Abstract
Protein disulfide isomerase (PDI)-like proteins act as oxido-reductases and chaperones in the endoplasmic reticulum (ER). How oligomerization of the PDI-like proteins control these activities is unknown. Here we show that dimerization of ERp29, a PDI-like protein, regulates its protein unfolding and escort activities. We have demonstrated previously that ERp29 induces the local unfolding of polyomavirus in the ER, a step required for viral infection. We now find that, in contrast to wild-type ERp29, a mutant ERp29 (D42A) that dimerizes inefficiently is unable to unfold polyomavirus or stimulate infection. A compensatory mutation that partially restores dimerization to the mutant ERp29 (G37D/D42A) rescues ERp29 activity. These results indicate that dimerization of ERp29 is crucial for its protein unfolding function. ERp29 was also suggested to act as an escort factor by binding to the secretory protein thyroglobulin (Tg) in the ER, thereby facilitating its secretion. We show that this escort function likewise depends on ERp29 dimerization. Thus our data demonstrate that dimerization of a PDI-like protein acts to regulate its diverse ER activities.
INTRODUCTION
Oligomerization of proteins has been described as a regulatory mechanism for many cellular processes, including cell signaling (Hubbard and Till, 2000), gene expression (Warren and O'Shea, 1998), and cytosolic quality control processes (Pearl and Prodromou, 2006). Events in the endoplasmic reticulum (ER) may also be regulated by oligomerization of ER resident proteins. For instance, the ER chaperones calnexin and calreticulin form dimers under conditions that enhance their chaperone activity, although it is not known whether this activity requires dimerization per se (Rizvi et al., 2004).
Protein disulfide isomerase (PDI)-like proteins belong to a large protein family in the ER characterized by the presence of a thioredoxin domain. They function as oxido-reductases, isomerases, and chaperones in this compartment (Ellgaard and Ruddock, 2005). The oxido-reductase activity of members of this family including PDI, ERp72, and ERp57 depends on the active site double-cysteine motif, which is absent in other redox-inactive PDI-like proteins (e.g., ERp27, ERp29, PDILT, TMX2; Ellgaard and Ruddock, 2005). Under certain conditions, PDI and ERp29 were shown to form dimers or higher oligomers (Pace and Dixon, 1979; Yu et al., 1994; Mkrtchian et al., 1998b). Other studies indicated that PDI (Solovyov and Gilbert, 2004; Li et al., 2006) and ERp57 (Frickel et al., 2004) exist as monomers. The discrepancy of PDI's dimerization state is unclear, but may reflect the different in vitro conditions of the purification procedures.
Evidence that PDI-like proteins exist as dimers and that dimerization is required for function has emerged from the prokaryotic Dsb system (Bader et al., 2001) and from more recent studies on Drosophila Wind (Ma et al., 2003; Barnewitz et al., 2004). ERp29, the mammalian homologue of Wind, consists of an N-terminal domain (NTD) with a thioredoxin fold and a C-terminal domain (CTD) with a novel, all-helical fold (Mkrtchian et al., 1998b; Liepinsh et al., 2001). An NMR study of rat ERp29 and a crystal structure of Wind indicated that the NTD mediates dimerization such that the C-terminal domains point in opposite directions (Liepinsh et al., 2001; Ma et al., 2003). Whether dimerization regulates ERp29 activity is unknown.
We demonstrated previously that the protein unfolding activity of ERp29 is hijacked by the murine polyomavirus (Py) during its entry into cells (Magnuson et al., 2005). To cause infection, Py is transported from the cell surface to the ER. In this compartment, the virus penetrates the ER membrane to reach the cytosol and then the nucleus, where transcription and replication of the viral DNA ensue, leading to lytic infection and cell transformation. Our data implicated the ERp29-dependent unfolding of Py as a step necessary for viral penetration across the ER membrane. Apart from its Py unfolding activity, the endogenous function of ERp29 is connected with protein secretion. In the ER, ERp29 binds to the secretory protein thyroglobulin (Tg) (Sargsyan et al., 2002) and is required for its secretion (Baryshev et al., 2006). We have shown that overexpression of the ERp29 NTD attenuates Tg secretion and Py infection (Magnuson et al., 2005; Baryshev et al., 2006). Such dominant-negative features of ERp29 NTD led us to suspect that its inhibitory action may be due to the disruption of the active ERp29 homodimer. Hence, we asked whether dimerization of ERp29 is required for both the viral unfolding and Tg secretion processes.
Here we provide evidence that homodimerization of ERp29 is necessary for both of these functions. We demonstrate that an ERp29 mutant (D42A) that fails to dimerize but folds properly is unable to induce Py unfolding or to stimulate Py infection, in contrast to the wild-type ERp29 homodimer. Partial restoration of ERp29 dimerization by the addition of a second, compensatory mutation (G37D/D42A) restores the unfolding reaction partially and infection. Furthermore, overexpression of the wild-type ERp29 increases Tg secretion, whereas overexpression of the D42A mutant attenuates this process because the mutant D42A ERp29-Tg complex is trapped in the ER. Collectively, these data demonstrate that dimerization of ERp29, a PDI-like protein, serves as a general mechanism to regulate its ER activities.
