Abstract
Forebrain activation patterns in normal and spinal-injured Sprague–Dawley (SD) rats were determined by measuring regional cerebral blood flow as an indicator of neuronal activity. Data are compared to our previously published findings from normal and spinal-injured Long–Evans (LE) rats and reveal a striking degree of overlap, as well as differences, between strains in the basal (unstimulated) forebrain activation in normal animals. Specifically, 81% of the structures sampled showed similar activation in both strains, suggesting a consistent and identifiable pattern of basal cerebral activation in the rat. LE controls showed significantly greater basal activation in the remaining structures compared to SD control group, including the anterior dorsal thalamus, basolateral amygdala, SII cortex, and the hypothalamic paraventricular nucleus. In contrast, spinal cord injury (SCI) resulted in strain-specific changes in forebrain activation categorized by structures that showed significant increases in: (1) only LE SCI rats (posterior, ventrolateral, and ventroposterolateral thalamic nuclei); (2) only SD SCI rats (anterior–dorsal and medial thalamus, basolateral amygdala, cingulate and retrosplenial cortex, habenula, interpeduncular nucleus, hypothalamic paraventricular nucleus, periaqueductal gray); or (3) both strains (arcuate nucleus, ventroposteromedial thalamus, SI and SII somatosensory cortex). These results provide information related to the remote, i.e. supraspinal, effects of spinal cord injury and suggest that genetic differences play an important part in the forebrain response to such injury. Brain activation studies therefore provide a useful tool in understanding the full extent of secondary consequences following spinal injury and for identifying potential central mechanism responsible for the development of pain.
Keywords: Plasticity, Somatosensory, Diencephalon, Cortex, Quisqualic acid, Genetics, Neuroimaging, Strain differences, Brain
Introduction
Damage to the central nervous system (CNS) by traumatic injury, ischemia, infection, disease, or surgery can lead to neuropathic pain in humans (Yezierski, 2002). Symptoms of neuropathic pain include spontaneous pain, increased responsiveness to noxious stimuli (hyperalgesia), and stimulus-evoked pain to previously innocuous stimuli (allodynia). The development of neuropathic pain after injury to a peripheral nerve or as a result of CNS injury in humans is highly variable (Boivie, 1989; Tasker, 1990; Bennett, 1994). Experimental studies in animals indicate that genetic factors underlie at least some of this variability (Mills et al., 2001; Gorman et al., 2001).
A growing literature documents the importance of genetics on the development of neuropathic pain-like behaviors (see Mogil et al., 1999). Early studies showed that the development of autonomy, a presumed sign of neuropathic pain, differs markedly in different strains of rat after total nerve transection (Inbal et al., 1980; Wiesenfeld and Hallin, 1981). Such strain-dependent variability in autonomy has subsequently been confirmed in both rats and mice (Panerai et al., 1987; Devor and Raber, 1990; Cohn and Seltzer, 1991; Carr et al., 1992; Defrin et al., 1996; Mogil et al., 1999). The role of genetic factors in neuropathic pain has also been confirmed in studies looking at spontaneous and/or evoked pain-like behaviors, allodynia, and hyperalgesia in rats or mice with peripheral nerve injury or in different models of spinal cord injury (SCI) (Mogil et al., 1999; Lovell et al., 2000; Mills et al., 2001; Yoon et al., 1999; DeLeo and Rutkowski, 2000; Xu et al., 2001; Wiesenfeld-Hallin et al., 1993; Gorman et al., 2001).
In spite of the long-standing presence of pain associated with CNS disease and/or trauma, little is known about the central mechanism(s) responsible for these pain syndromes. One approach to study the impact of injury on the CNS is the use of autoradiographic estimates of regional cerebral blood flow (rCBF) as a measure of neuronal activity in supraspinal regions. This technique can be used to simultaneously identify pain-related alterations in the activation of multiple forebrain structures following peripheral or central injury (Morrow et al., 1998). Previously, we characterized the injury-induced alterations in basal (unstimulated) forebrain activation in response to SCI in Long–Evans rats (Morrow et al., 2000) and in response to chronic constriction injury (CCI) of the sciatic nerve in Sprague–Dawley rats (Paulson et al., 2000; Paulson et al., 2002). Spinal injury in Long–Evans rats resulted in significant alterations in the activation of supraspinal structures associated with the processing of somatosensory information (Morrow et al., 2000). In contrast, CCI of the sciatic nerve in Sprague–Dawley rats produced alterations in brain activation primarily in limbic forebrain structures (Paulson et al., 2002). These differences in response to peripheral versus CNS injury may be due to differences in the type and/or location of the injury or to strain differences in response to injury. In order to address this question of genetic influences on SCI-induced changes in forebrain activation, we imaged the brains of Sprague–Dawley rats following an excitotoxic spinal lesions. In this report, we present the comparison of SCI-induced changes in forebrain activation in Sprague–Dawley rats to our previous results in Long–Evans rats (Morrow et al., 2000). Preliminary findings from this study were presented at the annual meeting of the Society for Neuroscience.
Methods
Experimental procedures were approved by the Animal Care and Use Committees of the University of Florida and the Ann Arbor Veterans Affairs Medical Center. All experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals 1996.
Animal subjects
Forty-one male Sprague–Dawley rats (Charles River) weighing 225–250 g were used in these experiments. All animals were housed in groups of three and maintained on a 12 h on–12 h off light–dark cycle. Ambient temperature in the animal facility was kept at 22°C. Food and water were given ad libitum. Subjects were divided into 2 groups: an un-operated control group (n = 20) and a spinal cord injury (SCI) group (n = 21).
Surgical preparation for intraspinal injection
Surgical and injection procedures were similar to previous studies (Yezierski et al., 1998; Morrow et al., 2000; Gorman et al., 2001). Rats were anesthetized with a mixture of ketamine, acepromazine, and rhompum (0.65 ml/kg, sc.). Supplemental doses of anesthetic were given, if necessary, when rats responded to a noxious pinch applied to the glabrous skin of the hindpaws. Animals were placed in a stereotaxic instrument, and spinal cords were immobilized with a vertebral clamp. One window for intraspinal injection was made by laminectomy between spinal segments T12 and L3. Once the cord was exposed, the dura was incised longitudinally and reflected bilaterally. A small hole for unilateral injections was made in the pia matter. After injections, muscles were closed in layers, and the skin closed with wound clips. Throughout surgery and during the post-operative recovery period, animals were maintained at normal body temperature (36.5°C) with a feedback-controlled heating blanket.
