Abstract
Nerve growth factor (NGF) induces neurite outgrowth and differentiation in a process that involves NGF binding to its receptor TrkA and endocytosis of the NGF–TrkA complex into signaling endosomes. Here, we find that biogenesis of signaling endosomes requires inactivation of Rab5 to block early endosome fusion. Expression of dominant-negative Rab5 mutants enhanced NGF-mediated neurite outgrowth, whereas a constitutively active Rab5 mutant or Rabex-5 inhibited this process. Consistently, inactivation of Rab5 sustained TrkA activation on the endosomes. Furthermore, NGF treatment rapidly decreased cellular level of active Rab5-GTP, as shown by pull-down assays. This Rab5 down-regulation was mediated by RabGAP5, which was shown to associate with TrkA by coimmunoprecipitation assays. Importantly, RNA interference of RabGAP5 as well as a RabGAP5 truncation mutant containing the TrkA-binding domain blocked NGF-mediated neurite outgrowth, indicating a requirement for RabGAP5 in this process. Thus, NGF signaling down-regulates Rab5 activity via RabGAP5 to facilitate neurite outgrowth and differentiation.
INTRODUCTION
Nerve growth factor (NGF) is a neurotrophin that is essential for survival and differentiation of neuronal cells (Huang and Reichardt, 2001; Segal, 2003). Endocytosis and intracellular trafficking of NGF–TrkA (a high-affinity NGF receptor) complex is necessary for a successful NGF signal transduction process to induce neurite outgrowth (Grimes et al., 1996; Zhang et al., 2000), a hallmark of neuron differentiation, in PC12 cells (a rat pheochromocytoma cell line) (Greene and Tischler, 1976). However, molecular components of the endocytic machinery involved are yet to be identified and characterized.
Rab5 is a small GTPase localized on early endosomes, and it controls early endosome fusion along the endocytic pathway (Gorvel et al., 1991; Bucci et al., 1992; Li and Stahl, 1993; Rybin et al., 1996; Li and Liang, 2001). A population of Rab5-positive early endosomes also contains endocytosed NGF–TrkA complexes, and these endosomes are called signaling endosomes (Delcroix et al., 2003). It is suggested that the endocytosed and activated TrkA may recruit proteins to activate the small GTPase Rap1 on the signaling endosomes (York et al., 2000; Zhang et al., 2000). One such pathway may involve the FRS-2/Crk/C3G adaptor system (Meakin et al., 1999; Nosaka et al., 1999). Activated Rap1 in turn activates B-Raf, leading to prolonged activation of extracellular signal-regulated kinases (York et al., 1998), which is associated with neurite outgrowth and cell differentiation. Furthermore, blocking the endocytosis of NGF-TrkA with a dynamin mutant is known to inhibit NGF-induced neurite outgrowth in PC12 cells (Zhang et al., 2000). Thus, NGF signaling on signaling endosomes is thought to be important for neurite outgrowth and differentiation.
An important question to be resolved is the nature and biogenesis of the signaling endosomes. Although signaling endosomes contain early endosomal markers such as Rab5, they are long-lived, and they are suggested to undergo long-distance retrograde transport from the axon to the cell body of neurons (Howe and Mobley, 2004). Their relationship with conventional early endosomes is unclear, although it is possible that they are specialized early endosomes that are temporally diverted from the conventional endocytic/degradation pathway to sustain the NGF signaling. In this regard, Rab5 controls the entry to early endosomes and endocytic pathway (Ceresa and Schmid, 2000; Rink et al., 2005). Thus, we have initiated an investigation into the activity and function of Rab5 in NGF-mediated neurite outgrowth in PC12 cells.
MATERIALS AND METHODS
Plasmids and cDNAs
pBI and pBI/EGFP were purchased from BD Biosciences (San Jose, CA). The cDNAs of Rab5:wild type (WT), Rab5:Q79L, Rab5:S34N, and Rab5:N133I were generated by polymerase chain reaction (PCR) with the previously made pGEX or pH2J1 constructs as templates (Liang et al., 2000; Li and Liang, 2001) and subcloned into the MluI restriction site of pBI and pBI/EGFP. Rat RabGAP5 cDNA was purchased from Invitrogen (Carlsbad, CA). The TrkA, RN-tre, and TSC2 cDNAs were kindly provided by Brian Rudkin (Laboratoire de Biologie Moleculaire et Cellulaire, Lyon, France), P. Paolo Di Fiore (European Institute of Oncology, Milan, Italy), and Kun-Liang Guan (Department of Biological Chemistry, University of Michigan, Ann Arbor, MI), respectively. We subcloned the TrkA cDNA into the MluI site of pBI. The RabGAP5 and RN-tre cDNAs were subcloned in pcDNA3 and pBI/TrkA vectors, either with or without the Myc tag.
Antibodies
The affinity-purified rabbit anti-RabGAP5 antibody was kindly provided by Francis Barr's laboratory (Max-Planck Institute of Biochemistry, Martinsried, Germany). Monoclonal antibodies for actin, FLAG, and Myc were purchased from Sigma-Aldrich (St. Louis, MO), whereas the anti-Rab5 monoclonal antibody (mAb), anti-hemagglutinin (HA) mAb, and anti-TrkA rabbit antiserum were from BD Biosciences, Santa Cruz Biotechnology (Santa Cruz, CA), and Upstate Biotechnology (Lake Placid, NY), respectively. The anti-pTrkA rabbit antiserum was from Cell Signaling Technology (Beverly, MA).
Cell Culture and Transfection
Tet-Off PC12 cells (BD Biosciences) were grown in 35-mm culture dishes in DMEM (Invitrogen) supplemented with 10% heat-inactivated horse serum (Invitrogen), 5% heat-inactivated fetal bovine serum (FBS; Invitrogen), 20 U/ml penicillin/streptomycin (Invitrogen), 1 mM l-glutamine (Invitrogen), and 200 μg/ml Geneticin (G-418; Invitrogen). Cells were incubated at 37°C in a humidified incubator with 10% CO2. For transfection, cells were seeded at a density of 2 × 105 cells/dish, grown to 70–80% confluence, and transfected with the indicated plasmids by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol.
Neurite Outgrowth Assay
After cotransfection with pBI/EGFP, cells were allowed to recover in full growth medium and to express the recombinant proteins for 24 h. The growth medium was then replaced with a medium containing only 0.5% horse serum (no FBS) and 50 ng/ml NGF. The medium was incubated at 37°C for 6 d, with replenishment of NGF every 2 d until the sixth day. The NGF concentration was 50 ng/ml unless indicated otherwise. On different days as indicated, cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) for 30 min, and neurite outgrowth was observed with either an inverted fluorescence microscope (transiently transfected cells) or a phase-contrast microscope (cloned cell lines). We used a Nikon Diaphot 300 microscope for both purposes. The images were captured by a digital camera, stored in a connected computer, and analyzed with the Nikon ACT-1 software. Differentiated cells were defined as those containing at least one neurite twice as long as the cell body diameter. The percentage of differentiated cells in each case was determined from transfected cells (i.e., cells expressing green fluorescent protein). Standard error of the mean (SEM) was calculated from three to five independent experiments.