MATERIALS AND METHODS
Materials
Polyclonal antibodies against PDI and Grp94 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and polyclonal antibodies against ERp72 and calnexin were purchased from StressGen Biotechnologies (San Diego, CA). Monoclonal antibodies against BiP were purchased from BD Biosciences (San Jose, CA), and polyclonal antibodies against ERp57 were a gift from S. High (University of Manchester, Manchester, England). Polyclonal VP1 antibodies were a gift from T. Benjamin (Harvard Medical School). Monoclonal antibodies against rat ERp29 (for immunoprecipitation) were produced by ASLA BIOTECH (Riga, Latvia), and polyclonal antibodies against rat ERp29 (for immunoblotting) were obtained as reported earlier (Mkrtchian et al., 1998a). Polyclonal antibodies against rat thyroglobulin were a gift from Dr. B. Di Jeso (University of Lecce, Italy).
Heterologous Expression and Purification of His-tagged Rat ERp29 NTD and CTD
His-ERp29 NTD and His-ERp29 CTD were expressed and purified as described previously (Magnuson et al., 2005).
Mutagenesis
Mutagenesis of ERp29 was performed with the Stratagene Quikchange II Site-directed Mutagenesis Kit (La Jolla, CA), using the pcDNA3.1(+)-ERp29 plasmid construct (cloning described in Baryshev et al., 2006) as template for the Asp42 to Ala42 mutation. The resulting pcDNA3.1(+)-D42A ERp29 construct was then used as template to create the pcDNA3.1(+)-G37D/D42A ERp29 construct. The mutated ERp29 constructs were confirmed by sequencing.
Cell Culture, Transfection, and Cell Extract Preparation
Cells were cultured in DMEM with 10% fetal calf serum (FCS) and penicillin/streptomycin. The ERp29 expression constructs were transiently transfected into 293T cells or NIH3T3 cells using Lipofectamine 2000. For the cross-linking assay, 293T cell were harvested with trypsin 48 h after transfection and pelleted, and the pellets were lysed with 1% Triton X-100 and protease inhibitors in a physiological buffer [150 mM KOAc, 250 mM sucrose, 50 mM HEPES, pH 7.5, 2 mM Mg(Oac)2] for 30 min on ice. Cell debris was cleared from the extracts by centrifugation at 13,000 rpm for 15 min. For the unfolding assay, 3T3 cell extracts were prepared 48 h after transfection by the same method as the 293T cell extracts, but excluding the protease inhibitors in the lysis buffer.
Affinity Pulldown
An ER lumenal extract was prepared from pancreatic microsomes as before (Tsai et al., 2001). The lumenal extract was incubated with or without His-ERp29 NTD or CTD for 20 min at 37°C. Ni-NTA beads (Qiagen, Chatsworth, CA) were added and rotated for 30 min at 4°C. The beads were washed three times in physiological buffer and then resuspended and boiled in 2% SDS-containing sample buffer. The eluted samples were analyzed by SDS-PAGE and immunoblotted with an ERp29 antibody.
Chemical Cross-linking
To demonstrate the formation of a NTD-ERp29 heterodimer, a lumenal extract and purified His-ERp29 NTD were incubated with a final concentration of 0.5 mM sulfo-succinimidyl 4(N-maleimidomethyl)cyclohexane-1-carboxylate (Sulfo-SMCC) for 30 min at room temperature. To test the dimerization of wild-type or mutant ERp29, 293T cell extracts from cells overexpressing wild-type or mutant ERp29 were incubated with 50 μg/ml Dithiobis(succinimidyl)propionate (DSP) for 30 min at room temperature. All cross-linking reactions were quenched following the room temperature incubation with a final concentration of 50 mM Tris, pH 7.5 on ice for 10 min. The cross-linked samples were analyzed by reducing SDS-PAGE (for Sulfo-SMCC) or nonreducing SDS-PAGE (for DSP) and immunoblotted with the corresponding antibody.
Proteolytic Analysis To Detect Gross Misfolding
Extracts harvested from 293T cells overexpressing wild-type or D42A ERp29 were incubated with a physiological buffer or denatured with 8 M urea for 10 min at 37°C, followed by incubation with 100 μg/ml trypsin for 30 min on ice. Trypsin was inhibited with TLCK on ice for 10 min. Samples were analyzed by reducing SDS-PAGE and immunoblotted with an ERp29 antibody.
Coimmunoprecipitation
Extracts harvested from 293T cells overexpressing wild-type or D42A ERp29 were incubated with monoclonal ERp29 antibody overnight at 4°C. Protein A agarose beads were added for 1 h and then washed three times in a physiological buffer. Complexes were eluted by boiling the beads in a 2% SDS-containing sample buffer. The eluted samples were analyzed by reducing SDS-PAGE and immunoblotted with calnexin, Grp94, or ERp29 antibodies.
Trypsin Sensitivity Assay
Extracts from NIH3T3 cells overexpressing wild-type or mutant ERp29 were used in the trypsin sensitivity assay as described previously (Magnuson et al., 2005).
Py Infection Assay
NIH3T3 cells transfected with wild-type or mutant ERp29 expression constructs were washed and infected with Py at 48 h posttransfection. After 2 h, the cells were washed to remove extracellular virus and either harvested to assess ERp29 expression or allowed to incubate for 24 h. At 24 h after infection, the infected cells were split onto coverslips at a density of 3 × 103 cells/well in a six-well plate. At 48 h after infection, cells were fixed and prepared for immunofluorescence by incubating with a primary rabbit antibody against murine Py large T antigen and an FITC-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA). Each condition depicted in Figures 4 and 5C represents the average value of four independent experiments. In each experiment, 1000 cells/condition were scored for the absence or presence of large T antigen expression using standard fluorescence microscopy, as before (Tsai et al., 2003). A Nikon epifluorescence microscope (Model Eclipse TS100, Melville, NY) equipped with a Piston GFP Bandpass emission filter and a 40× objective was used to determine whether a cell stained positive or negative for the nuclear expression of T antigen.
