Abstract
Stress exposure, depending on its intensity and duration, affects cognition and learning in an adaptive or maladaptive manner. Studies addressing the effects of stress on cognitive processes have mainly focused on conditioned fear, since it is suggested that fear-motivated learning lies at the root of affective and anxiety disorders. Inhibition of fear-motivated response can be accomplished by experimental extinction of the fearful response to the fear-inducing stimulus. Converging evidence indicates that extinction of fear memory requires plasticity in both the medial prefrontal cortex and the amygdala. These brain areas are also deeply involved in mediating the effects of exposure to stress on memory. Moreover, extensive evidence indicates that gamma-aminobutyric acid (GABA) transmission plays a primary role in the modulation of behavioral sequelae resulting from a stressful experience, and may also partially mediate inhibitory learning during extinction. In this review, we present evidence that exposure to a stressful experience may impair fear extinction and the possible involvement of the GABA system. Impairment of fear extinction learning is particularly important as it may predispose some individuals to the development of posttraumatic stress disorder. We further discuss a possible dysfunction in the medial prefrontal cortex-amygdala circuit following a stressful experience that may explain the impaired extinction caused by exposure to a stressor.
1. INTRODUCTION
Pavlovian fear conditioning is an extensively studied model for stress and anxiety-like disorders [1]. In this form of learning, an animal is exposed to pairings of a neutral conditioned stimulus (CS) such as a light or tone, with a fear-inducing unconditioned stimulus (US), such as a mild foot shock, and comes to exhibit a conditioned fear response (CR) to the CS. The CR includes freezing, increased startle reflexes, autonomic changes, analgesia, and behavioral response suppression. Experimental extinction is a behavioral technique leading to suppression of the acquired fear, that is, a decrease in the amplitude and frequency of a CR as a function of nonreinforced CS presentations. Experimental extinction is assumed to reflect an active learning process that is distinct from acquisition of fear and requires additional training to develop [2–5].
While clearly of importance to survival, the expression of emotional associations may become disadvantageous when the conditioned cue ceases to predict the appearance of danger. In that respect, the ability to extinguish emotional responses in the face of a no-longer relevant conditioned cue is an essential part of a healthy emotional memory system, particularly with respect to phobias, panic disorders, and posttraumatic stress disorder [PTSD; [4, 6–8]]. Thus, the suppression of the fear response (i.e., extinction) receives increasing attention, since it could become an effective intervention for the treatment of fear-related disorders.
Extinction suppresses, rather than erases, the original CS-US association. For example, even the completely extinguished fear can be recovered spontaneously after the passage of time [9, 10], or be “reinstated” by presentations of the US alone [11, 12], or be renewed by placing the animal in a context different from the one in which it was extinguished [13]. This is congruent with the notion that extinction is a form of relearning (of a CS-no US or “inhibitory” association) rather than unlearning (of the CS-US association) [14]. Accordingly, one suggestion put forward that extinction suppresses the expression of an intact underlying fear response, and extinction memory is labile and weak compared with the fear conditioning itself. Hence, understanding the factors that facilitate or impair extinction may aid in accelerating behavior therapy for the treatment of anxiety disorders.
Despite the efficacy of behavior therapy for human anxiety disorders, extinction-like treatments require repeated cue exposures and are vulnerable to reversal by a number of environmental factors, particularly stress.
The effects of stressful experiences on cognition are manifested through the activation of multiple mechanisms and operating over different time courses and have been linked to the onset of a variety of affective disorders. Stress can produce deleterious effects on the brain and behavior, and it contributes towards impaired health and an increased susceptibility to disease and mental disorders [15, 16]. Investigations into the interaction between stressful experiences and memory haves focused mainly on the behavioral and neural mechanisms of memory acquisition (i.e., fear conditioning), but not on memory extinction, even though extinction is used for the treatment of psychiatric conditions based on learned fear, such as phobias, panic, generalized anxiety, as well as PTSD.
Extensive evidence indicates that the amygdala and the prefrontal cortex are key structures in the response to stress and its effects on learning and memory. Importantly, it has been shown that extinction of fear memory requires plasticity in both the medial prefrontal cortex (mPFC) and the basolateral amygdala [BLA; [17–19]]. In this review, we will discuss the relevance of the prefrontal cortex-amygdala circuit as a key mechanism for understanding stress-induced alterations occurring during the extinction of fear.
2. STRESS AND EXTINCTION
There are intricate relationships between stress and cognitive processes [20]. On the one hand, cognitive processes are necessary to cope adequately with a stressor, both actively and passively, in that a subject has to be aware that there is a stressor and at the same time it has to learn that the stressor can be controlled by an appropriate response. Adaptation to stress occurs when the acquired response is successful in reducing the impact of the stressor. If not, maladaptation may occur. On the other hand, there is strong evidence that stress and stress hormones play an important role in the modulation of cognitive processes. It should be noted that in the fear conditioning paradigm, stress plays a role during conditioning and at least during the first stages of extinction training. Thus, we differentiate here between the aversive situation in the learning paradigm itself, for example, exposure to a foot shock, and the effects of additional exposure to an out-of-context stressor on fear extinction.
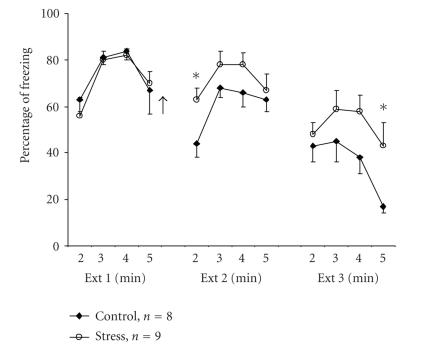
When examining the effects of exposure to an out-of-context stressor on fear extinction, we found that the stressor increased resistance to extinction (H. Reizel, I. Akirav, and M. Maroun, unpublished observation; Figure 1). Specifically, after contextual fear conditioning (using a US of 3 foot shocks of 0.5 mA each), control rats gradually extinguished their freezing (CR) when placed in the extinction box (CS) for 3 consecutive days, for 5 minutes each time. By contrast, the experimental rats were exposed to the out-of-context stressor on being placed on an elevated platform for 30 minutes immediately after the first extinction session. Animals placed on the platform exhibited behavioral “freezing,” that is, immobility for up to 10 minutes, defecation, and urination [21, 22]. This stressor was found to increase plasma corticosterone levels by 38% as compared with naïve rats [23] and we have recently found that it impairs long-term potentiation in the CA1 area of the hippocampus and in the BLA-medial prefrontal pathway [24]. In the contextual fear extinction experiment, the stressed rats showed increased levels of freezing in the extinction box even 48 hours after a single exposure to the elevated platform. This suggests that exposure to the stressor had the long-term effect of impairing the extinction of fear.