Confocal Fluorescence Microscopy
We used a Leica confocal laser scanning microscope with Ar-488 and Kr-568 laser excitation in the Flow and Image laboratory on campus and followed a procedure described previously (Li and Liang, 2001). Briefly Tet-Off PC12 cells were grown on coverslips coated with collagen IV and transfected with pBI and/or pcDNA3 constructs expressing various enhanced green fluorescent protein (EGFP)-Rab5:S34N, EGFP-Rab5:Q79L, red fluorescent protein (RFP)-Rab5:Q79L, TrkA, or TrkA-EGFP as indicated. RFP represents ds-Red monomer from BD Biosciences. At 24 h posttransfection, the cells were treated with 50 ng/ml NGF for the indicated times, and then they were processed for immunofluorescence microscopy. Cells were rinsed three times with phosphate-buffered saline (PBS) and fixed for 20 min with 4% paraformaldehyde (wt/vol in PBS) at room temperature, followed by permeabilization with 0.1% Triton X-100 (in PBS) for 5 min. The cells were then stained with the anti-pTrkA antibody that specifically recognizes the cytoplasmic domain of activated TrkA and a secondary antibody (goat anti-rabbit IgG conjugated with Alexa568; Invitrogen). The coverslips were then mounted in PBS on glass slides and viewed with the Leica confocal microscope.
Glutathione S-Transferase (GST) Pull-Down Assay
We cloned the cDNA of the Rab5-binding domain (R5BD, residues 739-862) of Rabaptin5 into the pGEX vector (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). The resulting construct was termed pGEX/Rabaptin-5(R5BD), which expressed the fusion protein GST-R5BD in the Escherichia coli strain DH5α upon isopropyl β-d-thiogalactoside induction. GST-R5BD was then affinity purified with glutathione-Sepharose 4B resin (GE Healthcare). Rab5 proteins (WT, Q79L, and S34N) were expressed in Tet-Off PC12 cells by transfection of corresponding pBI constructs and incubation at 37°C for 24 h. Cells were then treated with 50 ng/ml NGF for the indicated times (untreated cells served as controls), followed by washing with ice-cold PBS and lysis for 5 min in the lysis buffer, which contained 25 mM HEPES, pH 7.4, 100 mM NaCl, 5 mM MgCl2, 0.1% NP-40, 10% glycerol, 1 mM dithiothreitol, and protease inhibitor cocktail (Sigma-Aldrich). Lysates were clarified by centrifugation at 10,000g for 2 min at 4°C, and an aliquot (200 μl) of the supernatant was incubated with 20 μl of GST-R5BD bound to the glutathione-Sepharose 4B resin for 10 min at 4°C on a rotating mixer. The resin was subsequently rinsed with the lysis buffer, resuspended in SDS sample buffer, boiled for 3 min, and subjected to SDS-PAGE (15% gel), followed by immunoblot analysis with the anti-Rab5 mAb. The results were quantified by densitometry using Densitometer SI (GE Healthcare).
In Vivo GTPase-activating Protein (GAP) Assay
Cells were cotransfected with pBI/Rab5 and a pBI or pcDNA3 construct expressing one of the GAPs. At 24 h posttransfection, the Rab5-GTP level in the cell was determined by the GST pull-down assay described above.
Establishment of Stable PC12 Cell Lines
Tet-Off PC12 cells were cotransfected with pBI/FLAG-Rab5:Q79L (or pBI/FLAG-Rab5:S34N) and pTK-hyg at a 20:1 ratio by using the Lipofectamine 2000-mediated procedure as described above. The cells were then selected with 150 μg/ml hygromycin (BD Biosciences) in the presence of 1 μg/ml doxycycline (Dox; BD Biosciences). After 3 wk, hygromycin-resistant colonies began to grow. Individual colonies were isolated and transferred to 24-well plates in triplicates, with two samples maintained in the presence of Dox and one sample without Dox to induce the expression of cloned Rab5 proteins for 2 d. Recombinant Rab5 proteins containing the FLAG epitope were identified by immunoblot analysis with the anti-FLAG antibody, and clones with inducible recombinant protein expression were selected and scaled up for further assays.
Cell Growth Rate
Cells were seeded at a density of 1 × 105 cells/well in a six-well plate and incubated at 37°C. Cell numbers were counted each day up to 6 d with a hemacytometer (Hausser Scientific, Horsham, PA), after trypsinization and resuspension in the medium at each time point. The results were averaged from triplicate samples, and error bars represented SEM from three independent experiments.
Coimmunoprecipitation (coIP) Assay
Cells were transfected with pBI/TrkA constructs that coexpressed RabGAP5 or mutants (all contained Myc tag) via Lipofectamine 2000 as described above. Cells were allowed to recover in full growth medium and to express the recombinant proteins for 24 h at 37°C. Before cell lysis, cells were starved in serum-free medium for 24 h and then either treated or not treated with 50 ng/ml NGF for 5 min as indicated. Cells were then rinsed with ice-cold PBS, pH 7.4, and lysed on ice for 45 min in the lysis buffer, which contained 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 2 mM sodium orthovanadate, 2 mM para-nitrophenol phosphate, and protease inhibitor cocktail (Sigma- Aldrich). After clearing at 10,000 × g for 15 min, cell lysates were incubated with anti-Myc antibody-conjugated agarose beads (Sigma-Aldrich) for 4 h at 4°C. The beads were then washed four times with the lysis buffer and boiled for 3 min in SDS sample buffer, followed by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblot analysis with anti-TrkA, anti-pTrkA, and anti-Myc antibodies. The results were quantified by densitometry using Densitometer SI (GE Healthcare).
RNA Interference (RNAi) of RabGAP5
We used the pSUPER vector (Oligoengine) to express specific short hairpin RNAs (shRNAs) to knock down RabGAP5 expression in PC12 cells. The 19- or 21-mer oligonucleotides were designed, annealed, and then cloned into pSUPER according to the manufacturer's instructions. The targeting regions included four sequences in rat RabGAP5 and one sequence in its human counterpart as a control, including 5′-GCATCTGGGACCTGTTCTTCT-3′ (for rat shRNA1), 5′-GCCCTATTTGAACATGGATTG-3′ (for rat shRNA2), 5′-GGCAAAGAACATCAAACAA-3′ (for rat shRNA3), 5′-GGCCCTATTTGAACATGGA-3′ (for rat shRNA4), and 5′-GCAGAGCAACCAGAGTTCTAC-3′ (for human shRNA).
The effectiveness of RabGAP5 knockdown was confirmed by immunoblot analysis. Because of low transfection efficiency, the shRNA constructs were cotransfected with pBI/myc-RabGAP5, followed by immunoblot analysis 48 h later with the anti-myc mAb to examine the level of myc-RabGAP5 expression. For neurite outgrowth analysis, PC12 cells were transfected with either the pSUPER vector alone as a negative control or with each of the shRNA constructs. In this case, pBI/EGFP was cotransfected to identify the transfected cells by fluorescence microscopy, and neurite outgrowth was measured at 48 h posttransfection and for 5 days thereafter with the assay described above.
RESULTS
Transient Expression of Rab5 and a Constitutively Active Mutant (Rab5:Q79L) Blocks NGF-mediated Neurite Outgrowth
We used constitutively active as well as dominant-negative mutants of Rab5 in this study. Rab5:Q79L is a constitutively active mutant, because it is defective in GTP hydrolysis and it is locked in the active GTP-bound conformation (Li and Stahl, 1993; Stenmark et al., 1994; Li and Liang, 2001). Rab5:S34N and Rab5:N133I are dominant-negative mutants, because they are defective in GTP binding and they are either nucleotide free or guanosine diphosphate (GDP) bound (Li and Stahl, 1993; Li et al., 1994; Stenmark et al., 1994; Hoffenberg et al., 1995). Their dominant-negative phenotype is thought to be due to sequestration of Rab5 guanine-nucleotide exchange factor (GEF)(s), which is normally required for activating the endogenous Rab5 via stimulating GDP dissociation. We expressed WT Rab5 and each of the mutants in a Tet-Off PC12 cell line by using the bidirectional expression vector pBI/EGFP, which expressed EGFP and each cloned Rab5 protein simultaneously. Protein expression in this system was controlled by Dox (a stable analogue of tetracycline). On removal of Dox from the medium, the cloned WT and mutant Rab5 proteins were induced to show robust expression as confirmed by immunoblot analysis (Figure 1A).