Figure 4.
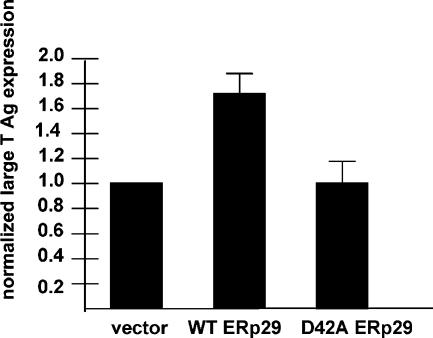
Overexpression of wild-type ERp29, but not D42A ERp29, enhances Py infection. 3T3 cells were transfected with empty vector, a wild-type ERp29 expression construct, or a D42A ERp29 expression construct, followed by infection with Py. Large T antigen expression was analyzed by standard fluorescence microscopy.
Figure 5.
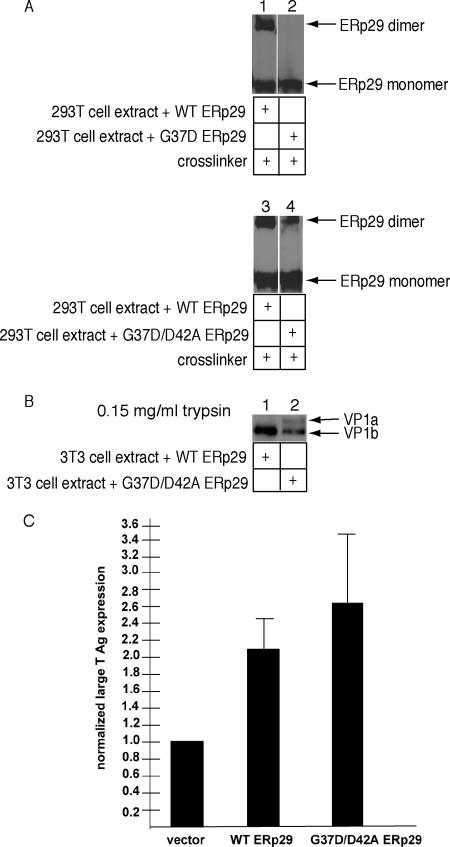
A compensatory mutation that partially restores dimerization to D42A ERp29 rescues ERp29 activity concomitantly. (A) Extracts harvested from 293T cells overexpressing wild-type, G37D, or G37D/D42A ERp29 were incubated with the cross-linking reagent DSP. Samples were analyzed by nonreducing SDS-PAGE and immunoblotted with an ERp29 antibody. (B) Py was incubated with extracts from 3T3 cells overexpressing wild-type or G37D/D42A ERp29 in the presence of DTT and EGTA, followed by trypsin addition (0.15 mg/ml). Samples were analyzed by reducing SDS-PAGE and immunoblotted with a VP1 antibody. (C) 3T3 cells were transfected with an empty vector, a wild-type or a G37D/D42A ERp29 expression constructs, followed by infection with Py. Large T antigen expression was analyzed by standard fluorescence microscopy.
Thyroglobulin Secretion Assay
Transient transfection, metabolic labeling of FRTL-5 rat thyroid epithelial cells, and immunoprecipitation of Tg and ERp29 were performed as previously described (Baryshev et al., 2006). For analysis of endoglycosidase H (endo H) sensitivity, immunoprecipitated thyroglobulin was treated with endo H according to the manufacturer's direction (Roche, Indianapolis, IN).
RESULTS
ERp29 and ERp29 NTD Form a Heterodimer
ERp29 induces local unfolding of Py, an event required for Py infection of host cells (Magnuson et al., 2005). We previously designed a trypsin sensitivity assay to monitor unfolding of the major viral coat protein, VP1. VP1 forms the outer surface of the viral capsid and is subject to initial unfolding events. Based on the crystal structure of Py (Stehle et al., 1994), disulfide bonds and calcium ions are thought to stabilize the VP1 pentamer; thus, reduction of disulfide bonds and the removal of calcium ions should partially destabilize the VP1 coat. Hence, in this assay, Py was first incubated with the reducing agent dithiothreitol (DTT), the calcium-chelating agent EGTA, and the control protein bovine serum albumin (BSA), followed by the addition of trypsin. Under this condition, a cleavage product derived from VP1 called VP1a is observed (Figure 1A, lane 1). Incubation of Py with an extract containing ER lumenal proteins derived from pancreatic microsomes, henceforth referred to as lumenal extract (LE), instead of BSA generated a further cleavage product, called VP1b (Figure 1A, lane 2). This increase in trypsin sensitivity indicates a further unfolding event that reveals previously cryptic trypsin cleavage sites in the virus. Importantly, formation of the VP1b peptide was shown to be ERp29-dependent (Magnuson et al., 2005). Therefore generation of VP1b in the trypsin sensitivity assay serves as a simple measure of the ERp29-induced unfolding activity.
Figure 1.