Figure 1.
Stress impairs extinction of contextual fear conditioning. Rats were given 3 mild foot shocks in the conditioning chamber. On the next day, the rats were placed in the extinction (Ext) chamber for 5 minutes and no shock was administered (Ext 1; the last 4 minutes are presented since all animals showed high levels of freezing in the first minute). Immediately afterwards, the animals were returned to their home cage (control) or placed on an elevated platform for 30 minutes (stress). Animals were exposed to additional 5 minutes in the extinction chamber, without shocks, on days 3 (Ext 2) and 4 (Ext 3). The stressed animals showed significantly higher levels of freezing compared with the control group during the second minute of Ext 2 (∗; P < .05) and the fifth minute of Ext 3 (∗; P < .05). Arrow denotes time of exposure to stress.
We found that exposure to stress had a similar effect on consolidation of the extinction of auditory fear conditioning (see later, see below, or ahead). The impairing effects of the elevated platform on auditory fear extinction also persisted for 48 hours following exposure to the stressor. Consistent with our results, Izquierdo et al. [25] reported that exposure to three episodes of stress ending 24 hours before fear conditioning significantly attenuated the rate of cued fear extinction relative to nonstressed controls. Shumake et al. [26] showed that rats that were selectively bred for increased susceptibility to learned helplessness show resistance to extinction of conditioned fear. Furthermore, Kellett and Kokkinidis [27] showed that amygdala kindling, which enhances emotionality, impaired the extinction of fear-potentiated startle, and rats showed increased levels of fear. They also found that electrical stimulation of the amygdala restored extinguished fear responses and that the fear reinstatement was specific to the extinction context. In a study with rainbow trout, Moreira et al. [28] compared two lines of fish that exhibit divergent endocrine responsiveness to stressors: the high-responders (HR) and low-responders (LR; the “stressed”). Postconditioning, the fish were tested by presentation of the CS at weekly intervals for 4 weeks, with no further reinforcement, and the extinction of the CR in the two lines was compared. The number of individuals within each line whose plasma cortisol levels indicated a stress response when exposed to the CS was significantly greater among the LR than HR fish at 14 and 21 days, with no HR fish falling into the stress-response category at 21 days. Thus, the stressed fish did not extinguish as well as the HR fish.
It is important to understand why exposure to stress impairs extinction learning, and here we put forward four possible explanations. One possibility is that extinction memory is labile and weak compared with fear conditioning itself, and thus exposure to a stressful experience interferes with the process of extinction learning or with the retrieval of information. Second, it has been shown that a stressful experience following or preceding a threatening or fear-related learning event enhances retention [29]. However, in extinction, the animals need to learn to suppress their fear response that is associated with the CS. Thus, the aversiveness of the stressful experience may counteract the extinguished emotional response. Further, it is possible that preexposure to the stressful experience increases resistance to extinction through sensitization, leading to the occurrence of a conditioned fear response even to a less intense “reminder” of the original US. Thus, retrieval of the CS-US association (i.e., acquisition) overcomes the CS-no US association (i.e., extinction) following the sensitization effect, making extinction more difficult to learn. However, this can hardly explain why exposure to an unrelated stressful experience, such as an elevated platform, should sensitize the animals to respond as if to the US during extinction training. A fourth possibility is that resistance to extinction is not related to sensitization or to the enhancement of an unspecific fear response. Accordingly, if the enhanced fear memory is expressed only when stressed animals are exposed to the CS, it may indicate that this response is sustained by associative learning, and thus the increased freezing behavior of stressed animals could be attributable to an attenuation of the extinction process, rather than to enhanced fear acquisition, although the latter remains a possibility [4].
It is usually assumed that stressful life events interfere with our ability to acquire new information. Yet, previous exposure to both acute and chronic stressful events can positively affect classical conditioning tasks, including fear conditioning [29–33]. Reports to date regarding the effects of stress on fear extinction show that exposure to stress increases resistance to extinction, that is, it impairs extinction acquisition and consolidation, which reduces the extent to which extinction is able to offset a fear response. In contrast, studies addressing the relationship between stress and the acquisition of new fear memories show that exposure to a stressful experience facilitates fear learning, so further enhancing the fear response. For example, previous exposure to a restraint session increased fear conditioning in a contextual fear paradigm [33]. Similarly, Rau et al. [34] have shown that preexposure to a stressor of repeated foot shocks enhanced conditional fear responses to a single context-shock pairing. Cordero et al. [29] have shown that a single exposure to an aversive stimulus is sufficient to facilitate context-dependent fear conditioning, and suggested increased glucocorticoid release at training in the mechanisms mediating the memory-facilitating effects induced by prior stressful experiences. These studies corroborate others showing that if an animal learns a stressful task, then the consolidation of this task may be enhanced by stress and that its end product, corticosterone, may be secreted during the task [35–37]. This was found to be the case in a variety of emotionally arousing tasks, such as inhibitory avoidance, spatial learning, discrimination learning, and fear conditioning [38–44].
3. THE NEURAL BASIS OF FEAR EXTINCTION
The basolateral amygdala (BLA) plays a pivotal role in the consolidation of memories related to fear and emotions, and in the initiation of responses to stressful events [37, 45–50]. Moreover, the BLA is significantly involved in both the formation and extinction of fear memory [17, 51–54]. For example, microinfusions of a protein synthesis inhibitor to the amygdala prevented recall of extinction after 30 minutes, and infusion of N-methyl-D-aspartate (NMDA) receptor antagonists or mitogen-activated protein kinase inhibitors to the BLA prevented across-day extinction of fear-potentiated startle [17, 54–56]. In another study [57], BLA lesions severely attenuated expression of previously acquired fear memory. Also, infusion of an NMDA agonist into the amygdala facilitated fear extinction [58, 59].
Another brain structure that is known to play an important role, not only in the regulation of emotion, but also in the integration of affective states with appropriate modulation of autonomic and neuroendocrine stress regulatory systems [60], is the medial prefrontal cortex (mPFC). The mPFC provides an interface between limbic and cortical structures [61] and regulates the stress-induced activity of the hypothalamus-pituitary-adrenal (HPA) axis [62, 63].
