–336. doi: 10.1186/ar432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferber I.A., et al. Mice with a disrupted INF-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J. Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 8.Vermeire K., et al. Accelerated collagen-induced arthritis in IFN-gamma receptor-deficient mice. J. Immunol. 1997;158:5507–5513. [PubMed] [Google Scholar]
- 9.Manoury-Schwartz B., et al. High susceptibility to collagen- induced arthritis in mice lacking IFN-gamma receptors. J. Immunol. 1997;158:5501–5506. [PubMed] [Google Scholar]
- 10.Guedez Y.B., et al. Genetic ablation of interferon-gamma up-regulates interleukin-1beta expression and enables the elicitation of collagen-induced arthritis in a nonsusceptible mouse strain. Arthritis Rheum. 2001;44:2413–2424. doi: 10.1002/1529-0131(200110)44:10<2413::aid-art406>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Guillonneau C., et al. CD40Ig treatment results in allograft acceptance mediated by CD8+CD45RClow T cells, IFN-γ, and indoleamine 2,3-dioxygenase. . J. Clin. Invest. 2007;117:1096–1106. doi: 10.1172/JCI28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z., et al. Role of IFN-γ in induction of Foxp3 and conversion of CD4+ CD25– T cells to CD4+ Tregs. . J. Clin. Invest. 2006;116:2434–2441. doi: 10.1172/JCI25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan B., et al. Aberrant regulation of synovial T cell activation by soluble co-stimulatory molecules in rheumatoid arthritis. J. Immunol. 2006;177:8844–8850. doi: 10.4049/jimmunol.177.12.8844. [DOI] [PubMed] [Google Scholar]
- 14.Zhu L., et al. Synovial autoreactive T cells in rheumatoid arthritis resist IDO-mediated inhibition. J. Immunol. 2006;177:8226–8233. doi: 10.4049/jimmunol.177.11.8226. [DOI] [PubMed] [Google Scholar]
- 15.Sawitzki B., et al. 2005IFN-gamma production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo . J. Exp. Med. 2011925–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komiyama Y., et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 17.Aringer M., Smolgen J.S. Tumour necrosis factor and other proinflammatory cytokines in systemic lupus erythematosus: a rationale for therapeutic intervention. Lupus. 2004;13:344–347. doi: 10.1191/0961203303lu1024oa. [DOI] [PubMed] [Google Scholar]
- 18. Chan, W.T. 1969.A source book in Chinese philosophy. Princeton University Press. Princeton, New Jersey, USA. 481–488. [Google Scholar]
- 19.Panitch H.S., Hirsch R.L., Haley A.S., Johnson K.P. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1:893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 20.Hong J., Li N., Zhang X., Zheng B., Zhang J.Z. Induction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc. Natl. Acad. Sci. U. S. A. 2005;102:6449–6454. doi: 10.1073/pnas.0502187102. [DOI] [PMC free article] [PubMed] [Google Scholar]