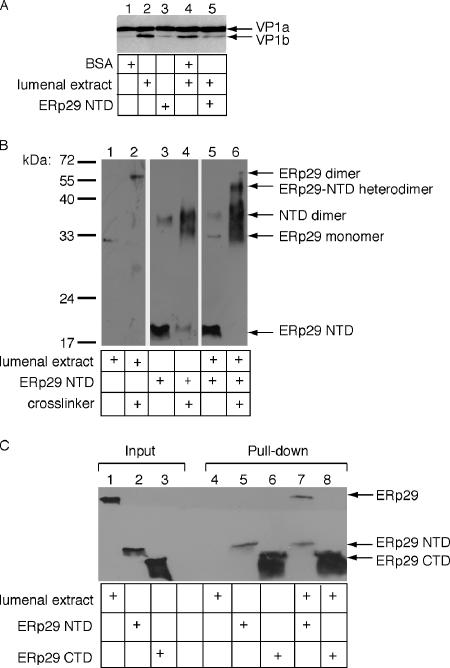
ERp29 NTD and full-length ERp29 heterodimerize. (A) Py (100 ng) was incubated with either a control protein (bovine serum albumin [BSA], 30 μg), a lumenal extract (30 μg), purified His-ERp29 NTD (1 μg), or a combination, as well as DTT (3 mM) and EGTA (10 mM), followed by trypsin addition (0.2 mg/ml). Samples were analyzed by reducing SDS-PAGE and immunoblotted with a VP1 antibody. (B) Lumenal extract alone, His-ERp29 NTD alone, or lumenal extract plus His-ERp29 NTD were incubated at 37°C, followed by addition of the cross-linking reagent Sulfo-SMCC. Samples were analyzed by reducing SDS-PAGE and immunoblotted with an ERp29 antibody. (C) Lumenal extract alone, His-ERp29 NTD alone, His-ERp29 CTD alone, or lumenal extract plus His-ERp29 NTD or CTD were incubated at 37°C, followed by isolation of His-containing complexes with Ni-NTA beads. The bound fractions were analyzed by reducing SDS-PAGE and immunoblotted with an ERp29 antibody.
We have shown that addition of purified His-tagged ERp29 NTD (and not BSA) to the LE decreased the VP1b level (Magnuson et al., 2005; Figure 1A, cf. lane 5 to 4), indicating that the NTD acted in a dominant-negative manner to disrupt the unfolding reaction. This result led us to suspect that, by forming a heterodimer with ERp29, the NTD may thereby disrupt the active ERp29 homodimer. To test whether the NTD forms a heterodimer with ERp29, a cross-linking experiment was performed. When the LE was incubated with a cross-linker and then immunoblotted with an antibody against ERp29 (∼30 kDa), a ∼60 kDa band appeared, indicating the formation of an ERp29 homodimer (Figure 1B, cf. lane 2 to 1), consistent with previous reports (Mkrtchian et al., 1998b; Ferrari et al., 1998; Lippert et al., 2007). Similarly, when purified ERp29 NTD (∼17 kDa) was incubated with a cross-linker, a ∼35-kDa species appeared (Figure 1B, cf. lane 4 to 3), representing NTD dimer. A low level of SDS-resistant NTD dimer was found in the absence of the cross-linker (Figure 1B, lane 3). When the NTD was incubated with LE followed by the addition of the cross-linker, a ∼45-kDa species appeared (Figure 1B, cf. lane 6 to 5). The size of this species is consistent with the predicted ERp29-ERp29 NTD heterodimer and suggests that NTD hetero-dimerizes with ERp29.
It is possible that NTD is complexed with a ∼30-kDa protein in the LE other than ERp29. To establish further the NTD-ERp29 interaction, the His-tagged NTD or CTD (as a control) was incubated alone or with LE, followed by isolation of the NTD or CTD using Ni-NTA beads. We found that ERp29 is present in the NTD but not CTD isolates (Figure 1C, cf. lane 7 to 8), indicating that NTD and ERp29 formed a complex. These results demonstrate that ERp29 NTD can heterodimerize with ERp29 and suggest that its inhibitory action in the viral unfolding reaction and in Tg secretion (Magnuson et al., 2005; Baryshev et al., 2006) may be through the disruption of the active ERp29 homodimer.
The D42A ERp29 Mutant Dimerizes Inefficiently
The dimer interfaces suggested by the NMR structure of ERp29 (Liepinsh et al., 2001) and the crystal structure of Wind (Ma et al., 2003) do not agree. This discrepancy may reflect species-specific difference in dimerization. Therefore, to determine if ERp29 dimerization regulates its viral unfolding activity, we rationally designed alanine mutants of ERp29 based on both the NMR structure of rat ERp29 and the crystal structure of Wind and Wind mutants (Ma et al., 2003; Sevvana et al., 2006). We initially generated alanine mutants of rat ERp29 whose positions, based on the NMR structure, represent the putative dimerization interface. However, based on chemical cross-linking analysis, all of the point mutants remained a dimer (not shown). We then examined the crystal structure of Wind, which identified four residues at the Wind dimerization interface (G26, V28, D31, and R41) that are conserved in ERp29 (Ma et al., 2003). (The corresponding sequence numbering in ERp29 differs by 11 residues.) Individual and combinatorial mutations to V28, D31, and R41 have been shown to impair Wind dimerization to differing degrees (Ma et al., 2003; Barnewitz et al., 2004). The four corresponding residues in ERp29 were individually mutated to alanine (i.e., G37A, L39A, D42A, and K52A) and the mutant ERp29 along with wild-type ERp29 expressed in 293T cells. Dimerization of the ERp29 mutants was compared with that of wild-type ERp29 by incubating whole cell extracts derived from the 293T cells with a cross-linking reagent. As the level of endogenous ERp29 in the 293T extracts used in this study is below the detection limit of the immunoblot analysis (Figure 2B, lane 1), the observed ERp29 is the overexpressed wild-type or mutant ERp29 (Figure 2B, lanes 2 and 3).