The mPFC is important in long-term fear extinction memory. Specifically, lesions or inhibition of protein synthesis in the infralimbic part of the medial PFC impair recall of extinction of conditioned fear [18, 19, 64, 65]. Furthermore, mPFC stimulation that mimics extinction-induced tone responses reduces conditioned fear [66, 67], and stimulating the mediodorsal thalamic inputs to the mPFC is associated with extinction maintenance [68, 69]. Moreover, functional imaging studies in human subjects indicate that the mPFC is engaged during extinction [70] and that subjects with PTSD have reduced mPFC activity during trauma recall [71]. Furthermore, Miracle et al. [72] have shown that one week of restrained stress had the effect of impairing recall of extinction of conditioned fear, and suggested that this is due to deficits in the mPFC caused by exposure to stress. Recently, it has been reported that stress exposure that impairs fear extinction also caused retraction of terminal branches of apical dendrites of infralimbic neurons [25].
4. THE ROLE OF GABA IN EXTINCTION OF FEAR
In addition to evidence indicating that extinction of fear memory requires plasticity in both the mPFC and the BLA [17–19], recent studies further point to a dysfunctional interaction between the prefrontal cortex and the amygdala in the failure to extinguish conditioned fear. These studies indicate that the mPFC has a function in the inhibition of emotions through its projections to the amygdala [73] and are in line with Pavlov's [74] view that extinction learning involves inhibitory cortical circuits that reduce the CS-evoked conditioned response.
The glutamatergic efferents from the mPFC synapse on amygdala gamma-aminobutyric acid (GABA)ergic neurons [75], and through this, may provide important inhibitory input to the amygdala. Of particular interest is the projection from the infralimbic region of the PFC (which, together with the prelimbic cortex, comprises the ventromedial PFC) to the capsular division of the central nucleus of the amygdala [76]. The capsular division of the central nucleus contains GABA-ergic intercalated cells that have been shown to exert powerful inhibitory control over central nucleus neurons that project out of the amygdala [77–79]. Infralimbic input to intercalated cells could be a pathway by which infralimbic tone responses inhibit the expression of conditioned fear (e.g., reduce freezing) [80].
The anatomical data described for the interaction between these two structures pinpoint the crucial role the neurotransmission of GABA may play in the extinction of fear. Indeed, a substantial number of studies have demonstrated that the BLA contains a powerful inhibitory circuit that uses GABA as a neurotransmitter [81–83]. Moreover, the BLA has larger amounts of benzodiazepine/GABAA receptors than any other amygdala nucleus [84], explaining why the infusion of benzodiazepines or GABAA agonists into the BLA reduces fear conditioning and anxiety [85–88]. Coincidently, local blockade of these receptors attenuates the anxiolytic influence of systemic benzodiazepines [89]. Recently, Rodríguez Manzanares et al. [33] have shown that stress attenuates inhibitory GABA-ergic control in the BLA, leading to neuronal hyperexcitability and increased plasticity that facilitates fear learning. Based on these data, it can be concluded that GABA-ergic mechanisms in the amygdala play a major role in controlling the emotional consequences of stress, and may thus affect extinction of fear.
Benzodiazepines have long been used to treat anxiety and are particularly appropriate in short-term treatment situations [8]. Direct modulation of GABA-ergic neurons, through the benzodiazepine-binding site, down regulates memory storage processes and specifically affects learned fear responses. On the other hand, benzodiazepine release could be modulated by the anxiety and/or stress associated with different types of learning [90].
Much research is directed at exploring the involvement of GABA in inhibiting learned fear responses. Although several studies support the central role GABA neurotransmission plays in extinction, there are different reports regarding whether this role is to facilitate or impair extinction [26, 91–95]. Using direct modulation of GABA-ergic neurons, it has been shown that the benzodiazepine inverse agonist FG7142, which attenuates the effect of GABA at its receptor, retards extinction of conditioned fear [91, 96]. Likewise, McCabe et al. [97] have shown that benzodiazepine agonists administered to mice following training significantly facilitated extinction during a food-reinforced lever-press procedure. Potentiation of GABA by the benzodiazepine agonist chlordiazepoxide administered prior to extinction sessions facilitated extinction in a paradigm of operant responding for food reinforcement [98]. By contrast, systemic administration of the GABAA antagonist picrotoxin, after the extinction of inhibitory avoidance learning, enhanced extinction retention during testing [93], and the GABAA-positive allosteric modulator diazepam impaired extinction retention when administered before extinction in a shuttle avoidance task [95].
There are also a number of ways of modulating GABA-ergic functions indirectly. For example, cannabinoid (CB1) receptors and gastrin-releasing peptide receptors are both located on GABA-containing interneurons. Endogenous cannabinoids, acting at the CB1 receptor, facilitated the extinction of aversive memories [92], and blocking the action of gastrin-releasing peptide, by genetically removing its receptor, retards extinction of learned fear responses [26]. Recently, Azad et al. [99] have shown that CB1 receptors reduce GABA-ergic synaptic transmission in the amygdale, and consequently facilitate extinction of aversive memories. Chhatwal et al. [100] showed that gephyrin mRNA and protein levels in the BLA significantly increased after fear extinction training, suggesting that the modulation of gephyrin and GABAA receptor expression in the BLA may play a role in the experience-dependent plasticity underlying extinction.