Figure 1.
Neurite outgrowth in PC12 cells transfected with Rab5 mutants in response to NGF treatment. (A) Immunoblot analysis of the expression of the indicated Rab5 proteins in the absence or presence of 1 μg/ml Dox. Top, expression levels of the transfected Rab5 constructs (WT, Q79L, and S34N) as detected by the anti-Rab5 antibody. Bottom, actin level in each sample as the loading control (anti-actin). Lane PC12 shows endogenous levels of Rab5 or actin in cells transfected with the empty vector. Molecular mass standards are indicated on the left (in kilodaltons). (B) Fluorescence microscopy images of the cells after a 6-d treatment with 50 ng/ml NGF. Cells were transfected either with the empty pBI/EGFP vector (vector) or with constructs expressing the indicated Rab5 proteins. Bar, 50 μm. (C) Quantification of PC12 cell differentiation upon expression of WT and mutant Rab5 proteins. Percentage of differentiated cells with extended neurites was determined among the transfected cells expressing EGFP and thus the indicated WT or mutant Rab5 proteins. In each case, the total number of transfected cells measured is as follows: vector, >100; WT, >80; S34N, >80; Q79L, >80; and N133I, >100. Error bars represent SEM of three independent experiments.
We determined the effect of overexpressing WT and mutant Rab5 proteins on NGF-mediated neurite outgrowth. Tet-Off PC12 cells were transfected with either the pBI/EGFP vector itself or the various pBI/EGFP constructs expressing WT or mutant Rab5 proteins, in the absence of Dox to promote expression. NGF was added the next day to induce neurite outgrowth, and cells expressing EGFP were identified by fluorescence microscopy. EGFP expression here served as a marker for transfected cells, which should also express the indicated Rab5 protein from the same vector. After 6 d of NGF treatment, the neurite length in the EGFP-expressing cells was measured, and the percentage of differentiated cells was determined. In control cells transfected with the pBI/EGFP vector, 60% of them differentiated into sympathetic neuron-like phenotype with long neurites (Figure 1, B and C). Overexpression of either dominant-negative mutant (Rab5:S34N or Rab5:N133I) did not significantly affect the neurite outgrowth under this condition (i.e., 50 ng/ml NGF) (Figure 1, B and C). In contrast, overexpression of WT or the constitutively active Rab5:Q79L mutant dramatically inhibited neurite outgrowth, with <10% of the cells containing extended neurites (Figure 1, B and C). When NGF concentration was reduced to 10 ng/ml, cells expressing either dominant-negative mutant (Rab5:S34N or Rab5:N133I) remained able to grow neurites, whereas control PC12 cells failed to do so (data not shown). Together, these results suggest that high Rab5 activity is unfavorable to neurite outgrowth and that endogenous Rab5 function may need to be suppressed during NGF-induced neurite outgrowth and cell differentiation.
Inactivation of Rab5 Facilitates Intracellular NGF Signaling
We further investigated directly the effect of expressing Rab5:S34N and Rab5:Q79L on NGF signaling by determining the phosphorylation profile of TrkA upon NGF treatment. In this case, we coexpressed TrkA with either Rab5:S34N or Rab5:Q79L to enhance the ratio of relevant TrkA detection signal in transfected cells versus TrkA background in untransfected cells. Cells were treated with NGF for different times, followed by immunoblot analysis of cell lysates with anti-phospho-TrkA (pTrkA) and anti-TrkA antibodies (Figure 2). The former antibody recognizes the activated, Tyr490-phosphorylated form only. A striking observation was that TrkA phosphorylation/activation was dramatically decreased in Rab5:Q79L-expressing cells, whereas the activation signal was enhanced in Rab5:S34N-expressing cells and sustained for a longer period, in comparison with control cells that expressed TrkA only (Figure 2A). There was a steady-state, basal level of pTrkA, which increased upon NGF treatment. The pTrkA level in Rab5:Q79L-expressing cells was always lower than that in control and Rab5:S34N-expressing cells before and after NGF treatment (Figure 2A). Even the NGF-enhanced pTrkA level in Rab5:Q79L-expressing cells was much lower than that of the basal pTrkA level in Rab5:S34N-expressing cells (Figure 2A). Indeed, there was much higher percentage of pTrkA among total TrkA in Rab5:S34N-expressing cells than in Rab5:Q79L-expressing cells (Figure 2, B and C). Because the total TrkA level in control, Rab5:S34N-expressing, and Rab5:Q79L-expressing cells was similar (Figure 2B), the low pTrkA level seen in Rab5:Q79L-expressing cells was most likely due to rapid dephosphorylation in Rab5:Q79L endosomes rather than to receptor degradation.
Figure 2.
Effect of Rab5 mutants on TrkA activation. (A) Immunoblot analysis of activated TrkA with the anti-pTrkA antibody. PC12 cells coexpressing TrkA and the indicated Rab5 mutants (Rab5:S34N or Rab5:Q79L) were treated with 50 ng/ml NGF for the indicated times, followed by cell lysis and immunoblot analysis with the anti-pTrkA antibody. Control cells expressed TrkA only. The signal for Rab5:Q79L samples is low and can be detected only after longer exposure (10 times) of the blot as indicated. Molecular mass standards (in kilodaltons) are indicated on the left of each panel. (B) Immunoblot analysis of total TrkA with the anti-TrkA antibody on the same membranes after stripping off the previous anti-pTrkA antibody. (C) Relative amounts of pTrkA versus total TrkA in each sample determined by densitometry of the blots in A and B. Because of different antibodies used, the ratio does not reflect absolute percentage of pTrkA, but it serves the purpose to compare the relative levels of pTrkA in control, Rab5:S34N, and Rab5:Q79L cells.