Figure 2.
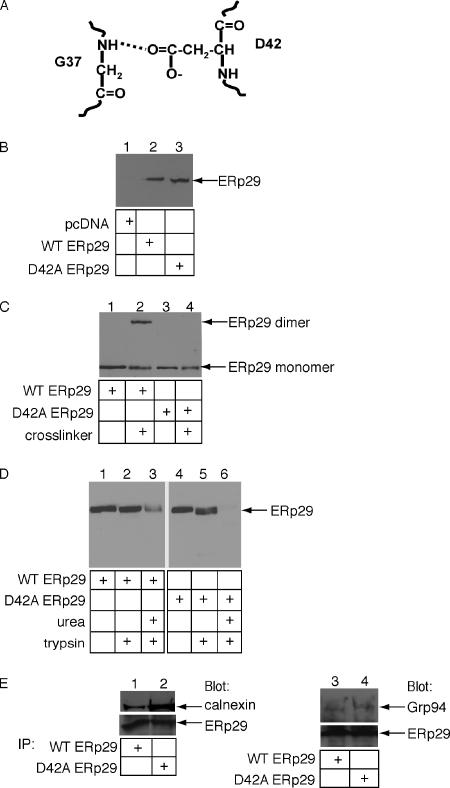
D42A ERp29 does not form a dimer. (A) The dimerization interface of ERp29 is stabilized, in part, by a hydrogen bond formed between the carboxylic acid group of D42 on one ERp29 molecule and the backbone amide group of G37 on the opposite ERp29 molecule. (B) 293T cells were transfected with empty vector, a wild-type ERp29 expression construct, or a D42A ERp29 expression construct. Expression of wild-type and D42A ERp29 were analyzed by reducing SDS-PAGE and immunoblotted with an ERp29 antibody. (C) Extracts harvested from 293T cells overexpressing wild-type or D42A ERp29 were incubated with the cross-linking reagent DSP. Samples were analyzed as in B except in non-reducing conditions. (D) Extracts harvested from 293T cells overexpressing wild-type or D42A ERp29 were incubated with trypsin alone or with urea followed by trypsin. Samples were analyzed as in B. (E) Extracts harvested from 293T cells overexpressing wild-type or D42A ERp29 were incubated with mAb against ERp29, followed by addition of protein A-agarose beads. Immune complexes were analyzed by reducing SDS-PAGE and immunoblotted with calnexin, Grp94, and ERp29 antibodies.
The cross-linking assay revealed that, in contrast to wild-type ERp29, mutation of aspartic acid 42 to alanine (D42A) prevented ERp29 dimerization (Figure 2C, cf. lane 4 to lane 2). The other three point mutants displayed no effect on dimerization (data not shown). Based on information from the crystal structure of the Wind dimer, the carboxyl group of D42 contributes to dimerization by forming a hydrogen bond with the amide backbone of G37 on the opposing ERp29 molecule (Figure 2A). Mutation of D42 to alanine presumably disrupted the hydrogen bond, leading consequently to a marked loss of dimer stability. Consistent with our observation that the D42A rat ERp29 mutant failed to dimerize, the corresponding D31N mutant in Wind (Ma et al., 2003) and the D42N mutant of human ERp29 (Lippert et al., 2007) are also significantly defective in dimerization.
To test whether the D42A mutant is misfolded grossly, D42A and wild-type ERp29 were subjected to limited proteolysis (Figure 2D). Grossly misfolded proteins are generally more sensitive to proteolysis due to exposure of internal proteolytic sites. We found that both wild-type and mutant ERp29 are largely resistant to trypsin digestion (Figure 2D, cf. lane 2 to 1 and lane 5 to 4). Under this condition, unfolded cholera toxin is degraded completely (Tsai et al., 2001; Forster et al., 2006). When the proteins were denatured artificially with urea, degradation of both wild-type and D42A ERp29 was observed (Figure 2D, lanes 3 and 6), although the wild-type protein was slightly less sensitive to degradation possibly due to the protective nature of dimerization. We conclude that the D42A mutant is not grossly misfolded.
To assess its structural integrity further, we asked whether the D42A ERp29 is able to complex with calnexin and Grp94, established ERp29 binding partners (Park et al., 2005). We found that calnexin and Grp94 coimmunoprecipitated with both wild-type and mutant ERp29 (Figure 2E, cf. lane 1 to 2 and lane 3 to 4); a higher level of calnexin was bound to the mutant ERp29. We conclude that introducing an alanine to residue 42 of ERp29, a predicted site of dimerization interface, perturbed its ability to dimerize without disrupting its overall structural integrity.