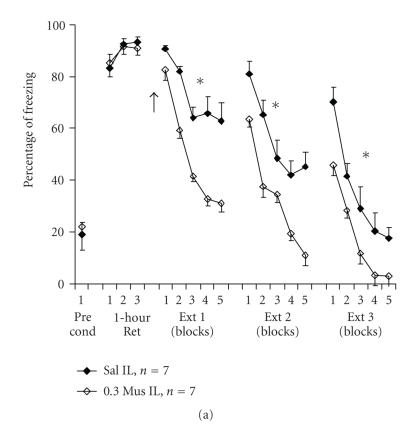
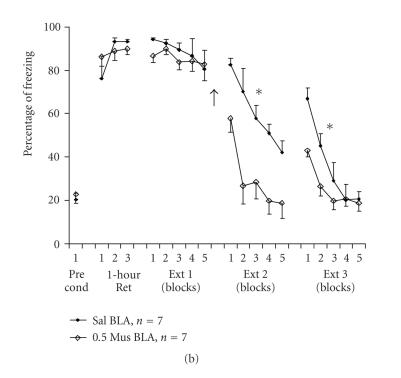
Using a low dose of the GABAA agonist muscimol, we recently found [51] that muscimol infused to the infralimbic area before extinction training (see Figure 2(a)) resulted in long-term facilitation of extinction. By contrast, where infusion of muscimol to the infralimbic area followed extinction training, no such effect was observed, regardless of the length of the extinction training period (5 or 15 trials; data not shown). However, infusion of muscimol to the BLA following a short (5-trial) extinction session facilitated extinction for at least 48 hours post-drug-infusion (see Figure 2(b)). The differences between the temporal parameters of the effects of muscimol in the infralimbic cortex compared to the BLA suggest differential involvement of these structures in long-term extinction of fear memory. We propose that GABAA neurotransmission in the infralimbic cortex plays a facilitatory role in triggering the onset of fear extinction and its maintenance, whereas in the BLA, GABAA neurotransmission facilitates extinction consolidation.
Figure 2.
(a) A low volume of muscimol microinfused into the infralimbic cortex before extinction training facilitates extinction learning. Rats received 7 pairings of a tone with a foot shock in the conditioning chamber. After 1 hour, three tones were delivered in the absence of foot shock (1-hour Ret). On the next day, the animals were microinfused with a total of 0.3 μl saline (Sal) or muscimol (0.3 Mus) to the infralimbic cortex (IL) and were exposed to 15 tones without foot shocks (Ext 1; presented as 5 blocks of 3 trials). Animals were exposed to additional 15 tones on days 4 (Ext 2) and 5 (Ext 3), without further administration of the drug. Muscimol IL animals showed significantly lower levels of freezing compared with the saline group in Ext 1 (∗; P < .001), Ext 2 (∗; P < .01) and Ext 3 (∗; P < .05). This supports a selective involvement of the IL in facilitating extinction of conditioned fear (see Akirav et al. [51]). Arrow denotes time of drug infusion. The Pre cond data points indicate the amount of freezing exhibited by rats prior to commencement of fear conditioning. (b) A low volume of muscimol microinfused to the basolateral amygdala following a short extinction training session facilitates extinction consolidation. Rats received 7 pairings of a tone with a foot shock in the conditioning chamber. After 1 hour, three tones were delivered in the absence of foot shock (1-hour Ret). On the next day, the animals underwent a short extinction training session consisting of 5 tones (Ext 1; presented as 5 trials), and were thereafter microinfused with a total volume of 0.5 μl saline (Sal) or muscimol (0.5 Mus) to the basolateral amygdala (BLA). On days 4 and 5 (Ext 2 and Ext 3, resp.), the animals were exposed to 15 tones without foot shocks (presented as 5 blocks of 3 trials). The BLA muscimol group showed significantly reduced levels of freezing compared with the other two groups during Ext 2 (∗; P < .001) and Ext 3 (∗; P < .05). This supports the selective involvement of the BLA in facilitating consolidation of extinction of conditioned fear (see Akirav et al. [51]). Arrow denotes time of drug infusion. The Pre cond data points indicate the amount of freezing exhibited by rats prior to commencement of fear conditioning.
Overall, the data suggest that manipulation of GABA transmission may have very different effects depending on whether it is administered pre- or postextinction training or before a retention test, and depending also on the behavioral paradigm used. Future studies are required to understand these discrepancies.
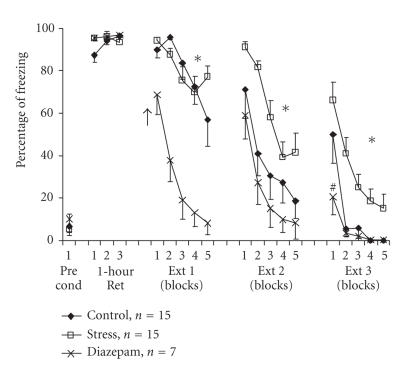
While examining the involvement of GABA in the effects of stress on fear extinction, we found that systemic administration of the benzodiazepine agonist diazepam reversed the resistance to extinction induced by exposure to an out-of-context stressor (see Figure 3). After classical auditory fear conditioning (3 CS-US pairings of a tone with a foot shock of 0.5 mA), control rats that were exposed to the tone without shock gradually extinguished their freezing (CR) in response to the tone during extinction training. At the end of the third extinction session, their freezing levels dropped to zero. Rats that were exposed to an out-of-context stressor (i.e., animals that were placed on an elevated platform for 30 minutes) before the first extinction training session showed increased levels of freezing in response to the tone even 48 hours after the stressor (i.e., showed resistance to extinction). A single injection of diazepam (2 mg/kg, IP) 20 minutes before exposure to the out-of-context stressor significantly facilitated extinction compared with the control and stress groups as manifested by reduced freezing levels in the first extinction session. On the second and third sessions of extinction training, the response of the diazepam-stress group was no different to that of the control group, with the former group also exhibiting significantly less freezing than the stressed rats that had not first received diazepam. Hence, treatment with diazepam reversed the impairing effect of exposure to stress on fear extinction. Further experiments to elucidate the possible role GABA plays in the BLA and the mPFC in preventing stress-associated impairments of extinction are required.
Figure 3.
Diazepam overcomes stress-induced impairment of the extinction of auditory fear. Rats were exposed to 3 pairings of a tone with a mild foot shock in the conditioning chamber. On the next day, control animals remained in their home cages, “diazepam” group animals were injected with diazepam (2 mg/kg, IP) 20 minutes before being placed on an elevated platform for 30 minutes, while “stress” group animals were placed directly onto the elevated platform for 30 minutes, without prior administration of the drug. Immediately afterwards, animals were taken for extinction training and were exposed to15 tones (Ext 1) with no shock. Animals were exposed to an additional 15 tones on days 3 (Ext 2) and 4 (Ext 3) with no drug or shock. There were significant differences between the diazepam group and the other groups during Ext 1 (P < .001). On Ext 2 and Ext 3, the stress group was significantly different from the control (Ext 2: P < .05, Ext 3: P < .01) and the diazepam (Ext 2: P < .01, Ext 3: P < .001) groups. Arrow denotes time of drug infusion. The Pre cond data points indicate the amount of freezing exhibited by rats prior to commencement of fear conditioning.