To test the aforementioned contention, we performed confocal fluorescence microscopy to determine whether pTrkA would accumulate or diminish in Rab5:S34N- or Rab5:Q79L-containing endosomes. In control PC12 cells transfected with TrkA alone, activated pTrkA was detected at plasma membrane as well as dispersed intracellular structures at steady state (Figure 3A). NGF induced endocytosis and accumulation of pTrkA near the nucleus, presumably on signaling endosomes (Figure 3A). There seemed to be an immobile fraction of pTrkA that remained on the plasma membrane and that was not internalized into endosomes (Figure 3A, 30 min), even after 1-h NGF treatment (data not shown). The plasma membrane-localized pTrkA may not be relevant to neurite growth, because endocytosis and signaling on endosomes is required for neurite outgrowth (Zhang et al., 2000). We focused on the internalized pool of pTrkA. In Rab5:S34N-expressing cells, endocytosed pTrkA colocalized to Rab5:S34N-containing endosomes and increasingly concentrated at the perinuclear region upon NGF treatment (Figure 3B). In contrast, there was no strong accumulation of pTrkA at the perinuclear region in Rab5:Q79L-expressing cells with or without NGF treatment (Figure 3B). The Rab5:Q79L-containing endosomes are more heterogeneous in size depending on the number of fusion events, and there are small/young endosomes as well as large/mature endosomes. Importantly intracellular pTrkA showed no colocalization with Rab5:Q79L on the large/mature endosomes and only partially localized to the small/young endosomes (Figure 3B), even though total TrkA (in the form of TrkA-EGFP) colocalized well with Rab5:Q79L on both types of endosomes (Figure 3C). TrkA-EGFP was previously shown to follow the same endocytic pathway as TrkA (Jullien et al., 2002). The data provide further evidence that the activation status of pTrkA is more transient in Rab5:Q79L endosomes that contain high Rab5 activity for endosome fusion than in Rab5:S34N endosomes where Rab5 activity and endosome fusion is blocked. The pTrkA is progressively inactivated by dephosphorylation as it enters Rab5:Q79L endosomes, probably by gaining access to the endosome-associated phosphatases. Along this line, pTrkA in Rab5:S34N endosomes avoids dephosphorylation and inactivation leading to sustained signaling and higher steady level of pTrkA, because it cannot get access to the phosphatases due to a block of Rab5 activity and early endosome fusion. Thus, enhanced TrkA signaling in cells expressing Rab5:S34N and compromised signaling in cells expressing Rab5:Q79L are consistent with the effects of these mutants on neurite outgrowth (Figure 1). Like Rab5:S34N, NGF also down-regulates Rab5 activity, via RabGAP5, in PC12 cells to facilitate neurite outgrowth (Figure 6; see below).
Figure 3.
Accumulation of activated pTrkA on endosomes where Rab5 activity is blocked. (A) Confocal microscopic images showing the localization of pTrkA in control PC12 cells transfected with pBI/TrkA upon 0-, 5-, and 30-min NGF treatment as indicated. The cells were stained by the anti-pTrkA antibody and goat anti-rabbit IgG conjugated with Alexa568. Bar, 16 μm. (B) Confocal microscopic images showing the localization of EGFP-Rab5:S34N and pTrkA or EGFP-Rab5:Q79L and pTrkA in PC12 cells cotransfected with pBI/EGFP-Rab5:S34N and pBI/TrkA or pBI/EGFP-Rab5:Q79L and pBI/TrkA upon 0-, 15-, and 30-min NGF treatment as indicated. Arrows indicate the large Rab5:Q79L vesicles devoid of pTrkA. Bar, 16 μm. (C) Confocal microscopic images showing the localization of TrkA-EGFP and RFP-Rab5:Q79L in PC12 cells transfected with TrkA-EGFP and pBI/RFP-Rab5:Q79L upon 30-min NGF treatment as indicated. Bar, 16 μm.
Figure 6.
NGF-induced reduction of Rab5-GTP level in PC12 cells. (A) PC12 cells were transfected with plasmids that express Rab5:WT, Rab5:S34N, and Rab5:Q79L as indicated. Cells were then lysed, and cell lysates were incubated with GST-R5BD. GTP-bound Rab5 fraction bound to GST-R5BD (pull-down) and total amount of Rab5 proteins in the lysates (cell lysate, the amount loaded was 5% that used for the pull-down) were detected by immunoblot analysis with the anti-Rab5 antibody. (B) The cells that overexpressed Rab5:WT were treated with 50 ng/ml NGF for the indicated times, followed by cell lysis and pull-down assays as described in A. Control cells (control) were not treated with NGF.
The defective NGF signaling and neurite outgrowth seen in cells overexpressing WT or Rab5:Q79L was not due to any negative effect on cell growth. This point was confirmed by the following experiments in which we established cell lines expressing Rab5:Q79L and Rab5:S34N, respectively.
Cloned PC12 Cell Lines: Rab5:Q79L Expression Inhibits, but Rab5:S34N Expression Promotes, NGF-mediated Neurite Outgrowth
To extend the transient expression results, we established a number of Tet-Off PC12 cell lines that expressed the Rab5 mutants upon Dox removal (Supplemental Figure 1). Consistent with the transient expression data (Figure 1), expression of Rab5:Q79L inhibited NGF-induced neurite outgrowth, whereas expression of Rab5:S34N facilitated neurite outgrowth (Figure 4A). In this case, we observed and quantified neurite outgrowth each day upon NGF treatment. Rab5:Q79L-expressing cell lines showed slower neurite outgrowth. The extent of inhibition was less than the transient expression results, possibly due to the lower expression level and/or adjustment and adaptation of the cells. Rab5:S34N facilitated neurite outgrowth in the sense that these cells were more sensitive to NGF treatment. Although it usually took 5 d for the control parental cells to reach maximal neurite outgrowth, it only took 2–3 d for the Rab5:S34N-expressing cells to reach a similar level (Figure 4A). Although the results in Figure 4A were obtained from clone 1 of FLAG-Rab5:Q79L cell lines and clone 1 of FLAG-Rab5:S34N cell lines (Supplemental Figure 1), we also examined clone 2 of both cell lines and obtained the same results. Furthermore, we determined growth rates of the Rab5:Q79L- and Rab5:S34N-expresssing cell lines over a 6-d period and found that the Q79L-expressing cells grew faster and the S34N-expressing cells grew slower than the control parental PC12 cells (Figure 4B). Again, both cell clones for each mutant showed similar results. In PC12 cells, NGF-induced neurite outgrowth and cell differentiation is usually accompanied by decreased cell growth rate (Greene and Tischler, 1976). The fact that the S34N cells grew slower suggested that these cells may favor cell differentiation, consistent with the observation that S34N indeed enhanced the neurite outgrowth (Figure 4A). The faster growth rate of the Q79L cells correlated well with the block in neurite outgrowth (Figure 4A). Furthermore, that the Q79L cells grew well indicated that the expression of Rab5:Q79L did not cause cell death or other pleiotropic cytopathic effects, and the inability of these cells to grow neurites was likely a physiological consequence of Rab5:Q79L.
Figure 4.
Cloned PC12 cell lines expressing FLAG-Rab5:Q79L and FLAG-Rab5:S34N: neurite outgrowth and cell growth rate. (A) Comparison of neurite outgrowth in the parental (PC12) cell line and the cell lines expressing FLAG-Rab5:S34N and FLAG-Rab5:Q79L as indicated, in the absence of Dox to induce protein expression. On addition of 50 ng/ml NGF, neurite outgrowth was quantified each day as described in the legend to Figure 1. (B) Comparison of cell growth rates in the PC12 cell line and the cell lines expressing FLAG-Rab5:S34N and FLAG-Rab5:Q79L as indicated. Each cell line was seeded at 105 cells/35-mm dish and grown in the absence of Dox. Each day thereafter, cells were trypsinized, and the cell number was determined by using a hemacytometer. Error bars represent SEM of three independent experiments.