Wild-type ERp29, but Not D42A ERp29, Triggers Local Unfolding of Py
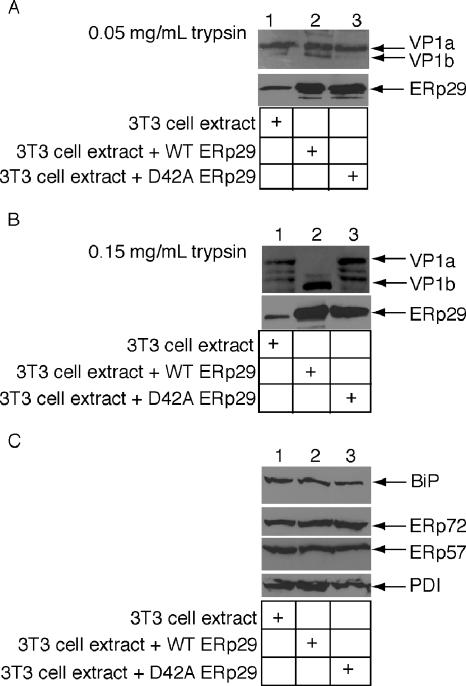
What is the functional consequence of reduced dimerization to ERp29 unfolding activity? To address this question, we compared the Py unfolding activity of D42A ERp29 to wild-type ERp29 using the trypsin sensitivity assay. We demonstrated previously that a whole cell extract derived from mouse 3T6 cells overexpressing wild-type ERp29 stimulated VP1 unfolding (Magnuson et al., 2005). We now find that a 3T3 cell extract overexpressing wild-type ERp29, but not an extract overexpressing the D42A mutant (Figure 3A, bottom panel, cf. lane 3 to 2), triggered the generation of the VP1b fragment when compared with a mock-transfected extract (Figure 3A, top panel, cf. lane 2 to lanes 3 and 1), indicating the mutant ERp29 is unable to induce the VP1 conformational change. These results are more pronounced when the digestion is performed with a higher trypsin concentration (Figure 3B). Under this condition, the wild-type but not the mock-transfected or the mutant ERp29 extracts stimulated potent production of VP1b (Figure 3B, top panel, cf. lane 2 to lanes 1 and 3); partial degradation of the VP1a fragment was seen using the mock-transfected and the D42A extracts (Figure 3B, top panel, lanes 1 and 3). The increased unfolding activity of the wild-type ERp29 extract is not due to nonspecific up-regulation of other ER chaperones, because the levels of BiP, ERp72, ERp57, and PDI are similar in each of the extracts (Figure 3C, lanes 1–3). The inability of D42A mutant to generate the VP1b fragment indicates that dimerization of ERp29 plays a role in its protein unfolding activity.
Figure 3.
Wild-type ERp29, but not D42A ERp29, triggers local unfolding of Py VP1. (A) Py (100 ng) was incubated with extracts from mock-transfected 3T3 cells (30 μg), 3T3 cells overexpressing wild-type ERp29 (30 μg), or 3T3 cells overexpressing D42A ERp29 (30 μg) in the presence of DTT and EGTA, followed by trypsin addition (0.05 mg/ml). Samples were analyzed by reducing SDS-PAGE and immunoblotted with a VP1 antibody. The 3T3 cell extracts were also analyzed for ERp29 content by reducing SDS-PAGE and immunoblotted with an ERp29 antibody. (B) As in A, except 0.15 mg/ml trypsin was used. (C) Extracts were harvested from 3T3 cells mock-transfected or overexpressing wild-type or D42A ERp29. These extracts were analyzed for the levels of ER chaperones by reducing SDS-PAGE and immunoblotted with antibodies against BiP, ERp72, ERp57, and PDI.
Overexpression of Wild-Type ERp29, but Not D42A ERp29, Enhances Py Infection
The ERp29-dependent unfolding reaction enables Py to penetrate the ER membrane to reach the cytosol and then the nucleus, where the virus initiates infection (Magnuson et al., 2005). We asked whether the ERp29 dimer is required for infection by overexpressing wild-type and D42A mutant ERp29 in 3T3 cells, and subsequently monitored infection by measuring the expression of large T antigen. Large T antigen is encoded by the Py genome, and its expression indicates successful arrival of the virus to the nucleus. Compared with transfection with an empty vector, overexpression of wild-type ERp29 increased by ∼65% the number of cells expressing large T antigen (Figure 4), demonstrating that the unfolding activity of ERp29 is the rate-limiting step in the Py infection pathway. By contrast, overexpression of D42A ERp29 did not stimulate large T antigen expression when compared with expression of an empty vector (Figure 4). These results are consistent with the inability of D42A ERp29 to stimulate Py unfolding. Together, the data indicate that the ERp29 dimer regulates Py infection.
Partial Restoration of Dimerization to D42A ERp29 Rescues ERp29 Activity
We have shown that the inability of the mutant D42A ERp29 to dimerize is correlated with its inability to trigger Py unfolding and to stimulate viral infection. To determine if ERp29 dimerization per se is required for these processes, we designed a compensatory mutation in an effort to restore dimerization to D42A ERp29. As discussed above, the Wind crystal structure suggests that the ERp29 dimer is stabilized by formation of a hydrogen bond between the carboxyl group of D42 on one ERp29 molecule and the backbone amino group of G37 on the other ERp29 molecule (Figure 2A). Mutation of D42 to alanine hence disrupts hydrogen bond formation between D42 and G37. We predicted that, by introducing an aspartic acid residue to position 37 in the D42A mutant, generating the G37D/D42A double mutant, a hydrogen bond could be established between the carboxyl group of the aspartic acid at position 37 on one molecule of ERp29 and the backbone amide group of alanine at position 42 on the opposing ERp29 molecule.