A problem associated with the use of anxiolytic and anxiogenic compounds in studies of extinction, however, is the possibility of state dependency as opposed to a true effect on the suppression of the learning process [101]. That is, it is possible that a drug administered before or immediately following extinction produces an internal state, or drug context, that is discriminable to the animal [102]. However, in our experiment, the effect was probably not due to state dependence because the stressed animals that were treated with diazepam showed less freezing (i.e., more extinction) than the stressed animals that were treated with saline, even 24 and 48 hours after a single injection.
To conclude, the present results demonstrate that pretreatment with the benzodiazepine tranquilizer diazepam reverses the CR-enhancing effects of the elevated platform experience. These findings suggest that benzodiazepines may prevent the augmentation of the trauma-related symptoms seen in phobia and PTSD patients that are caused by exposure to a stressful experience.
5. EXTINCTION OF FEAR: INTERPLAY FOR DOMINANCE BETWEEN THE AMYGDALA AND THE PREFRONTAL CORTEX
Recent observations provide direct physiological support that the mPFC reduces fear responses by reducing amygdala output [66, 103, 104]. For example, Milad and Quirk [66] found that stimulation of the mPFC decreases the responsiveness of central amygdala neurons that regularly fire in response to the CS only when animals are recalling extinction of a fear task learned using that CS. Additionally, Morgan et al. [64] reported that rats with mPFC lesions had an increased resistance to extinction. They proposed that connections between the mPFC and amygdala normally allow the organism to adjust its emotional behavior when environmental circumstances change, and that some alteration in this circuitry, causing a loss of prefrontal control of the amygdala, might underlie the inability of persons with anxiety disorders to regulate their emotions.
If the mPFC normally inhibits the amygdala as an active component of extinction of fear conditioning, then when the mPFC is inhibited or suppressed, emotional associations mediated by the amygdala may be not inhibited during nonreinforcement. As a result, conditioned responding may be prolonged over time [64].
A combination of changes throughout this circuit is important in generating stress-induced changes in emotionality. The mPFC may have a regulatory role in stress-induced fear and anxiety-like behaviors through inhibitory effects on amygdala output and processing [105]. Indeed, extensive evidence supports the notion that the BLA is a site of plasticity for fear conditioning [104, 106], and that the BLA is extensively connected with the central nucleus of the amygdala [107, 108]. In turn, the central nucleus projects to the paraventricular nucleus of the hypothalamus [109], thereby providing the most likely route for any BLA-dependent effects on stress-induced HPA output.
We would like to take this a step further, and suggest a possible mode of action for the mPFC-amygdala circuit in fear extinction under stressful conditions. Accordingly, under normal conditions of fear suppression, the mPFC is activated and inhibits amygdala output. This dominance of the mPFC results in normal suppression of fear, and in consequence promotes extinction of fear. However, exposure to a stressful experience may reduce medial PFC inhibition of the amygdala, and as a result the amygdala takes control to assure defensive behaviors and becomes dominant. The expected consequence is interference in the suppression of the fear response, that is, impaired extinction learning. Therefore, exposure to a stressful experience would result in reduced mPFC activity leading to resistance to extinction and inappropriate and exaggerated fear responses, as seen in PTSD patients. Indeed, abnormally low PFC activity together with abnormally high amygdala activity were found in PTSD patients, when reexposed to traumatic reminders [110]. Accordingly, deficits in extinction of conditioned fear as a result of exposure to a stressful experience are proposed to contribute to the sustained anxiety responses seen in PTSD.
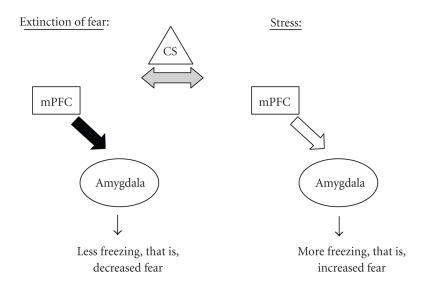
Figure 4 schematically summarizes this idea and shows that during extinction of fear, the mPFC is activated and acts to inhibit the amygdala in order to reduce fear, resulting in less freezing (i.e., extinction). However, exposure to stress at a critical time with respect to extinction learning activates the amygdala to increase fear and the result is more freezing (i.e., resistance to extinction). Therefore, according to our proposed model, the stressor shifts the dominance from the mPFC to the amygdala and, as a consequence, extinction of fear is impaired.
Figure 4.
A possible mode of action for the medial prefrontal cortex-amygdala circuit in fear extinction under normal and stressful conditions. Under normal conditions of fear suppression, the medial prefrontal cortex (mPFC) is activated and inhibits amygdala output (filled arrow). This dominance of the mPFC results in less freezing in response to a conditioned stimulus (CS; i.e., extinction). However, under stressful conditions, the inhibitory action of the mPFC on the amygdala is reduced (empty arrow), the amygdala dominates (indicated by the bold circle around the amygdala) and the result is more freezing in response to a CS (i.e., impaired extinction).
Our model is consistent with the data shown in Figure 1, which demonstrate that exposure to a stressful experience results in resistance to extinction in the stressed group compared with the nonstressed group. Whether this effect is due to a reduction in mPFC modulation of amygdala output, and to the involvement of GABA-based mechanisms acting on the PFC-amygdala circuit, still needs to be examined. Our model is also consistent with the suggestion put forward by Quirk and Gehlert [111] that deficient inhibitory tone in the amygdala due to decreased inhibition from the prefrontal cortex could lead to overexpression of conditioned responses, producing pathological states such as anxiety disorders and drug-seeking behavior.
6. PERSPECTIVES
Pathological fear and anxiety, such as that exhibited by PTSD sufferers, may be the manifestation of abnormal modulations in the activity of the amygdala and the mPFC, and in their interaction. PTSD is defined as symptoms of reexperiencing the trauma, avoidance of associated stimuli and hyperarousal symptoms, suggesting a heightened fear response, and it has been proposed that PTSD symptoms reflect amygdala hyperresponsivity to fear-related stimuli, with a concomitant lack of “top-down” prefrontal inhibition. This proposal is supported by neuroimaging studies of PTSD patients, which observed abnormal reductions in mPFC activity [71, 112, 113], as well as enhanced and distinctive amygdala engagement [114, 115], particularly for combat PTSD veterans [113]. In line with this, fMRI and PET data have shown significant inverse correlations between the functional activity of the mPFC and the amygdala [116, 117]. Collectively, these data provide strong support for the hypothesis that PTSD is characterized by a failure of the mPFC to sufficiently inhibit the amygdala.