Transient Expression of Rabex-5 Blocks NGF-mediated Neurite Outgrowth
Constitutive activation of Rab5 exhibited negative effect on NGF-induced neurite outgrowth, as evidenced by the Rab5:Q79L results (Figures 1–4). To test further the specific role of Rab5 in the neurite outgrowth process, we expressed Rabex-5, a specific Rab5 GEF that activates Rab5, in PC12 cells and determined whether it may exhibit the same negative effect on neurite outgrowth as Rab5:Q79L. The Myc-tagged Rabex-5 was expressed via the pBI vector and was detected by immunoblot analysis with the anti-Myc antibody (Figure 5A). The pBI/EGFP vector was cotransfected to mark the transfected cells with EGFP expression. On NGF treatment, the transfected cells were identified by fluorescence microscopy, and neurite outgrowth was scored each day for 5 days. Like Rab5:Q79L, the expression of Rabex-5 blocked neurite outgrowth (Figure 5, B and C). Interestingly, it took 2–3 d for Rabex-5 to show inhibitory effect in comparison with the more acute inhibition by Rab5:Q79L, possibly due to the fact that the Rabex-5 effect is manifested via its activation of endogenous Rab5 and is less direct than the constitutive active Rab5:Q79L itself.
Figure 5.
Rabex-5-mediated inhibition of neurite outgrowth in PC12 cells. (A) Immunoblot of Myc-Rabex-5 expressed in PC12 cells via the pBI vector in the absence (−) or presence (+) of Dox as indicated. Vector alone served as a negative control. Molecular mass standards (in kilodaltons) are indicated on the left. (B) Comparison of neurite outgrowth in control PC12 cells transfected with vector and cells expressing Myc-Rabex-5 as indicated, in the absence of Dox to induce protein expression. On addition of 50 ng/ml NGF, neurite outgrowth was quantified each day as described in Figure 1 legend. More than 150 transfected cells were measured in each case, and SEMs were obtained from three independent experiments. (C) Fluorescence microscopy images of control cells (vector) and cells expressing Myc-Rabex-5 after 5-d treatment with NGF.
NGF Rapidly Down-Regulates Rab5 Activity
The data so far indicated that high Rab5 activity (Rab5:Q79L and Rabex-5) blocked neurite outgrowth. Low Rab5 activity (Rab5:S34N) enhanced this process. Thus, we directly examined the possibility that NGF signaling itself may down-regulate Rab5 activity during neurite outgrowth and differentiation.
As a GTPase, Rab5 activity in the cell is reflected by the level of active GTP-bound Rab5, which we determined by developing and using a GST pull-down assay. The assay was based on the specific binding of Rab5-GTP by the R5BD of Rabaptin5 (a Rab5 effector) (Stenmark et al., 1995; Zhu et al., 2004). We made GST-R5BD fusion protein and used it to pull-down Rab5-GTP in PC12 cell lysates, followed by immunoblot analysis with a Rab5 antibody to determine the relative amount of Rab5-GTP. Endogenous Rab5-GTP level was too low to be detected in this assay; thus, we transfected the cells with pBI/Rab5 to overexpress Rab5. As positive and negative controls, we expressed Rab5:Q79L and Rab5:S34N mutants, respectively. Rab5:Q79L showed the most robust pull-down signal that reflected high level of GTP-bound form, consistent with its defect in GTP hydrolysis (Figure 6A). WT Rab5 also showed pull-down signal but at a level sevenfold lower than that of Rab5:Q79L (Figure 6A). In contrast, Rab5:S34N showed no pull-down signal, consistent with its defect in GTP binding (Figure 6A). These results demonstrated the feasibility of the GST-R5BD pull-down assay in determining the relative amount of Rab5-GTP in the cell.
To determine whether NGF may regulate the Rab5-GTP level in PC12 cells, we added NGF to the cells overexpressing Rab5:WT for different times, followed by the pull-down assay to determine the amount of Rab5-GTP at each time point. NGF rapidly decreased the Rab5-GTP level by fivefold within 5 min and kept the low level for at least 1 h (Figure 6B). This correlated with the activation and endocytosis of TrkA (Jullien et al., 2002). Because the activation of TrkA is transient and it is inactivated by dephosphorylation after endocytosis, we examined Rab5 activity at later time points and found that the steady-state Rab5-GTP level recovered to a level slightly higher than the control by 24- and 48-h post-NGF treatment (Figure 6B). The transient reduction of Rab5-GTP level is likely underestimated, considering the overexpression of Rab5. In normal cells without Rab5 overexpression, NGF signaling may lead to a more complete inactivation of endogenous Rab5.
RabGAP5 Activity in PC12 Cells
NGF-mediated down-regulation of Rab5 may require TrkA to recruit a Rab5 GAP, which inactivates Rab5 by stimulating GTP hydrolysis. The Rab5-specific GAP (RabGAP5) was recently identified (Haas et al., 2005). In addition, other proteins such as RN-tre (Lanzetti et al., 2000) and TSC2 (Xiao et al., 1997) were also reported to have GAP activity toward Rab5. Thus, we examined whether these GAPs can actually down-regulate Rab5 in vivo in PC12 cells. We coexpressed each of the GAPs with Rab5:WT in PC12 cells and determined their effect on the level of Rab5-GTP by the GST-R5BD pull-down assay. RabGAP5 and RN-tre showed Rab5 GAP activity and efficiently reduced Rab5-GTP level in the cell (Figure 7A). In contrast, TSC2 showed no Rab5 GAP activity in the cell (Figure 7A). In each case, the total Rab5 expression was the same (Figure 7A). In additional control experiments, we confirmed the expression of each GAP protein (tagged with either Myc or HA epitope) by immunoblot analysis with anti-Myc and anti-HA antibodies (Figure 7B). Importantly, the RabGAP5 protein was abundant in PC12 cells, and we were able to detect endogenous RabGAP5 by immunoblot analysis with the affinity-purified anti-RabGAP5 antibody (Haas et al., 2005). The antibody recognized a major protein band at ∼75 kDa with two smaller minor species (Figure 7C). The 75-kDa protein correlated with the full-length RabGAP5 (Haas et al., 2005), whereas the smaller proteins were likely degradation products. Overexpression of RabGAP5 in these cells, via transfection of a plasmid containing rat RabGAP5 cDNA, enhanced production of the 75-kDa protein species (Figure 7C), confirming that it is indeed RabGAP5.
Figure 7.
RabGAP5 activity and expression in PC12 cells. (A) PC12 cells were cotransfected with pBI/Rab5WT, and each of the potential Rab5 GAPs (with Myc or HA tag and in pcDNA3) as indicated. Shown are the Rab5-GTP levels (Rab5-GTP) and total amount of Rab5 (total Rab5) in these cells, determined by the GST-R5BD pull-down assay described in Figure 6. Control cells (control) were transfected with pBI/Rab5WT and empty pcDNA3 vector. Molecular mass standards (in kilodaltons) are indicated on the left. (B) Additional controls, indicating that each putative GAP protein is indeed expressed in the transfected cells by immunoblot analysis of cell lysates with anti-Myc and anti-HA antibodies for detection of Myc-tagged RabGAP5, RN-tre, and HA-tagged TSC2, respectively. Molecular mass standards (in kilodaltons) are indicated on the left of each panel. (C) Endogenous (PC12) and overexpressed RabGAP5 in control and transfected PC12 cells by immunoblot analysis with the anti-RabGAP5 antibody, as indicated. Immunoblot of actin (as indicated) on the same membrane serves as the loading control.