Intraspinal injection procedure
Quisqualic acid (QUIS), 125 mM, was mixed fresh prior to injection, corrected to physiological pH, and injected unilaterally at one level of the cord (T12–L2). These injections produce pathological changes comparable to those described following traumatic SCI, i.e. neuronal loss, cavitation, demyelination, glial response (Yezierski et al., 1993; Yezierski et al., 1998). Injections were made at depths ranging from 600 to 1200 μm below the surface, between the dorsal root entry zone and the dorsal vein. These parameters placed injections in the center of the dorsal gray matter between spinal laminae IV and VI. The total volume of QUIS injected was 1.2 μl in 3 tracks (0.4 μl/track; distance between tracks 0.3 mm).
Brain imaging
Three to six weeks following SCI, on the day of the brain imaging procedure, rats were placed in a towel restraint to which they had been habituated. A flexible 24-gauge intravenous catheter was inserted through the skin into the tail vein. A rubber-capped injection port was attached to the catheter and flushed with 0.25 ml of saline solution. Each animal was then permitted to rest quietly in the restraint for 30 to 40 min to recover from the stress induced by tail vein catheterization.
For imaging regional cerebral blood flow, 10–12 mCi of technetium ([99m]Tc) exametazime in a 0.5–1 ml total volume was injected through the tail vein catheter as a bolus over 10 to 15 s. Two to five minutes following tracer injection, the rat was overdosed with chloral hydrate (300 mg/kg, iv), removed from the restraint, and decapitated. The brain was removed and prepared for sectioning by rapid freezing with powdered dry ice. Twenty-micron coronal frozen sections were cut at −18°C using a Hacker-Bright™ cryostat. Three to four consecutive sections taken at fixed intervals (240 μm) were mounted on glass slides and rapidly desiccated on a hotplate at 60°C. Slides were arranged in a standard X-ray cassette, and an autoradiogram was generated by direct apposition of the tissue to the emulsion side of Kodak BioMax™ MR-1 Imaging film (Kodak Inc., Rochester, NY) for a period of 1.5 to 2.0 h (for a more detailed description of the imaging technique, see Morrow et al., 1998).
Densitometric analysis of autoradiograms was performed using a microcomputer-assisted video imaging densitometer (MCID system, Imaging Research Inc., St. Catherines, Ontario, Canada). Anatomic locations of selected regions of interest (ROIs) were determined using an approach designed to ensure accuracy and consistency of structures identified (Morrow et al., 1998). Briefly, for each brain section, we overlaid a matching transparent stereotaxic template, adapted from the atlas of Paxinos and Watson (1986), on the digitized brain images displayed on the video monitor and aligned the images using prominent anatomical landmarks. The template served as a guide to sample ROIs present at a given anterior–posterior level of the brain.
As described previously (Morrow et al., 1998), an index of activation (AI) was calculated for each ROI. Briefly, the MCID system converted sampled film optical densities to apparent tissue radioactivity concentrations (nCi/mg) by comparison with the optical densities of [14C] standards also imaged on each film. The average total brain activity was then estimated by sampling all pixels in each brain section and averaging activity across all sections for a given animal. AI values were then calculated for each sampled ROI as a percent difference from the average total activity of the entire brain using the following formula:
An average AI was computed for each ROI in each animal. Finally, the within subject mean AIs for each sampled region were averaged across all subjects in an experimental group to compute within group means for each ROI. Distinctly bilateral structures were examined for side-to-side differences in activation using a paired t test (P ≤ 0.05). For each ROI, significant differences in AI between groups were tested using a one-way ANOVA. All statistical analyses were performed using the software package SPSS for Windows (SPSS Inc., Chicago, Illinois).
For the purpose of this study, regions of interest (ROIs) were limited to twenty-one structures. Table 1 lists all structures sampled and provide a key for all anatomical abbreviations. Structures known to participate in pain processing and others that are not generally included in pain pathways were sampled (Morrow et al., 2000). Some ROIs were previously identified in human PET as showing increased activation during acute pain resulting from noxious thermal stimuli (Talbot et al., 1991; Casey et al., 1994). Due to our interest in the forebrain, sampling was restricted primarily to cortical and thalamic structures as well as the PAG in the mesencephalon.
Table 1.