There is clinical interest in the effects of stress on fear extinction learning as a model for the mechanisms operating in PTSD, as well as interest in means to improve therapeutic outcomes following fear-extinction-based strategies. Future therapies aimed at increasing the inhibitory tone in the amygdala, either locally or via the prefrontal cortex, may accelerate extinction and may help in the treatment of anxiety disorders.
ACKNOWLEDGMENTS
We thank Professor Joram Feldon from Laboratory of Behavioral Biology, Zurich, Switzerland, for his generous help with the digitized freezing box. We thank Noam Hikind and Hagit Raizel for their technical help. This research is supported by a grant from the Ebelin and Gerd Bucerius ZEIT Foundation to the second author.
References
- 1.Charney DS. Psychobiological mechanism of resilience and vulnerability: implications for successful adaptation to extreme stress. American Journal of Psychiatry. 2004;161(2):195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 2.Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science. 2001;291(5512):2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- 3.Bouton ME, Nelson JB. Context-specificity of target versus feature inhibition in a feature-negative discrimination. Journal of Experimental Psychology: Animal Behavior Processes. 1994;20(1):51–65. [PubMed] [Google Scholar]
- 4.Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36(4):567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- 5.Rescorla RA. Preservation of pavlovian associations through extinction. Quarterly Journal of Experimental Psychology Section B: Comparative and Physiological Psychology. 1996;49(3):245–258. [Google Scholar]
- 6.Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M. Psychobiologic mechanisms of posttraumatic stress disorder. Archives of General Psychiatry. 1993;50(4):295–305. doi: 10.1001/archpsyc.1993.01820160064008. [DOI] [PubMed] [Google Scholar]
- 7.Fyer AJ. Current approaches to etiology and pathophysiology of specific phobia. Biological Psychiatry. 1998;44(12):1295–1304. doi: 10.1016/s0006-3223(98)00274-1. [DOI] [PubMed] [Google Scholar]
- 8.Gorman JM. Treating generalized anxiety disorder. Journal of Clinical Psychiatry. 2003;64(supplement 2):24–29. [PubMed] [Google Scholar]
- 9.Rescorla RA. Experimental extinction. In: Mowrer RR, Klein S, editors. Handbook of Contemporary Learning Theories. Mahwah, NJ, USA: Erlbaum; 2001. pp. 119–154. [Google Scholar]
- 10.Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learning and Memory. 2002;9(6):402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. Journal of Experimental Psychology: Animal Behavior Processes. 1975;1(1):88–96. [PubMed] [Google Scholar]
- 12.Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5(4):368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- 13.Bouton ME, King DA. Contextual control of the extinction of conditioned fear: tests for the associative value of the context. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9(3):248–265. [PubMed] [Google Scholar]
- 14.Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301(5636):1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- 15.McEwen BS. The brain is an important target of adrenal steroid actions: a comparison of synthetic and natural steroids. Annals of the New York Academy of Sciences. 1997;823:201–213. doi: 10.1111/j.1749-6632.1997.tb48392.x. [DOI] [PubMed] [Google Scholar]
- 16.Baum A, Posluszny DM. Health psychology: mapping biobehavioral contributions to health and illness. Annual Review of Psychology. 1999;50:137–163. doi: 10.1146/annurev.psych.50.1.137. [DOI] [PubMed] [Google Scholar]
- 17.Falls WA, Miserendino MJD, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. The Journal of Neuroscience. 1992;12(3):854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behavioral Neuroscience. 1995;109(4):681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- 19.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. The Journal of Neuroscience. 2000;20(16):6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prickaerts J, Steckler T. Effects of glucocorticoids on emotion and cognitive processes in animals. In: Steckler TS, Kalin NH, Ruel JMHM, editors. Handbook of Stress and the Brain. Amsterdam, The Netherlands: Elsevier; 2005. pp. 359–385. [Google Scholar]
- 21.Xu L, Anwyl R, Rowan MJ. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387(6632):497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, Anwyl R, Rowan MJ. Spatial exploration induces a persistent reversal of long-term potentiation in rat hippocampus. Nature. 1998;394(6696):891–894. doi: 10.1038/29783. [DOI] [PubMed] [Google Scholar]
- 23.Kavushansky A, Richter-Levin G. Effects of stress and corticosterone on activity and plasticity in the amygdala. Journal of Neuroscience Research. 2006;84(7):1580–1587. doi: 10.1002/jnr.21058. [DOI] [PubMed] [Google Scholar]
- 24.Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. The Journal of Neuroscience. 2003;23(11):4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. The Journal of Neuroscience. 2006;26(21):5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shumake J, Barrett D, Gonzalez-Lima F. Behavioral characteristics of rats predisposed to learned helplessness: reduced reward sensitivity, increased novelty seeking, and persistent fear memories. Behavioural Brain Research. 2005;164(2):222–230. doi: 10.1016/j.bbr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Kellett J, Kokkinidis L. Extinction deficit and fear reinstatement after electrical stimulation of the amygdala: implications for kindling-associated fear and anxiety. Neuroscience. 2004;127(2):277–287. doi: 10.1016/j.neuroscience.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Moreira PSA, Pulman KGT, Pottinger TG. Extinction of a conditioned response in rainbow trout selected for high or low responsiveness to stress. Hormones and Behavior. 2004;46(4):450–457. doi: 10.1016/j.yhbeh.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats: evidence for a role of corticosterone. Hormones and Behavior. 2003;44(4):338–345. doi: 10.1016/s0018-506x(03)00160-0. [DOI] [PubMed] [Google Scholar]
- 30.Shors TJ, Weiss C, Thompson RF. Stress-induced facilitation of classical conditioning. Science. 1992;257(5069):537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- 31.Beylin AV, Shors TJ. Stress enhances excitatory trace eyeblink conditioning and opposes acquisition of inhibitory conditioning. Behavioral Neuroscience. 1998;112(6):1327–1338. doi: 10.1037//0735-7044.112.6.1327. [DOI] [PubMed] [Google Scholar]
- 32.Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiology of Learning and Memory. 2001;75(1):10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. The Journal of Neuroscience. 2005;25(38):8725–8734. doi: 10.1523/JNEUROSCI.2260-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neuroscience and Biobehavioral Reviews. 2005;29(8):1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 35.De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocrine Reviews. 1998;19(3):269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 36.De Kloet ER, Oitzl MS, Joëls M. Stress and cognition: are corticosteroids good or bad guys? Trends in Neurosciences. 1999;22(10):422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 37.Roozendaal B. Systems mediating acute glucocorticoid effects on memory consolidation and retrieval. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27(8):1213–1223. doi: 10.1016/j.pnpbp.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Kovacs GL, Telegdy G, Lissak K. Dose dependent action of corticosteroids on brain serotonin content and passive avoidance behavior. Hormones and Behavior. 1977;8(2):155–165. doi: 10.1016/0018-506x(77)90032-0. [DOI] [PubMed] [Google Scholar]