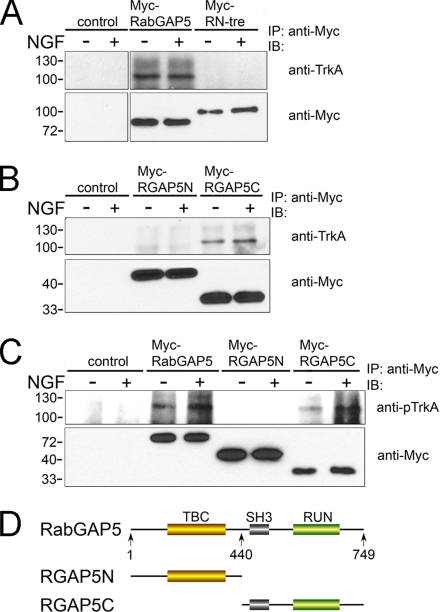
RabGAP5 Is Associated with TrkA
We next determined whether RabGAP5 or RN-tre is associated with TrkA by coIP assays. Each GAP (containing the Myc tag) was coexpressed with TrkA in PC12 cells. The cells were then either treated or not treated with NGF, followed by cell lysis and incubation with Myc antibody-conjugated Sepharose beads to immunoprecipitate the GAP proteins. Total TrkA or pTrkA associated with the GAP proteins was determined by immunoblot analysis with the anti-TrkA or anti-pTrkA antibody. We found that RabGAP5 was associated with TrkA, whereas RN-tre was not (Figure 8A). Although the total amount of TrkA associated with RabGAP5 seemed not changed upon NGF treatment (Figure 8A), the association of activated and pTrkA was markedly enhanced upon NGF treatment (Figure 8C). RabGAP5 contains several functional domains, with the GAP (or Tre2/Bub2/Cdc16 [TBC]) domain in the N-terminal half and the Scr homology (SH)3 and RPIP8/UNC14/NESCA (RUN) domains in the C-terminal half (Haas et al., 2005) (Figure 8D). To dissect further the domains involved in the association with TrkA, we made and expressed Myc-tagged RabGAP5-N (residues 1-440) and RabGAP5-C (residues 441-749) in PC12 cells and determined which one would associate with TrkA by the coIP assay. Our results showed that only the C-terminal half (RabGAP5-C), which contains SH3 and RUN domains, was associated with TrkA (Figure 8B). In this case, an increase in the total amount of TrkA associated with RabGAP5-C was detected upon NGF treatment (Figure 8B). More profound increase was seen in the association of pTrkA and RabGAP5-C upon NGF treatment (Figure 8C), consistent with the results on the association of pTrkA with full-length RabGAP5 (Figure 8C).
Figure 8.
CoIP of RabGAP5 with TrkA. (A) PC12 cell lysates containing coexpressed TrkA and each of the Myc-tagged GAPs (as indicated) were immunoprecipitated by anti-Myc antibody-conjugated Sepharose beads, followed by SDS-PAGE and immunoblot (IB) analysis with anti-TrkA and anti-Myc antibodies, respectively. Control cells expressed TrkA only without the GAPs. Top, association of TrkA with immunoprecipitated RabGAP5 but not with RN-tre. The NGF treatment was for 5 min (+). Bottom, controls indicating the successful immunoprecipitation of each of the Myc-tagged GAPs. Molecular mass standards (in kiloldaltons) are indicated on the left of each panel. (B) Same experiment as described in A except that RabGAP5 truncation mutants were expressed instead of their WT counterparts. RGAP5N, N-terminal half (residues 1-440) of RabGAP5; RGAP5C, C-terminal half (residues 441-749) of RabGAP5. (C) Same experiment as described in A and B except that anti-pTrkA instead of anti-TrkA antibody was used in the immunoblot analysis, as indicated. (D) Schematic illustration of the RabGAP5 structure. Locations of TBC, SH3, and RUN domains are indicated, and the residue numbers mark the truncation mutants.
RabGAP5 Truncation Mutant or Knockdown via shRNA Blocks NGF-mediated Neurite Outgrowth
To further examine the relevance of RabGAP5 in NGF-mediated neurite outgrowth, we expressed full-length and truncated RabGAP5 proteins in PC12 cells and determined the effect on neurite outgrowth (Figure 9), with the same assay as was used for determining the Rab5 effect (Figure 1). Each RabGAP5 protein was coexpressed with EGFP, which helped identify the transfected cells via fluorescence microscopy. Like the dominant-negative Rab5 mutants (Figure 1), full-length RabGAP5 showed no inhibitory effect on NGF-induced neurite outgrowth (Figure 9). It did not further enhance neurite outgrowth either, suggesting that if required, it is not rate limiting in PC12 cells. To determine whether abrogation of endogenous RabGAP5 function may affect neurite outgrowth, we expressed truncated RabGAP5 proteins (RabGAP5-N and RabGAP5-C) in PC12 cells and then determined NGF-mediated neurite outgrowth. Although RabGAP5-N had little effect, RabGAP5-C greatly reduced the neurite outgrowth from 60 to 20%, (Figure 9), possibly by competing with endogenous RabGAP5 for association with TrkA. To further demonstrate the requirement of RabGAP5 in neurite outgrowth, we transfected PC12 cells with pSUPER constructs that expressed shRNAs to knock down RabGAP5 expression. The four shRNAs targeting different regions in rat RabGAP5 all effectively knocked down coexpressed myc-RabGAP5 (rat cDNA) levels, whereas a shRNA targeting human RabGAP5 had only a small (but consistent) effect on the myc-RabGAP5 expression in comparison with the pSUPER vector control (Figure 9). The effect on endogenous RabGAP5 was difficult to assess with the immunoblot assay because of the low transfection efficiency in PC12 cells. Importantly, the transfected cells expressing the four rat shRNAs, as identified by coexpression of EGFP, showed 50% or more reduction in NGF-induced neurite outgrowth, with shRNA4 being the most potent inhibitor (Figure 9), whereas the control human shRNA had little effect in comparison with the vector control (Figure 9).
Figure 9.
Inhibition of NGF-induced neurite outgrowth by RabGAP5 truncation mutant and RNAi. (A) Fluorescence microscopy images of the cells after a 6-d treatment with 50 ng/ml NGF and with or without expression of RabGAP5 and the two truncation mutants, as indicated. (B) Quantification of the results from A. The percentage of differentiated cells was determined from >200 transfected cells expressing EGFP and the indicated RabGAP5 proteins in each case. Error bars represent SEM of three independent experiments. (C) Inhibitory effect of RabGAP5 RNAi on the expression of Myc-RabGAP5 in PC12 cells by immunoblot analysis with the anti-Myc antibody, as indicated. Cells were either not transfected (lane PC12) or cotransfected with pcDNA3/Myc-RabGAP5 (rat) and each of the pSUPER constructs expressing shRNAs for rat RabGAP5 (1-4), its human counterpart (control), or the empty vector (vector). Immunoblot of actin serves as the loading control. Molecular mass standards (in kilodaltons) are indicated on the left. (D) Inhibitory effect of RabGAP5 RNAi on NGF-induced neurite outgrowth. Cells were cotransfected with pBI/EGFP and each of the pSUPER constructs as indicated, followed by neurite outgrowth assays described in A and B.
We also examined RN-tre in this regard and found that expression of either WT or the GAP-dead RN-tre:R150A mutant (Lanzetti et al., 2000) induced severe cell rounding (data not shown). Whereas this RN-tre effect may be associated with its growth-promoting properties, it is not what is expected of a Rab5 GAP in facilitating neurite outgrowth. In addition, the RN-tre effect is independent of its GAP activity and thus is likely mediated through a function other than Rab5. Together with the fact that RN-tre is not associated with TrkA (Figure 8A), we conclude that RN-tre is not involved in NGF signaling-mediated Rab5 inactivation and neurite outgrowth.