Anatomical abbreviations and regional differences in cerebral blood flow (rCBF) expressed as the mean percent difference from the average whole brain activity, the activation index (AI)
| Region of interest | LE: Control (n = 12) | LE: SCI (n = 11) | SD: Control (n = 20) | SD: SCI (n = 21) |
|---|---|---|---|---|
| Anterior dorsal thalamic group (AD) | 30.88 ± 3.49* | 31.33 ± 2.51 | 19.74 ± 1.96 | 28.40 ± 1.72* |
| Arcuate nucleus (Arc) | 38.53 ± 5.02 | 94.02 ± 6.92 | 23.19 ± 6.63 | 71.03 ± 6.70* |
| Basolateral amygdala (BLA) | 4.46 ± 2.59* | 9.63 ± 3.02 | 21.77 ± 1.12 | 11.75 ± 2.28* |
| Caudate putamen—anterior (Cpu) | 7.52 ± 2.43 | 9.70 ± 1.09 | 11.47 ± 1.77 | 9.34 ± 1.72 |
| Cingulate cortex (CC) | 17.37 ± 2.57 | 16.37 ± 2.90 | 17.37 ± 2.95 | 21.58 ± 2.02 |
| Diagonal band of Broca (DB) | 37.76 ± 2.83 | 36.45 ± 2.74 | 32.32 ± 3.51 | 37.67 ± 2.62 |
| Dorsal hippocampus (HIP) | −6.17 ± 1.85 | −4.94 ± 2.80 | −4.64 ± 2.05 | −5.27 ± 1.57 |
| Habenular complex (HBc) | 54.20 ± 2.51 | 57.39 ± 3.64 | 45.73 ± 3.46 | 66.21 ± 3.99* |
| Hindlimb area of cortex (SI) | 23.67 ± 3.21 | 35.81 ± 3.84 | 17.65 ± 2.14 | 31.13 ± 2.70* |
| Interpeduncular nucleus (IPN) | 52.02 ± 4.84 | 52.33 ± 4.06 | 38.37 ± 4.91 | 59.19 ± 4.90* |
| Medial thalamic group (MT) | 10.70 ± 1.91 | 13.57 ± 2.48 | 5.68 ± 1.88 | 10.22 ± 1.25* |
| Parietal area of cortex (SII) | 29.04 ± 1.49* | 44.97 ± 2.04 | 18.96 ± 1.81 | 33.95 ± 1.78* |
| Periaquaductal gray (PAG) | −0.38 ± 3.23 | 4.09 ± 3.77 | −3.31 ± 2.37 | 4.93 ± 2.71* |
| Paraventricular nucleus (PVN) | 53.53 ± 3.49* | 55.26 ± 5.89 | 18.99 ± 5.12 | 55.05 ± 3.21* |
| Posterior thalamic group (PO) | 0.41 ± 2.91 | 9.25 ± 2.64 | 2.09 ± 1.59 | 5.44 ± 1.20 |
| Retrosplenial cortex (RS) | 30.36 ± 2.48 | 34.72 ± 3.13 | 25.60 ± 1.88 | 32.49 ± 1.27* |
| Subthalamic nucleus (SbTh) | 23.05 ± 2.31 | 25.79 ± 3.93 | 18.24 ± 2.21 | 22.23 ± 2.21 |
| Ventrolateral thalamus (VL) | 3.88 ± 2.06 | 11.38 ± 2.18 | 1.75 ± 1.49 | 1.01 ± 1.49 |
| Ventromedial thalamus (VM) | 7.12 ± 2.30 | 13.78 ± 2.47 | 3.09 ± 1.31 | 6.35 ± 1.37 |
| Ventroposterolateral thalamus (VPL) | 4.45 ± 1.77 | 15.79 ± 2.86 | 1.56 ± 1.68 | 4.05 ± 1.32 |
| Ventroposteromedial thalamus (VPM) | 12.56 ± 2.30 | 26.65 ± 3.43 | 12.99 ± 1.96 | 19.53 ± 1.68* |
Values calculated as group means (see Methods) ±SEM for all regions of interest (ROIs).
Bold italics indicates a significant difference between LE and SD SCI groups.
Underline indicates a significant difference from LE controls.
Indicates a significant difference from SD controls.
Histological assessment of the spinal cord
Spinal cord segments containing QUIS injection sites were removed, post-fixed in buffered formalin, and cut on a freezing microtome (75 μm). Coronal sections were mounted on glass slides and stained with cresyl violet. Reconstructions of the QUIS-induced neuronal loss were performed using a technique previously described (Yezierski et al., 1998; Gorman et al., 2001). Briefly, sections were examined with light microscopy, and drawings of damaged areas were made using of an overhead projector and camera lucida. For each animal, a schematic representation of the injury site was made on a representative drawing of the spinal cord. To compare areas of neuronal loss with those observed in a previous study (Morrow et al., 2000), the gray matter was divided into 11 regions representing the full cross-sectional dimensions of the cord.
Results
Histological analysis of the QUIS-injected spinal cords showed characteristics similar to those described previously (Yezierski et al., 1993, 1998; Morrow et al., 2000; Gorman et al., 2001). The standardized grid drawings in Fig. 1 show examples of typical spinal injuries induced by the unilateral injection of QUIS in two animals. Note that, although QUIS was injected into the dorsal horn on only one side of the cord, there was evidence of damage on the side ipsilateral and contralateral to the injection site. Although the behavioral consequences of QUIS injections were not evaluated in animals undergoing brain imaging, the pattern of neuronal loss was similar to that found in animals with spontaneous and evoked pain behaviors following similar excitotoxic spinal cord injury (Yezierski and Park, 1993; Yezierski et al., 1998; Gorman et al., 2001).
Fig. 1.
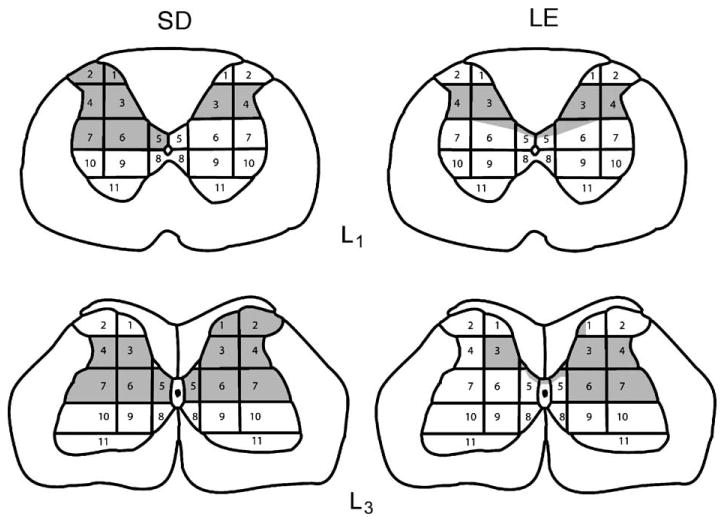
Schematic diagrams show the area of tissue damage for representative sections from SD and LE spinal-injured animals. The 11 regions of spinal gray matter are the same as those used in a previous study to examine the morphological correlate of spontaneous and evoked pain behaviors following QUIS injections (Yezierski et al., 1998).
The effects of strain on the basal activation of multiple forebrain structures in control and SCI groups are presented in Table 1 and Figs. 2 and 3. For comparison with Sprague–Dawley rats (SD), previously published values from Long–Evans (LE) control and LE SCI groups are presented (Morrow et al., 2000).
Fig. 2.