- 39.Flood JF, Vidal D, Bennett EL, Orme AE, Vasquez S, Jarvik ME. Memory facilitating and anti-amnesic effects of corticosteroids. Pharmacology Biochemistry and Behavior. 1978;8(1):81–87. doi: 10.1016/0091-3057(78)90127-2. [DOI] [PubMed] [Google Scholar]
- 40.Roozendaal B, McGaugh JL. Amygdaloid nuclei lesions differentially affect glucocorticoid-induced memory enhancement in an inhibitory avoidance task. Neurobiology of Learning and Memory. 1996;65(1):1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- 41.Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behavioral Neuroscience. 1997;111(3):503–511. [PubMed] [Google Scholar]
- 42.Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. European Journal of Neuroscience. 1997;9(4):637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 43.Cordero MI, Sandi C. A role for brain glucocorticoid receptors in contextual fear conditioning: dependence upon training intensity. Brain Research. 1998;786(1-2):11–17. doi: 10.1016/s0006-8993(97)01420-0. [DOI] [PubMed] [Google Scholar]
- 44.Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiology of Learning and Memory. 2004;81(1):67–74. doi: 10.1016/j.nlm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews Neuroscience. 2002;3(6):453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 46.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 47.McGaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends in Neurosciences. 2002;25(9):456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- 48.Paré D. Role of the basolateral amygdala in memory consolidation. Progress in Neurobiology. 2003;70(5):409–420. doi: 10.1016/s0301-0082(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 49.Pelletier JG, Paré D. Role of amygdala oscillations in the consolidation of emotional memories. Biological Psychiatry. 2004;55(6):559–562. doi: 10.1016/j.biopsych.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 50.Seidenbecher T, Reymann KG, Balschun D. A post-tetanic time window for the reinforcement of long-term potentiation by appetitive and aversive stimuli. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(4):1494–1499. doi: 10.1073/pnas.94.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABAA agonist muscimol into the rat prefrontal cortex and amygdala. European Journal of Neuroscience. 2006;23(3):758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- 52.Maren S. Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends in Neurosciences. 1999;22(12):561–567. doi: 10.1016/s0166-2236(99)01465-4. [DOI] [PubMed] [Google Scholar]
- 53.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406(6797):722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 54.Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. The Journal of Neuroscience. 2001;21(16):RC162. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. The Journal of Neuroscience. 1998;18(20):8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin C-H, Lee C-C, Gean P-W. Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Molecular Pharmacology. 2003;63(1):44–52. doi: 10.1124/mol.63.1.44. [DOI] [PubMed] [Google Scholar]
- 57.Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. The Journal of Neuroscience. 2005;25(42):9680–9685. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker DL, Ressler KJ, Lu K-T, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. The Journal of Neuroscience. 2002;22(6):2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behavioral Neuroscience. 2003;117(2):341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- 60.Sullivan RM. Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Stress. 2004;7(2):131–143. doi: 10.1080/102538900410001679310. [DOI] [PubMed] [Google Scholar]
- 61.Groenewegen HJ, Uylings HBM. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Progress in Brain Research. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- 62.Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: possible role of the bed nucleus of the stria terminalis. The Journal of Comparative Neurology. 2005;481(4):363–376. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- 63.Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27(1-2):99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- 64.Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neuroscience Letters. 1993;163(1):109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 65.Santini E, Ge H, Ren K, Peña de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. The Journal of Neuroscience. 2004;24(25):5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 67.Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behavioral Neuroscience. 2004;118(2):389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- 68.Herry C, Garcia R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. The Journal of Neuroscience. 2002;22(2):577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herry C, Garcia R. Behavioral and paired-pulse facilitation analyses of long-lasting depression at excitatory synapses in the medial prefrontal cortex in mice. Behavioural Brain Research. 2003;146(1-2):89–96. doi: 10.1016/j.bbr.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 70.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 71.Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biological Psychiatry. 1999;45(7):806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiology of Learning and Memory. 2006;85(3):213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 73.Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? Journal of Affective Disorders. 2002;70(1):1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pavlov IP. Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. London, UK: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. The Journal of Neuroscience. 2002;22(1):324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71(1):55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 77.Paré D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience. 1993;57(4):1077–1090. doi: 10.1016/0306-4522(93)90050-p. [DOI] [PubMed] [Google Scholar]
- 78.Royer S, Martina M, Paré D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. The Journal of Neuroscience. 1999;19(23):10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pinto A, Sesack SR. Prefrontal cortex projection to the rat amygdala: ultrastructural relationship to dopamine D1 and D2 receptors. Abstracts - Society for Neuroscience. 2002;28:587.6. [Google Scholar]
- 80.Paré D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. Journal of Neurophysiology. 2004;92(1):1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 81.Takagi M, Yamamoto C. The long-lasting inhibition recorded in vitro from the lateral nucleus of the amygdala. Brain Research. 1981;206(2):474–478. doi: 10.1016/0006-8993(81)90550-3. [DOI] [PubMed] [Google Scholar]