DISCUSSION
Endocytosis is essential for NGF-mediated neurite outgrowth and differentiation in PC12 cells (Grimes et al., 1996; Zhang et al., 2000). NGF signaling is initiated when it binds to its receptor TrkA at the cell surface, leading to transient activation of the Ras/MAPK pathway and phosphoinositide 3-kinase that promotes cell survival (Zhang et al., 2000). The NGF–TrkA complex is then endocytosed into the cell and found on the so-called signaling endosomes (Grimes et al., 1996) where TrkA is suggested to recruit new adaptors and to activate additional signaling processes to induce neurite outgrowth (York et al., 1998; Meakin et al., 1999). The biogenesis of signaling endosomes is not well understood. Although signaling endosomes contain early endosomal markers such as Rab5 (Delcroix et al., 2003), they are involved in long-distance retrograde transport of NGF in axons and have a much longer lifetime than conventional early endosomes, implying that Rab5 activity needs to be suppressed at this stage to avoid fusion with endosomes. Rink et al. (2005) recently described a model on the dynamic nature of the early endosomal network (EEN) and the transition to late endosomes. Rab5-dependent early endosome fusion is necessary for the entry to the EEN where cargoes are concentrated before the progression to late endosomes.
Our data shed light on the biogenesis of signaling endosomes (Figure 10). NGF binding and signaling induce endocytosis of its receptor TrkA. RabGAP5 associates with TrkA, such that the TrkA-containing endocytic vesicles have low Rab5 activity and thus less opportunity to fuse with early endosomes and enter the EEN. As a result, the TrkA-containing vesicles are diverted from the EEN and specialize to become signaling endosomes, which serve as a platform for signaling processes leading to neurite outgrowth and differentiation. Our model is supported by several lines of evidence. First, low Rab5 activity (Rab5:S34N) facilitates NGF signaling and neurite outgrowth, whereas high Rab5 activity (Rab5:Q79L and Rabex-5) inhibits this process. Second, high Rab5 activity (Rab5:Q79L) diminishes intracellular NGF signaling by rapid dephosphorylation of internalized pTrkA, possibly via gaining access to endosome-associated phosphatases, whereas low Rab5 activity (Rab5:S34N) sustains intracellular NGF signaling by blocking endosome fusion and consequently the access to the phosphatases. Third, NGF signaling leads to down-regulation of Rab5 activity as evidenced by the reduction of Rab5-GTP level in PC12 cells. Fourth, RabGAP5 is found to be associated with TrkA as evidenced by coIP assays. Finally, RabGAP5 RNAi and truncation mutant inhibit NGF-induced neurite outgrowth, strongly suggesting the requirement of RabGAP5 and thus down-regulation of Rab5 in this process. Other Rab5-related functions such as regulation of membrane ruffles (Lanzetti et al., 2004), could promote neurite growth. Although this possibility cannot be ruled out, it is inconsistent with our data that show Rab5:S34N, which should inhibit Rab5-mediated ruffles, actually increases NGF signaling and neurite outgrowth.
Figure 10.
Model of NGF/TrkA-mediated Rab5 inactivation and establishment of signaling endosomes. NGF binds TrkA and induces its endocytosis. TrkA-associated RabGAP5 inactivates Rab5 by promoting its GTP hydrolysis. As a result, the TrkA-containing endocytic vesicles cannot fuse with early endosomes and enter the endocytic pathway. This population of TrkA-containing endocytic vesicles is thus diverted from the conventional endocytic pathway and specializes to become the signaling endosomes.
RabGAP5 is associated with TrkA and pTrkA, although it is yet to be determined whether pTrkA has higher affinity. This physical coupling can ensure that each TrkA-containing endocytic vesicle also contains RabGAP5, which should suppress Rab5 activity and prevent the vesicle from entering the EEN and endocytic pathway. In this context, our observation that NGF rapidly reduces the Rab5-GTP level may be explained if NGF induces new recruitment of RabGAP5 to pTrkA and/or NGF-induced TrkA endocytosis brings the associated RabGAP5 with it from the plasma membrane into endocytic vesicles where most Rab5 molecules are targeted and activated (Ullrich et al., 1994), leading to the approximation of RabGAP5 to its substrate, i.e., Rab5-GTP. It is also possible that NGF may enhance the GAP activity of RabGAP5 already bound to TrkA, although such an activation mechanism has not been described for a Rab GAP.
The fate of signaling endosomes remains an open question. Because Rab5 is retained on signaling endosomes (Delcroix et al., 2004), the possibility exists that they may eventually reenter the EEN and endocytic pathway upon reactivation of Rab5, which requires the recruitment and action of Rab5 GEFs, e.g., Rabex5 (Horiuchi et al., 1997) and RIN proteins (Tall et al., 2001; Kajiho et al., 2003), to overcome the GAPs. In support of this contention, localized balance of GEFs and GAPs is shown to regulate the activities of Ras-like small GTPases in different compartments (Mochizuki et al., 2001; Bivona et al., 2003). In addition, our data show that NGF-induced down-regulation of Rab5 occurs in the initial phase, but cellular Rab5 activity (i.e., the Rab5-GTP level) recovers later, corresponding to the activation and inactivation phases of TrkA. Furthermore, TrkA does eventually reach lysosomes after NGF-induced endocytosis, despite the diversion into signaling endosomes (Zhou et al., 1995; Jullien et al., 2002). In this context, we notice a remarkable contrast between NGF signaling that promotes cell differentiation and EGF signaling that promotes cell growth and proliferation. NGF signaling suppresses Rab5 via the RabGAP5 to help establish the signaling endosomes and sustain the differentiation signals (this study), whereas epidermal growth factor signaling enhances Rab5 activity via the Rab5 GEFs to accelerate the entry into the EEN and endocytic pathway (Tall et al., 2001). In this regard, Rab5 may be considered as a switch in cell fate decision: differentiation versus proliferation.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Francis Barr, Kun-Liang Guan, P. Paolo Di Fiore, and Brian Rudkin for kindly providing the RabGAP5 antibody, TSC2, RN-tre, and TrkA-EGFP cDNAs, respectively. We thank Gillian Air, Francis Barr, Richard Cummings, and Ann Louise Olson for critical reading of this manuscript. We are indebted to Jim Henthorn for assistance with the confocal microscopy. This work was supported in part by an American Cancer Society grant RSG-03-164-01-MGO (to G.L.).