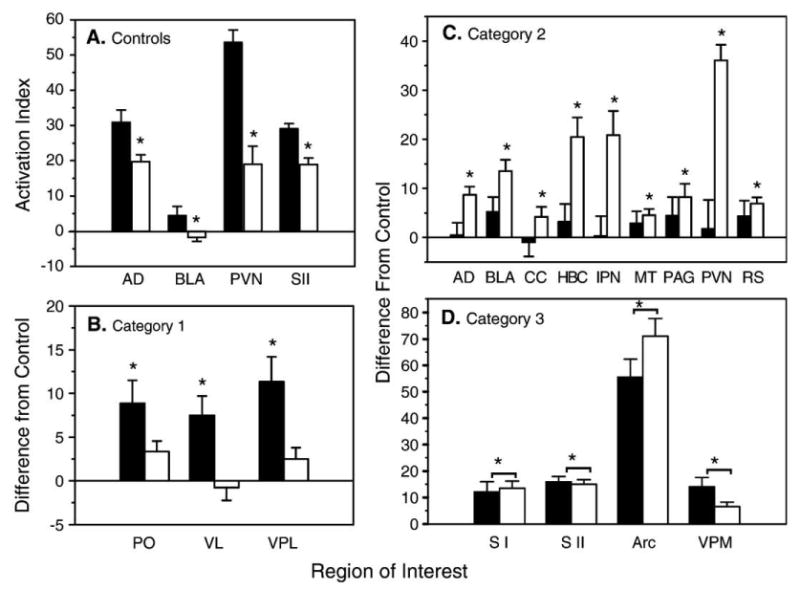
Average bilateral level of activation in basal (unstimulated) regional cerebral blood flow in Long–Evans (LE) and Sprague–Dawley (SD) rats. The filled bars represent the LE groups, and the open bars represent the SD groups in all panels. (A) The data shown are for ROIs exhibiting a significant difference in activation between the LE and the SD control (un-operated) groups. The relative levels of activation are expressed as AI, the mean percent difference from total brain activity. The asterisk (*) indicates a significant difference in activation between the two control groups (P < 0.05, ANOVA). (B) The data shown are category 1 ROIs, those exhibiting a significant increase in activation in only the LE SCI group when compared to the same-strain control group. (C) The data shown are category 2 ROIs, those exhibiting a significant increase in activation in only the SD SCI group when compared to the same-strain control group. (D) The data shown are category 3 ROIs, those exhibiting a significant increase in activation in both the SD and LE SCI groups when compared to the same-strain control group. The data displayed in panels (B–D) are expressed as the difference from the control, calculated by subtracting the control mean from the same-strain SCI group for each ROI. The asterisks (*) indicate a significant difference from the same strain control group (P < 0.05, ANOVA).
Fig. 3.
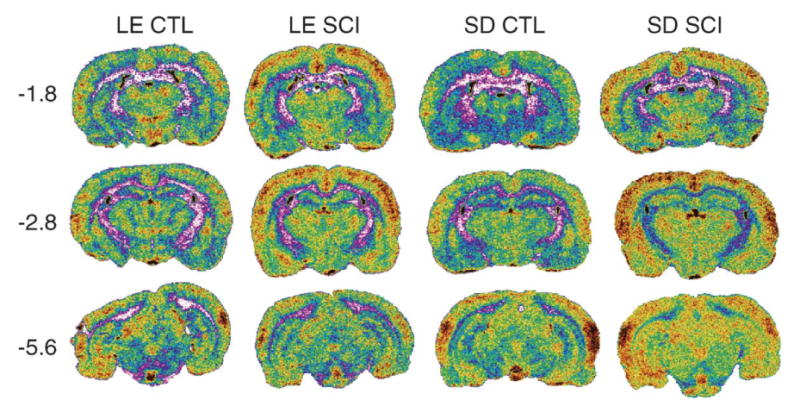
Sample coronal sections showing differences in resting brain activation between Long–Evans and Sprague–Dawley control (CTL) and spinal cord injury (SCI) groups, at three A –P levels: −1.8, −2.8, −5.6. Note: each image is taken from a single brain section in individual animals and therefore may not accurately depict group means presented in the table and graphs.
Animals in all groups displayed a regional heterogeneity in the pattern of forebrain activation. Neither control nor SCI groups showed any significant side-to-side differences in activation in any of the 21 structures sampled (data not shown). Because lateralized differences were absent in control and SCI groups, the index of activation (AI) from both sides for each ROI in all animals was averaged before computing group means. All subsequent analyses were performed on this pooled data.
The regional differences in activation for all ROIs sampled in the LE and SD control and SCI groups are shown in Table 1. Compared to SD controls, LE controls had significantly greater AI values in only 4 of the 21 regions sampled (Figs. 2A, 3). For example, LE rats had greater basal (unstimulated) activation in the anterior–dorsal nucleus of the thalamus (AD), basolateral amygdala (BLA), paraventricular nucleus (PVN) of the hypothalamus, and SII area of somatosensory cortex. No other comparisons in basal activation between LE and SD controls were significantly different.
Table 1 and Figs. 2 and 3 show that the pattern of SCI-induced changes in activation was significantly different between the two strains of rats in selected regions of the brain. Forebrain structures were divided into three categories that showed the following changes: (1) significant increases observed only in LE SCI rats (Fig. 2B), (2) significant increases observed only in SD SCI rats (Fig. 3C), or (3) significant increases observed in both strains (Fig. 2D).
Strain differences in forebrain activation following SCI were identified by calculating the AIs for the SD and LE SCI groups as differences from their respective control groups. Although decreases in activation in some ROIs were observed following spinal injury, none of these were significant in either strain.
The forebrain structures in category 1 (ROIs showing a significant increase only in LE SCI rats) were thalamic structures (Fig. 2B). As compared to controls, only the LE SCI rats showed a significant bilateral increase in activation in the posterior (PO), ventrolateral (VL), and the ventroposterolateral (VPL) thalamic nuclear groups. Note that all structures showing an increase in activation in LE SCI rats are sites associated with some aspects of somatosensory processing or sensory-motor integration.
In contrast to category 1, category 2 regions of interest (a significant increase in only SD SCI rats) included primarily limbic structures (Fig. 2C). Specifically, SD SCI rats showed a significant bilateral increase in activation in the anterior–dorsal thalamus (AD), basolateral amygdala (BLA), cingulate cortex (CC), habenular complex (HBC), interpeduncular nucleus (IPN), medial thalamus (MT), periaqueductal gray (PAG), paraventricular nucleus (PVN) of the hypothalamus, and retrosplenial cortex (RS).
Four structures were identified in category 3, ROIs showing a significant increase in both strains (Fig. 3D). These included two somatosensory cortical structures, the hindlimb area of the somatosensory cortex (SI) and parietal area of somatosensory cortex (SII), and two subcortical structures, the ventroposteromedial thalamus (VPM) and the arcuate nucleus of the hypothalamus (Arc).