Accordingly, whole cell extracts were prepared from 293T cells overexpressing wild-type ERp29, the G37D single mutant, and the G37D/D42A double mutant, and the extracts were used in a cross-linking assay to examine the dimerization of the ERp29 mutants. In contrast to the wild-type ERp29, G37D failed to dimerize (Figure 5A, cf. lane 2 to 1). Because the G37A mutant dimerized (not shown), introduction of a carboxylic acid side chain, but not a methyl group, at position 37 presumably disrupted hydrogen bonding between the amide backbone of residue 37 and the carboxyl group of D42. Importantly, the G37D/D42A double mutant is able to form the dimer species, although its efficiency is reduced compared with the wild-type ERp29 (Figure 5A, cf. lane 4 to 3). These results demonstrate that substitution of G37 with aspartic acid can partially restore the dimerization of D42A ERp29.
To determine if the G37D/D42A mutant unfolds Py, extracts from 3T3 cells overexpressing wild-type and G37D/D42A ERp29 were prepared and used in the trypsin sensitivity assay. Using the higher trypsin concentration shown in Figure 3B, we found that the G37D/D42A extract generated the VP1b fragment, although the level is lower than that produced by the wild-type ERp29 extract (Figure 5B, cf. lane 2 to 1). These data showed that partial restoration of ERp29 dimerization concomitantly restored its unfolding activity proportionally.
We next tested the effect of overexpressing the G37D/D42A ERp29 mutant on viral infection and found that this double mutant stimulated the expression of large T antigen to a similar extent as wild-type ERp29 (Figure 5C). As the compensatory mutation that was able to restore dimerization rescued both the viral unfolding and infection processes, we conclude that dimerization of ERp29 is essential for its ER unfolding activity.
Effects of Overexpressing Wild-type and Mutant ERp29 on Thyroglobulin Secretion
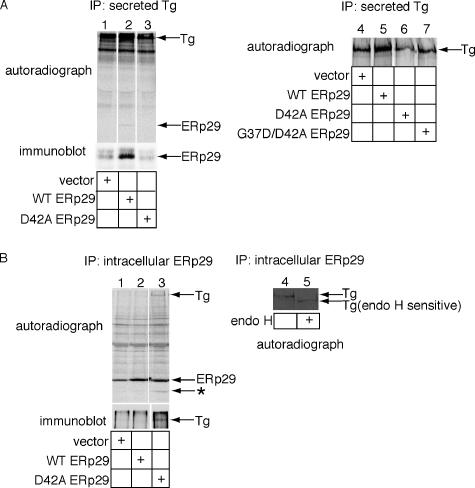
In addition to its role in mediating viral infection, ERp29 interacts with the secretory protein Tg in the ER (Sargsyan et al., 2002) to facilitate its secretion (Baryshev et al., 2006). As substantial levels of ERp29 can be detected extracellularly, it was suggested that ERp29 binds to Tg in the ER escorting it to the cell exterior (Sargsyan et al., 2002). To examine how ERp29 guides Tg secretion and whether dimerization of ERp29 is relevant to this process, wild-type and D42A ERp29 were transfected into FRTL-5 rat thyrocytes. At 48 h after transfection, cells were metabolically labeled with 35S methionine/cysteine for 1 h, and the medium was removed and replenished with normal medium. After 6 additional hours (to enable Tg secretion), the medium was collected, and the cells were harvested and lysed. Total secreted Tg was isolated from the medium by immunoprecipitation using a Tg antibody. A long-exposure autoradiograph of the SDS-PAGE resolved samples showed a twofold increase in secreted Tg from medium of cells overexpressing wild-type ERp29 (Figure 6A, top panel, cf. lane 2 to 1), as shown previously (Baryshev et al., 2006). Under this exposure, a faint 29-kDa band coimmunoprecipitated with Tg secreted from the cells overexpressing wild-type ERp29 (Figure 6A, top panel, lane 2). Immunoblotting with an ERp29 antibody showed that this band is ERp29 (Figure 6A, bottom panel, lane 2). That the ERp29-Tg interaction can be found both in the ER and in the medium suggests a simple mechanism by which ERp29 potentiates Tg secretion: ERp29 complexes with Tg in the ER, escorting it along the secretory pathway to the extracellular environment. We tested whether ERp29 dimer is crucial for its Tg escort activity and found that in cells overexpressing the D42A mutant, the level of extracellular Tg decreased when compared with mock-transfected cells (Figure 6A, top panel, cf. lane 3 to 1).
Figure 6.
Effects of overexpressing wild-type and mutant ERp29 on thyroglobulin secretion. (A) Cells were transfected with an empty vector, wild-type ERp29, D42A ERp29, or G37D/D42A ERp29 expression construct and labeled metabolically. Secreted Tg was immunoprecipitated from the culture media, and the immune complexes analyzed by SDS-PAGE, followed by autoradiography to detect Tg and by immunoblotting with an ERp29 antibody to detect coprecipitated ERp29. (B) Cells were transfected, labeled, and harvested as described above. ERp29 was immunoprecipitated from the cell lysates, and the immune complexes were analyzed by SDS-PAGE, followed by autoradiography to detect ERp29 and immunoblotting with a Tg antibody to detect coprecipitated Tg (left panel). Intracellular Tg that coimmunoprecipitated with the D42A ERp29 mutant was treated with endo H and subjected to SDS-PAGE, followed by autoradiography to detect Tg (right panel).