Abbreviations used:
- coIP
coimmunoprecipitation
- Dox
doxycycline
- EEN
early endosomal network
- EGFP
enhanced green fluorescent protein
- GAP
GTPase-activating protein
- GEF
guanine nucleotide exchange factor
- NGF
nerve growth factor
- pTrkA
phosphorylated TrkA
- RFP
red fluorescent protein
- R5BD
Rab5-bindng domain
- WT
wild-type.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-08-0725) on January 31, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Bivona T. G., Perez De Castro I., Ahearn I. M., Grana T. M., Chiu V. K., Lockyer P. J., Cullen P. J., Pellicer A., Cox A. D., Philips M. R. Phospholipase Cgamma activates Ras on the Golgi apparatus by means of RasGRP1. Nature. 2003;424:694–698. doi: 10.1038/nature01806. [DOI] [PubMed] [Google Scholar]
- Bucci C., Parton R. G., Mather I. M., Stunnenberg H., Simons K., Hoflack B., Zerial M. The small GTPase Rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Ceresa B. P., Schmid S. L. Regulation of signal transduction by endocytosis. Curr. Opin. Cell Biol. 2000;12:204–210. doi: 10.1016/s0955-0674(99)00077-0. [DOI] [PubMed] [Google Scholar]
- Delcroix J. D., Valletta J., Wu C., Howe C. L., Lai C. F., Cooper J. D., Belichenko P. V., Salehi A., Mobley W. C. Trafficking the NGF signal: implications for normal and degenerating neurons. Prog. Brain Res. 2004;146:3–23. doi: 10.1016/s0079-6123(03)46001-9. [DOI] [PubMed] [Google Scholar]
- Delcroix J. D., Valletta J. S., Wu C., Hunt S. J., Kowal A. S., Mobley W. C. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- Gorvel J.-P., Chavrier P., Zerial M., Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes M. L., Zhou J., Beattie E. C., Yuen E. C., Hall D. E., Valletta J. S., Topp K. S., LaVail J. H., Bunnett N. W., Mobley W. C. Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J. Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A. K., Fuchs E., Kopajtich R., Barr F. A. A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat. Cell Biol. 2005;7:887–893. doi: 10.1038/ncb1290. [DOI] [PubMed] [Google Scholar]
- Hoffenberg S., Sanford J. C., Liu S., Daniel D. S., Tuvin M., Knoll B. J., Wessling-Resnick M., Dickey B. F. Biochemical and functional characterization of a recombinant GTPase, Rab5, and two of its mutants. J. Biol. Chem. 1995;270:5048–5056. doi: 10.1074/jbc.270.10.5048. [DOI] [PubMed] [Google Scholar]
- Horiuchi H., et al. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Howe C. L., Mobley W. C. Signaling endosome hypothesis: a cellular mechanism for long distance communication. J. Neurobiol. 2004;58:207–216. doi: 10.1002/neu.10323. [DOI] [PubMed] [Google Scholar]
- Huang E. J., Reichardt L. F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien J., Guili V., Reichardt L. F., Rudkin B. B. Molecular kinetics of nerve growth factor receptor trafficking and activation. J. Biol. Chem. 2002;277:38700–38708. doi: 10.1074/jbc.M202348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiho H., Saito K., Tsujita K., Kontani K., Araki Y., Kurosu H., Katada T. RIN 3, a novel Rab5 GEF interacting with amphiphysin II involved in the early endocytic pathway. J. Cell Sci. 2003;116:4159–4168. doi: 10.1242/jcs.00718. [DOI] [PubMed] [Google Scholar]
- Lanzetti L., Palamidessi A., Areces L., Scita G., Di Fiore P. P. Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature. 2004;429:309–314. doi: 10.1038/nature02542. [DOI] [PubMed] [Google Scholar]
- Lanzetti L., Rybin V., Malabarba M. G., Christoforidis S., Scita G., Zerial M., Di Fiore P. P. The Eps8 protein coordinates EGF receptor signalling through Rac and trafficking through Rab5. Nature. 2000;408:374–377. doi: 10.1038/35042605. [DOI] [PubMed] [Google Scholar]
- Li G., Barbieri M. A., Colombo M. I., Stahl P. D. Structural features of the GTP-binding defective Rab5 mutants required for their inhibitory activity on endocytosis. J. Biol. Chem. 1994;269:14631–14635. [PubMed] [Google Scholar]
- Li G., Liang Z. Phosphate-binding loop and Rab GTPase function: mutations at Ser29 and Ala30 of Rab5 lead to loss-of-function as well as gain-of-function phenotype. Biochem. J. 2001;355:681–689. doi: 10.1042/bj3550681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Stahl P. D. Structure-function relationship of the small GTPase Rab5. J. Biol. Chem. 1993;268:24475–24480. [PubMed] [Google Scholar]
- Liang Z., Mather T., Li G. GTPase mechanism and function: new insights from systematic mutational analysis of the phosphate-binding loop residue Ala30 of Rab5. Biochem. J. 2000;346:501–508. [PMC free article] [PubMed] [Google Scholar]
- Meakin S. O., MacDonald J. I., Gryz E. A., Kubu C. J., Verdi J. M. The signaling adapter FRS-2 competes with Shc for binding to the nerve growth factor receptor TrkA. A model for discriminating proliferation and differentiation. J. Biol. Chem. 1999;274:9861–9870. doi: 10.1074/jbc.274.14.9861. [DOI] [PubMed] [Google Scholar]
- Mochizuki N., Yamashita S., Kurokawa K., Ohba Y., Nagai T., Miyawaki A., Matsuda M. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature. 2001;411:1065–1068. doi: 10.1038/35082594. [DOI] [PubMed] [Google Scholar]
- Nosaka Y., Arai A., Miyasaka N., Miura O. CrkL mediates Ras-dependent activation of the Raf/ERK pathway through the guanine nucleotide exchange factor C3G in hematopoietic cells stimulated with erythropoietin or interleukin-3. J. Biol. Chem. 1999;274:30154–30162. doi: 10.1074/jbc.274.42.30154. [DOI] [PubMed] [Google Scholar]
- Rink J., Ghigo E., Kalaidzidis Y., Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Rybin V., Ullrich O., Rubino M., Alexandrov K., Simon I., Seabra M. C., Goody R., Zerial M. GTPase activity of Rab5 acts as a timer for endocytic membrane fusion. Nature. 1996;383:266–269. doi: 10.1038/383266a0. [DOI] [PubMed] [Google Scholar]
- Segal R. A. Selectivity in neurotrophin signaling: theme and variations. Annu. Rev. Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Parton R. G., Steele-Mortimer O., Lutcke A., Gruenberg J., Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Vitale G., Ullrich O., Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- Tall G. G., Barbieri M. A., Stahl P. D., Horazdovsky B. F. Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev. Cell. 2001;1:73–82. doi: 10.1016/s1534-5807(01)00008-9. [DOI] [PubMed] [Google Scholar]
- Ullrich O., Horiuchi H., Bucci C., Zerial M. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature. 1994;368:157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- Xiao G. H., Shoarinejad F., Jin F., Golemis E. A., Yeung R. S. The tuberous sclerosis 2 gene product, tuberin, functions as a Rab5 GTPase activating protein (GAP) in modulating endocytosis. J. Biol. Chem. 1997;272:6097–6100. doi: 10.1074/jbc.272.10.6097. [DOI] [PubMed] [Google Scholar]
- York R. D., Molliver D. C., Grewal S. S., Stenberg P. E., McCleskey E. W., Stork P. J. Role of phosphoinositide 3-kinase and endocytosis in nerve growth factor-induced extracellular signal-regulated kinase activation via Ras and Rap1. Mol. Cell Biol. 2000;20:8069–8083. doi: 10.1128/mcb.20.21.8069-8083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York R. D., Yao H., Dillon T., Ellig C. L., Eckert S. P., McCleskey E. W., Stork P. J. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Moheban D. B., Conway B. R., Bhattacharyya A., Segal R. A. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J. Neurosci. 2000;20:5671–5678. doi: 10.1523/JNEUROSCI.20-15-05671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Valletta J. S., Grimes M. L., Mobley W. C. Multiple levels for regulation of TrkA in PC12 cells by nerve growth factor. J. Neurochem. 1995;65:1146–1156. doi: 10.1046/j.1471-4159.1995.65031146.x. [DOI] [PubMed] [Google Scholar]
- Zhu G., Zhai P., Liu J., Terzyan S., Li G., Zhang X. C. Structural basis of Rab5-Rabaptin5 interaction in endocytosis. Nat. Struct. Mol. Biol. 2004;11:975–983. doi: 10.1038/nsmb832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.