Discussion
In the present study, regional cerebral blood flow (rCBF) was measured as an indicator of neuronal activity to identify basal forebrain activation patterns in two strains of adult male rat following spinal injury. This is the first study to use rCBF imaging to identify strain differences in basal forebrain activation patterns before and after spinal injury. Our results reveal a striking degree of overlap between LE and SD strains in the spatial and intensity patterns of basal cerebral activation in awake, intact, normal control animals. Of the twenty-one structures sampled, seventeen (81%) showed similar basal activation in both strains. This comparison provides evidence that, in the absence of spinal injury, the pattern of baseline cerebral activity is largely comparable, at least between these two strains.
Rats from the Long–Evans strain showed greater resting neuronal activity in the somatosensory cortex (SII), basolateral amygdala (BLA), anterior dorsal thalamus (AD), and para-ventricular nucleus (PVN) of the hypothalamus. The amygdala is considered an essential link between sensory and limbic areas of the cerebral cortex and subcortical brain regions, and amygdaloid subnuclei participate in the modulation of fear, memory, and attention (for review, see McDonald, 1998; Rasia-Filho et al., 2000). The PVN also contributes to learning, memory, as well as having a role in stress, pain, and immune responses (Bodnar et al., 1986; Senba et al., 1993; Smith and Day, 1994; Matsumoto et al., 1997; Marquez et al., 2004; see Doris (1984) for review). The greater baseline activity in the SII, AD, BLA, and PVN of the LE may explain, in part, published reports that LE rats perform better in cognitive tasks (Lindner and Schallert, 1988; Tonkiss et al., 1992; Andrews et al., 1995; Harker and Whishaw, 2002), have higher baseline levels of locomotor activity (Aulakh et al., 1988; Onaivi et al., 1992; van Lier et al., 2003), and show less reactivity to external stimuli (Glowa and Hanson, 1994; Acri et al., 1995; Faraday, 2002). In addition, LE rats are more sensitive to painful stimuli, indicated by lower baseline responses to painful mechanical and thermal stimuli applied to the hindpaw (Mills et al., 2001). It is possible that the differences in basal forebrain activation shown in our study may be in part responsible for differences in behavioral response reported by others. Thus, our results suggest that a careful characterization of the differences in the pattern of forebrain activation and in behavior between rat strains will provide useful insights to evaluate neural mechanisms of behavior.
In contrast to the concordance in forebrain activation between rat strains of uninjured, intact, control animals, the majority (57% or 12 of 21) of forebrain structures showed significant inter-strain differences in response to SCI. These differences cannot be accounted for by differences in lesion size and placement as histological analysis showed comparable lesions in the two strains (Fig. 1).
Most of the SCI-induced strain differences fell into category 2: SCI-induced increases in activation found in only the SD strain, of which there were nine structures (43%). These forebrain structures were confined to limbic brain areas and included the anterior dorsal thalamic group, basolateral amygdala, cingulate cortex, habenular complex, interpeduncular nucleus, medial thalamus, periaqueductal gray, paraventricular nucleus, and retrosplenial cortex. Interestingly, these same limbic areas showed a significant increase in activation in SD rats in response to unilateral chronic constriction injury of the sciatic nerve (Paulson et al., 2000). Therefore, the increase in activation in these areas may be characteristic of how this strain of rat responds to nervous system trauma. Further studies using different models of nervous system injury would be necessary to verify this hypothesis.
Recently, we performed a study examining differences in behavioral responses to excitotoxic spinal injury in two strains of rat (Gorman et al., 2001). In this study, the onset of excessive grooming behavior was slower in SD males than LE males, but the intensity of excessive grooming was greater in SD males. These results agree with previous reports related to the response to injury that show that SD rats exhibit significantly greater spontaneous pain, in addition to significantly greater mechanical and cold allodynia compared to LE rats (Yoon et al., 1999; Mills et al., 2001). The pattern of increase in neuronal activity of limbic structures in the present study may underlie the enhancement of these neuropathic behaviors in the SD strain after spinal injury.
Category 1 forebrain structures (SCI-induced changes in activation found only in the LE strain) were limited to the thalamus and included the posterior, ventrolateral, and the ventroposterolateral nuclear groups. These thalamic nuclei all participate in somatosensory processing. Note, however, that these thalamic structures showed equal basal activity in both strains. The functional significance of increased activation in these thalamic structures following SCI cannot be determined from this study. The few reports that compare neuropathic pain in the LE and SD rat generally report an enhancement in neuropathic pain behaviors in the SD strain (see previous paragraph). A single report by Mills and colleagues (2001) did find that a greater percentage of LE rats (73%) developed mechanical allodynia compared to SD rats (60%) following a contusion spinal injury. Further research is needed to determine the relationship between thalamic activity in neuropathic pain behavior in the LE strain.
Despite the numerous strain differences in SCI-induced changes in basal forebrain activation patterns, 4 of 21 (20%) of the forebrain structures sampled showed similarities in their response to spinal cord injury. Category 3 regions (comparable SCI-induced increases in activation found in both LE and SD rats) included both cortical and subcortical areas. The subcortical areas included the ventroposteromedial thalamus and the arcuate nucleus of the hypothalamus. The increased activity in the VPM may be a reflection of our method of restraint during the imaging procedure. Animals are restrained in a soft towel, which produces some tactile stimulation of the face. Hyperresponsiveness at a site distant from the site of injury has previously been reported. For example, Christensen and colleagues reported that unilateral hemisection at T13 produced mechanical and thermal allodynia and hyperalgesia in both the ipsilateral and contralateral forelimbs and hindlimbs (Christensen et al., 1996). Since that study, there have been several reports of hyperresponsiveness of the forelimbs following SCI (Hulsebosch et al., 1998; Bennett et al., 2000a,b; Hains et al., 2000, 2001). Therefore, it is possible that our animals may also experience a hyperresponsiveness to stimulation of the face and whisker barrels.
The other subcortical structure that showed a significant increase in activation in both strains was the arcuate nucleus of the hypothalamus (Arc). This finding is consistent with the presence of an SCI-induced state of chronic pain. Recent studies have described direct spinohypothalamic projections from superficial laminae (I–II) and base of the dorsal horn (Burstein et al., 1991, 1996). Other studies also support a role for Arc in both pain modulation (Hamba, 1988; Wang et al., 1990; Takeshige et al., 1991; Bach, 1997) and physiological stress response induced by prolonged pain (Palkovits et al., 1986).