- 82.Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. The Journal of Neuroscience. 1992;12(10):4066–4079. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Washburn MS, Moises HC. Inhibitory responses of rat basolateral amygdaloid neurons recorded in vitro. Neuroscience. 1992;50(4):811–830. doi: 10.1016/0306-4522(92)90206-h. [DOI] [PubMed] [Google Scholar]
- 84.Niehoff DL, Kuhar MJ. Benzodiazepine receptors: localization in rat amygdala. The Journal of Neuroscience. 1983;3(10):2091–2097. doi: 10.1523/JNEUROSCI.03-10-02091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scheel-Kruger J, Petersen EN. Anticonflict effect of the benzodiazepines mediated by a GABAergic mechanism in the amygdala. European Journal of Pharmacology. 1982;82(1-2):115–116. doi: 10.1016/0014-2999(82)90564-7. [DOI] [PubMed] [Google Scholar]
- 86.Petersen EN, Braestrup C, Scheel-Kruger J. Evidence that the anticonflict effect of midazolam in amgydala is mediated by the specific benzodiazepine receptors. Neuroscience Letters. 1985;53(3):285–288. doi: 10.1016/0304-3940(85)90552-x. [DOI] [PubMed] [Google Scholar]
- 87.Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behavioral Neuroscience. 1997;111(4):683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- 88.Jasnow AM, Huhman KL. Activation of GABAA receptors in the amygdala blocks the acquisition and expression of conditioned defeat in Syrian hamsters. Brain Research. 2001;920(1-2):142–150. doi: 10.1016/s0006-8993(01)03054-2. [DOI] [PubMed] [Google Scholar]
- 89.Sanders SK, Shekhar A. Blockade of GABAA receptors in the region of the anterior basolateral amygdala of rats elicits increases in heart rate and blood pressure. Brain Research. 1991;567(1):101–110. doi: 10.1016/0006-8993(91)91441-3. [DOI] [PubMed] [Google Scholar]
- 90.Izquierdo I, Medina JH. GABAA receptor modulation of memory: the role of endogenous benzodiazepines. Trends in Pharmacological Sciences. 1991;12(7):260–265. doi: 10.1016/0165-6147(91)90567-c. [DOI] [PubMed] [Google Scholar]
- 91.Harris JA, Westbrook RF. Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology. 1998;140(1):105–115. doi: 10.1007/s002130050745. [DOI] [PubMed] [Google Scholar]
- 92.Marsicano G, Wotjak CT, Azad SC, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418(6897):530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 93.McGaugh JL, Castellano C, Brioni J. Picrotoxin enhances latent extinction of conditioned fear. Behavioral Neuroscience. 1990;104(2):264–267. doi: 10.1037//0735-7044.104.2.264. [DOI] [PubMed] [Google Scholar]
- 94.Pereira ME, Dalmaz C, Rosat RM, Izquierdo I. Diazepam blocks the interfering effect of post-training behavioral manipulations on retention of a shuttle avoidance task. Psychopharmacology. 1988;94(3):402–404. doi: 10.1007/BF00174697. [DOI] [PubMed] [Google Scholar]
- 95.Pereira ME, Rosat R, Huang CH, Godoy MGC, Izquierdo I. Inhibition by diazepam of the effect of additional training and of extinction on the retention of shuttle avoidance behavior in rats. Behavioral Neuroscience. 1989;103(1):202–205. doi: 10.1037//0735-7044.103.1.202. [DOI] [PubMed] [Google Scholar]
- 96.Stowell JR, Berntson GG, Sarter M. Attenuation of the bidirectional effects of chlordiazepoxide and FG 7142 on conditioned response suppression and associated cardiovascular reactivity by loss of cortical cholinergic inputs. Psychopharmacology. 2000;150(2):141–149. doi: 10.1007/s002130000443. [DOI] [PubMed] [Google Scholar]
- 97.McCabe C, Shaw D, Atack JR, et al. Subtype-selective GABAergic drugs facilitate extinction of mouse operant behaviour. Neuropharmacology. 2004;46(2):171–178. doi: 10.1016/j.neuropharm.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 98.Williams JH, Gray JA, Sinden J, Buckland C, Rawlins JNP. Effects of GABAergic drugs, fornicotomy, hippocampectomy and septal lesions on the extinction of a discrete-trial fixed ratio 5 lever-press response. Behavioural Brain Research. 1990;41(2):129–150. doi: 10.1016/0166-4328(90)90149-9. [DOI] [PubMed] [Google Scholar]
- 99.Azad SC, Monory K, Marsicano G, et al. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. The Journal of Neuroscience. 2004;24(44):9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. The Journal of Neuroscience. 2005;25(2):502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Overton DA. Basic mechanisms of state-dependent learning. Psychopharmacology Bulletin. 1978;14(1):67–68. [PubMed] [Google Scholar]
- 102.Davis M, Myers KM. The role of glutamate and gamma-aminobutyric acid in fear extinction: clinical implications for exposure therapy. Biological Psychiatry. 2002;52(10):998–1007. doi: 10.1016/s0006-3223(02)01507-x. [DOI] [PubMed] [Google Scholar]
- 103.Garcia R, Vouimba R-M, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402(6759):294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- 104.LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 105.Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Research Reviews. 2005;4(2):271–287. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 106.Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learning and Memory. 2001;8(5):229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- 107.Pitkanen A, Stefanacci L, Farb CR, Go G-G, LeDoux JE, Amaral DG. Intrinsic connections of the rat amygdaloid complex: projections originating in the lateral nucleus. The Journal of Comparative Neurology. 1995;356(2):288–310. doi: 10.1002/cne.903560211. [DOI] [PubMed] [Google Scholar]
- 108.Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends in Neurosciences. 1997;20(11):517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 109.Gray TS, Carney ME, Magnuson DJ. Direct projections from the central amygdaloid nucleus to the hypothalamic paraventricular nucleus: possible role in stress-induced adrenocorticotropin release. Neuroendocrinology. 1989;50(4):433–446. doi: 10.1159/000125260. [DOI] [PubMed] [Google Scholar]
- 110.Shin LM, Whalen PJ, Pitman RK, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry. 2001;50(12):932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 111.Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states? Annals of the New York Academy of Sciences. 2003;985:263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- 112.Shin LM, McNally RJ, Kosslyn SM, et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. American Journal of Psychiatry. 1999;156(4):575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 113.Shin LM, Orr SP, Carson MA, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61(2):168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 114.Liberzon I, Taylor SF, Amdur R, et al. Brain activation in PTSD in response to trauma-related stimuli. Biological Psychiatry. 1999;45(7):817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 115.Rauch SL, Whalen PJ, Shin LM, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 116.Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14(18):2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- 117.Dougherty DD, Rauch SL, Deckersbach T, et al. Ventromedial prefrontal cortex and amygdala dysfunction during an anger induction positron emission tomography study in patients with major depressive disorder with anger attacks. Archives of General Psychiatry. 2004;61(8):795–804. doi: 10.1001/archpsyc.61.8.795. [DOI] [PubMed] [Google Scholar]