In addition to the two subcortical structures, two cortical areas also showed a significant increase in activation in both strains. These were the hindlimb region of primary (SI) and the secondary (SII) areas of the somatosensory cortex. Not only were SI and SII significantly increased in both strains in response to spinal injury, but these same two cortical areas have previously been reported to be hyperactive in response to other neuronal trauma, such as the chronic constriction injury model of chronic pain (Paulson et al., 2000). Other imaging studies also report hyperactivity in SI and SII areas of the somatosensory cortex after CNS trauma in chronic pain patients (Casey et al., 2001). Therefore, a hyperactive cortex may be a common denominator of chronic pain resulting from neuronal trauma.
The changes in activation in forebrain structures we describe here add to the growing literature describing central reorganization following spinal cord injury. Previous reports have shown that excitotoxic damage to the SCI with QUIS results in anatomical, physiological, and molecular changes within the spinal cord and brain (Yezierski et al., 1998; Morrow et al., 2000; Abraham et al., 2001). In fact, central reorganization has been demonstrated following a variety of spinal injury models. For example, spinal cord injury produced by contusion results in changes in neuronal receptive field characteristics in the caudal brain stem (Hubscher and Johnson, 1999). In addition, hyperactive thalamic and cortical activity has been described following spinal injury in the rat, cat, and human (Koyama et al., 1993; Lenz et al., 1994; Faggin et al., 1997).
Most notably, the results from this study show that genetic factors can play an important role in the forebrain changes following damage to central nervous system injury. Subsequent investigations should not assume that changes in the response to CNS injury are uniform among rat strains. Carefully designed experiments and critical evaluations of the strain differences in behavioral and neural response may therefore provide insight into the functional significance of adaptive responses to CNS injury.
Acknowledgments
This work was supported by the Dept. of Veterans Affairs, the National Institutes of Health (PO1-HD33986), the Veterans Education and Research Association of Michigan, and NS40096 (RPY). We thank Dr. Milton Gross and the staff of the Nuclear Medicine Laboratory at the Ann Arbor VAMC for preparing the radiotracer kits used in these experiments. We also express our gratitude to Amersham Biosciences, Piscataway, NJ (a division of GE Healthcare) for their generous donation of the Ceretec™ radiotracer kits that were used in this study.
References
- Abraham KE, McGinty JF, Brewer KL. Spinal and supraspinal changes in opioid mRNA expression are related to the onset of pain behaviors following excitotoxic spinal cord injury. Pain. 2001;90:181. doi: 10.1016/s0304-3959(00)00402-4. [DOI] [PubMed] [Google Scholar]
- Acri JB, Brown KJ, Saah MI, Grunberg NE. Strain and age differences in acoustic startle responses and effects of nicotine in rats. Pharmacol Biochem Behav. 1995;50:191–198. doi: 10.1016/0091-3057(94)00285-q. [DOI] [PubMed] [Google Scholar]
- Andrews JS, Jansen JHM, Linders S, Princen A, Broekkamp CLE. Performance of four different rat strains in the autoshaping, two-object discrimination, and swim maze tests of learning and memory. Physiol Behav. 1995;57:785–790. doi: 10.1016/0031-9384(94)00336-x. [DOI] [PubMed] [Google Scholar]
- Aulakh CS, Hill JL, Murphy DL. A comparison of feeding and locomotion responses to serotonin agonists in three rat strains. Pharmacol Biochem Pharmacol Biochem Behav. 1988;31:567–571. doi: 10.1016/0091-3057(88)90231-6. [DOI] [PubMed] [Google Scholar]
- Bach FW. β-endorphin in the brain. A role in nociception. Acta Anaesthesiol Scand. 1997;41:133–140. doi: 10.1111/j.1399-6576.1997.tb04627.x. [DOI] [PubMed] [Google Scholar]
- Bennett GJ. Textbook of Pain. Churchill Livingstone; Edinburg: 1994. [Google Scholar]
- Bennett AD, Chastain KM, Hulsebosch CE. Alleviation of mechanical and thermal allodynia by CGRP(8–37) in a rodent model of chronic central pain. Pain. 2000a;86:163–175. doi: 10.1016/s0304-3959(00)00242-6. [DOI] [PubMed] [Google Scholar]
- Bennett AD, Everhart AW, Hulsebosch CE. Intrathecal administration of an NMDA or a non-NMDA receptor antagonist reduces mechanical but not thermal allodynia in a rodent model of chronic central pain after spinal cord injury. Brain Res. 2000b;859:72–82. doi: 10.1016/s0006-8993(99)02483-x. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Truesdell LS, Haldar J, Aral IA, Kordower JH, Nilaver G. Elimination of vasopressin analgesia following lesions placed in the rat hypothalamic paraventricular nucleus. Peptides. 1986;7:111–117. doi: 10.1016/0196-9781(86)90070-7. [DOI] [PubMed] [Google Scholar]
- Boivie J. On central pain and central pain mechanisms. Pain. 1989;38:121–122. doi: 10.1016/0304-3959(89)90229-7. [DOI] [PubMed] [Google Scholar]
- Burstein R, Dado RJ, Cliffer KD, Giesler GJ., Jr Physiological characterization of spinohypothalamic tract neurons in the lumbar enlargement of rats. J Neurophysiol. 1991;66:261–284. doi: 10.1152/jn.1991.66.1.261. [DOI] [PubMed] [Google Scholar]
- Burstein R, Falkowsky O, Borsook D, Strassman A. Distinct lateral and medial projections of the spinohypothalamic tract of the rat. J Comp Neurol. 1996;373:549–574. doi: 10.1002/(SICI)1096-9861(19960930)373:4<549::AID-CNE6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Carr MM, Best TJ, Mackinnon SE, Evans PJ. Strain differences in autonomy in rats undergoing sciatic nerve transection or repair. Ann Plast Surg. 1992;28:538–544. [PubMed] [Google Scholar]
- Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol. 1994;71:802–807. doi: 10.1152/jn.1994.71.2.802. [DOI] [PubMed] [Google Scholar]
- Casey KL, Lorenz J, Minoshima S, Morrow TJ, Paulson PE. Forebrain and spinal central pain (CP): comparative analysis of thalamo-cortical activity by PET. Abstr-Soc Neurosci. 2001;27:1329. [Google Scholar]
- Christensen MD, Everhart AW, Pickelman JT, Hulsebosch CE. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;68:97–107. doi: 10.1016/S0304-3959(96)03224-1. [DOI] [PubMed] [Google Scholar]
- Cohn S, Seltzer Z. Inherited propensity for neuropathic pain is mediated by sensitivity to injury discharge. NeuroReport. 1991;2:647–650. doi: 10.1097/00001756-199111000-00003. [DOI] [PubMed] [Google Scholar]
- Defrin R, Zeitoun I, Urca G. Strain differences in autonomy levels in mice: relation to spinal excitability. Brain Res. 1996;711:241–244. doi: 10.1016/0006-8993(95)01481-0. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Rutkowski MD. Gender differences in rat neuropathic pain sensitivity is dependent on strain. Neurosci Lett. 2000;282 (3):197–199. doi: 10.1016/s0304-3940(00)00880-6. [DOI] [PubMed] [Google Scholar]
- Devor M, Raber P. Heritability of symptoms in an experimental model of neuropathic pain. Pain. 1990;42:51–67. doi: 10.1016/0304-3959(90)91092-W. [DOI] [PubMed] [Google Scholar]
- Doris PA. Vasopressin and central integrative processes. Neuroendocrinology. 1984;38:75–85. doi: 10.1159/000123869. [DOI] [PubMed] [Google Scholar]
- Faggin BM, Nguyen KT, Nicolelis MA. Immediate and simultaneous sensory reorganization at cortical and subcortical levels of the somatosensory system. Proc Natl Acad Sci U S A. 1997;94:9428–9433. doi: 10.1073/pnas.94.17.9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraday MM. Rat sex and strain differences in responses to stress. Physiol Behav. 2002;75:507–522. doi: 10.1016/s0031-9384(02)00645-5. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Hanson CT. Differences in response to an acoustic startle stimulus among forty-six rat strains. Behavior Genetics. 1994;24:79–84. doi: 10.1007/BF01067931. [DOI] [PubMed] [Google Scholar]
- Gorman AL, Yu CG, Sanchez D, Ruenes GR, Daniels L, Yezierski R. Conditions affecting the onset, severity, and progression of a spontaneous pain-like behavior after excitotoxic spinal cord injury. J Pain. 2001;2:229–240. doi: 10.1054/jpai.2001.22788. [DOI] [PubMed] [Google Scholar]
- Hains BC, Chastain KM, Everhart AW, McAdoo DJ, Hulsebosch CE. Transplants of adrenal medullary chromaffin cells reduce forelimb and hindlimb allodynia in a rodent model of chronic central pain after spinal cord hemisection injury. Exp Neurol. 2000;164:426–437. doi: 10.1006/exnr.2000.7439. [DOI] [PubMed] [Google Scholar]
- Hains BC, Yucra JA, Hulsebosch CE. Reduction of pathological and behavioral deficits following spinal cord contusion injury with the selective cyclooxygenase-2 inhibitor NS-398. J Neurotrauma. 2001;18:409–423. doi: 10.1089/089771501750170994. [DOI] [PubMed] [Google Scholar]
- Hamba M. Effects of lesion and stimulation of rat hypothalamic arcuate nucleus on the pain system. Brain Res Bull. 1988;21:757–763. doi: 10.1016/0361-9230(88)90043-3. [DOI] [PubMed] [Google Scholar]
- Harker KT, Whishaw IQ. Place and matching-to-place spatial learning affected by rat inbreeding (Dark-Agouti, Fischer 344) and albinism (Wistar, Sprague–Dawley) but not domestication (Wild rat vs. Long–Evans, Fischer–Norway) Behav Brain Res. 2002;143:467–477. doi: 10.1016/s0166-4328(02)00083-9. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Johnson RD. Changes in neuronal receptive field characteristics in caudal brain stem following chronic spinal cord injury. J Neurotrauma. 1999;16:533–541. doi: 10.1089/neu.1999.16.533. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE, Dewitt DS, Jenkins LW, Prough DS. Traumatic brain injury in rats results in increased expression of Gap-43 that correlates with behavioral recovery. Neurosci Lett. 1998;255:83–86. doi: 10.1016/s0304-3940(98)00712-5. [DOI] [PubMed] [Google Scholar]
- Inbal R, Devor M, Tuchendler O, Lieblich I. Autonomy following nerve injury: genetic factors in the development of chronic pain. Pain. 1980;9:327–337. doi: 10.1016/0304-3959(80)90047-0. [DOI] [PubMed] [Google Scholar]
- Koyama S, Katayama Y, Maejima S, Hirayama T, Fujii M, Tsubokawa T. Thalamic neuronal hyperactivity following transection of the spinothalamic tract in the cat: involvement of N-methyl-D-aspartate receptor. Brain Res. 1993;612:345–350. doi: 10.1016/0006-8993(93)91684-k. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Kwan HC, Martin R, Tasker R, Richardson RT, Dostrovsky JO. Characteristics of somatotopic organization and spontaneous neuronal activity in the region of the thalamic principal sensory nucleus in patients with spinal cord transection. J Neurophysiol. 1994;72:1570–1587. doi: 10.1152/jn.1994.72.4.1570. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Schallert T. Aging and atropine effects on spatial navigation in the Morris water task. Behav Neurosci. 1988;102:621–634. doi: 10.1037//0735-7044.102.5.621. [DOI] [PubMed] [Google Scholar]
- Lovell JA, Stuesse SL, Cruce WLR, Crisp T. Strain differences in neuropathic hyperalgesia. Pharmacol Biochem Behav. 2000;65:141–144. doi: 10.1016/s0091-3057(99)00180-x. [DOI] [PubMed] [Google Scholar]
- Marquez C, Nadal R, Armario A. The hypothalamic–pituitary–adrenal and glucose responses to daily repeated immobilisation stress in rats: individual differences. Neuroscience. 2004;123:601–612. doi: 10.1016/j.neuroscience.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Koike K, Miyake A, Watanabe K, Konishi K, Kiyama H. Noxious stimulation enhances release of cytokine-induced neutrophil chemoattractant from hypothalamic neurosecretory cells. Neurosci Res. 1997;27:181–184. doi: 10.1016/s0168-0102(96)01144-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Mills CD, Hains BC, Johnson KM, Hulsebosch CE. Strain and model differences in behavioral outcomes after spinal cord injury in rat. J Neurotrauma. 2001;18:743–756. doi: 10.1089/089771501316919111. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Bon K. Heritability of nociception: I. Responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- Morrow TJ, Paulson PE, Danneman PJ, Casey KL. Regional changes in forebrain activation during the early and late phase of formalin nociception: analysis using cerebral blood flow in the rat. Pain. 1998;75:355–365. doi: 10.1016/s0304-3959(98)00016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow TJ, Paulson PE, Brewer KL, Yezierski RP, Casey KL. Chronic, selective forebrain responses to excitotoxic dorsal horn injury. Exp Neurol. 2000;161:220–226. doi: 10.1006/exnr.1999.7246. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Maguire PA, Tsai NF, Davies MF, Loew GH. Comparison of behavioral and central BDZ binding profile in three rat lines. Pharmacol Biochem Behav. 1992;43:825–831. doi: 10.1016/0091-3057(92)90414-b. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Lang T, Patthy A, Elekes I. Distribution and stress-induced increase of glutamate and aspartate levels in discrete brain nuclei of rats. Brain Res. 1986;373:252–257. doi: 10.1016/0006-8993(86)90339-2. [DOI] [PubMed] [Google Scholar]
- Panerai AE, Sacerdote P, Brini A, Blanchi M, Mantegazza P. Autonomy and central nervous system neuropeptides after section of the sciatic nerve in rats of different strains. Pharmacol Biochem Behav. 1987;28:385–388. doi: 10.1016/0091-3057(87)90458-8. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Morrow TJ, Casey KL. Bilateral behavioral and regional cerebral blood flow changes during painful peripheral mononeuropathy in the rat. Pain. 2000;84:233–245. doi: 10.1016/s0304-3959(99)00216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Casey KL, Morrow TJ. Long term changes in behavior and regional cerebral blood flow associated with painful peripheral mononeuropathy in the rat. Pain. 2002;95:31–40. doi: 10.1016/s0304-3959(01)00370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1986. [Google Scholar]
- Rasia-Filho AA, Londero RG, Achaval M. Functional activities of the amygdala: an overview. J Psychiatry Neurosci. 2000;25:14–23. [PMC free article] [PubMed] [Google Scholar]
- Senba E, Matsunaga K, Tohyama M, Noguchi K. Stress-induced c-fos expression in the rat brain: activation mechanism of sympathetic pathway. Brain Res Bull. 1993;31:329–344. doi: 10.1016/0361-9230(93)90225-z. [DOI] [PubMed] [Google Scholar]
- Smith DW, Day TA. c-fos expression in hypothalamic neurosecretory and brainstem catecholamine cells following noxious somatic stimuli. Neuroscience. 1994;58:765–775. doi: 10.1016/0306-4522(94)90453-7. [DOI] [PubMed] [Google Scholar]
- Takeshige C, Tsuchiya M, Zhao W, Guo S. Analgesia produced by pituitary ACTH and dopaminergic transmission in the arcuate. Brain Res Bull. 1991;26:788. doi: 10.1016/0361-9230(91)90175-j. [DOI] [PubMed] [Google Scholar]
- Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251:1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- Tasker RR. Pain resulting from central nervous system pathology (central pain) In: Bonica JJ, editor. The Management of Pain. 2. Lea & Febiger; Philadelphia, PA: 1990. pp. 264–286. [Google Scholar]
- Tonkiss J, Shultz P, Galler JR. Long–Evans and Sprague–Dawley rats differ in their spatial navigation performance during ontogeny and at maturity. Dev Psychobiol. 1992;25:567–579. doi: 10.1002/dev.420250804. [DOI] [PubMed] [Google Scholar]
- van Lier H, Drinkenburg WHIM, Coenen AML. Strain differences in hippocampal EEG are related to strain differences in behaviour in rats. Physiol Behav. 2003;78:91–97. doi: 10.1016/s0031-9384(02)00893-4. [DOI] [PubMed] [Google Scholar]
- Wang QA, Mao LM, Han JS. Analgesia from electrical stimulation of the hypothalamic arcuate nucleus in pentobarbital-anesthetized rats. Brain Res. 1990;526:221–227. doi: 10.1016/0006-8993(90)91225-6. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld Z, Hallin RG. Influence of nerve lesions, strain differences and continuous cold stress on chronic pain behavior in rats. Physiol Behav. 1981;27:735–740. doi: 10.1016/0031-9384(81)90249-3. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Hao JX, Xu XJ, Aldskogius H, Seiger A. Genetic factors influence the development of mechanical hypersensitivity, motor deficits and morphological damage after transient spinal cord ischemia in the rat. Pain. 1993;55 (2):235–241. doi: 10.1016/0304-3959(93)90152-F. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Plesan A, Yu W, Hao JX, Wiesenfeld-Hallin Z. Possible impact of genetic differences in the development of neuropathic pain-like behaviors after unilateral sciatic nerve ischemic injury in rats. Pain. 2001;89 (23):135–146. doi: 10.1016/s0304-3959(00)00356-0. [DOI] [PubMed] [Google Scholar]
- Yezierski RP. Central neuropathic pain Surgical Management of Pain. Thieme Medical Publishers; New York: 2002. pp. 42–64. [Google Scholar]
- Yezierski RP, Park SH. The mechanosensitivity of spinal sensory neurons following intraspinal injections of quisqualic acid in the rat. Neurosci Lett. 1993;157:115–119. doi: 10.1016/0304-3940(93)90656-6. [DOI] [PubMed] [Google Scholar]
- Yezierski RP, Santana M, Park SH, Madsen PW. Neuronal degeneration and spinal cavitation following intraspinal injections of quisqualic acid in the rat. J Neurotrauma. 1993;10:445–456. doi: 10.1089/neu.1993.10.445. [DOI] [PubMed] [Google Scholar]
- Yezierski RP, Liu S, Ruenes GL, Kajander KC, Brewer KL. Excitotoxic spinal cord injury: behavioral and morphological characteristics of a central pain model. Pain. 1998;75:141–155. doi: 10.1016/S0304-3959(97)00216-9. [DOI] [PubMed] [Google Scholar]
- Yoon YW, Lee DH, Lee BH, Chung K, Chung JM. Different strains and substrains of rats show different levels of neuropathic pain behaviors. Exp Brain Res. 1999;129:167–171. doi: 10.1007/s002210050886. [DOI] [PubMed] [Google Scholar]