We next ascertained the effect of overexpressing the partially dimerized ERp29 double mutant (G37D/D42A) on Tg secretion. A short-exposure autoradiograph showed that, in contrast to overexpression of the D42A ERp29 mutant, overexpression of the partially dimerized ERp29 double mutant (G37D/D42A) did not decrease the level of secreted Tg (Figure 6A, cf. lanes 6 and 7 to 4). This result is consistent with the idea that dimerization of ERp29 facilitates its exit out of the ER, enabling ERp29 to escort Tg to the cell exterior. Overexpression of wild-type ERp29 but not the double mutant increased thyroglobulin secretion (Figure 6A, cf. lanes 5 and 7 to 4), likely due to the fact that the double mutant is only dimerized partially. We note that this result is in contrast to the observation that the double ERp29 mutant restored viral unfolding and infection (Figure 5, B and C). The simplest explanation is that the expression level of the double ERp29 mutant is dramatically lower in the FRTL-5 cells used in the thyroglobulin secretion assay than in the 3T3 cells used in the viral infection assay (not shown). Nonetheless, these results indicate that dimerization of ERp29 facilitates Tg secretion.
How might the D42A mutant exert such dominant-negative action on Tg secretion? One likely explanation is that the D42A mutant binds to Tg in a subcellular compartment, but is unable to exit this compartment because of its improper assembly (i.e., absence of dimerization). To test whether the mutant ERp29 traps Tg in the cells, intracellular ERp29 was immunoprecipitated from mock-, wild-type–, and D42A ERp29-transfected lysates. The autoradiograph showed the twofold overexpression of wild-type and D42A ERp29 when compared with the endogenous ERp29 from the mock-transfected cells (Figure 6B, top panel, cf. lanes 2 and 3 to 1). Single high- and low-molecular-weight bands were detected in the ERp29-immunoprecipitate derived from cell lysates expressing the D42A but not wild-type ERp29 (Figure 6B, top panel, cf. lane 3 to 2). Immunoblotting with a Tg antibody confirmed that the high-molecular-weight band was Tg (Figure 6B, bottom panel, lane 3), whereas the low-molecular-weight band (indicated by *) remains unidentified. This finding indicates that the D42A ERp29 mutant binds to Tg but is unable to exit the cell, leading to reduced Tg secretion. Of note, that the D42A mutant complexes with Tg further demonstrates that it is not misfolded globally, consistent with our earlier analysis of this protein (Figure 2).
To identify the subcellular localization of the Tg that complexed with the D42A mutant, the intracellular Tg that coimmunoprecipitated with the D42A ERp29 mutant was treated with endo H and found to be entirely sensitive (Figure 6B, cf. lane 5 to 4). This finding indicates that the D42A ERp29 mutant binds to Tg in the ER, becomes trapped in this compartment, and is unable to reach the Golgi, presumably because the D42A mutant is not oligomerized properly. Collectively, our data demonstrate that the escort activity of ERp29, similar to the viral unfolding activity, requires its dimerization and suggest a general regulatory role for dimerization in ERp29 function.
DISCUSSION
Thioredoxin domain–containing ER proteins function in a wide variety of processes, both physiological and pathological. The normal processes of protein folding, ER quality control, and secretion rely on PDI-like proteins that distinguish between correctly folded, secretion-competent substrates and misfolded substrates that need to be retained and then refolded or degraded. Moreover, to cause disease, pathogens such as Py and cholera toxin traffic from the plasma membrane to the ER where they hijack PDI-like proteins (Magnuson et al., 2005; Forster et al., 2006; Gilbert et al., 2006) in order to gain access to the cytosol. The regulatory mechanisms that control the various functions of a given PDI-like protein have not been well characterized.
To assess whether dimerization could regulate the activities of a specific PDI-like protein, we elucidated the role of dimerization of ERp29, a PDI-like protein shown to have at least two distinct activities, one in mediating the entry of a viral pathogen and the other in the escorting of secretory proteins. Our studies revealed that homodimerization of ERp29 is essential for both its protein unfolding and escort functions. A single amino acid mutation in the N-terminal domain of ERp29 (D42A) abrogates dimerization, disrupting its ability to unfold Py. Why dimerization of ERp29 is required to stimulate Py unfolding is unclear. Because we have not observed physical binding between Py and ERp29, their interaction is assumed to be transient. Whether dimeric ERp29 is required for this transient interaction is unknown. After NTD-mediated dimerization, the two C-terminal domains of ERp29 are brought together and may become active only in close proximity to one another. This idea is supported by the observation that ERp29 NTD can block unfolding and infection. In this scenario, NTD acts in a dominant-negative manner by titration of ERp29 monomers, preventing the two CTD domains from joining together.
Through a rational design approach based on the crystal structure of the Drosophila ERp29 orthologue, we added a compensatory mutation to the D42A ERp29 mutant (G37D/D42A) that partially restored its ability to form dimers and found the unfolding activity to be rescued partially. Surprisingly, the G37D/D42A mutant is able to stimulate Py infection to a similar extent as wild-type ERp29. It is known that only a small fraction of virus taken up by cells during infection reaches the ER (Gilbert and Benjamin, 2004), and even fewer viral particles are transported out of the ER and into the nucleus to cause infection (Greber and Kasamatsu, 1996). Therefore, only a small percentage of G37D/D42A ERp29 overexpressed in the cell is needed to dimerize in order to provide the unfolding activity necessary to stimulate infection by one or a few viral particles. By contrast, in the in vitro unfolding assay, the virus:G37D/D42A ERp29 ratio is higher by necessity due to the detection limit of the immunoblot analysis. As a consequence, the higher virus:ERp29 ratio results in the unfolding of only a fraction of the viral particles.
In addition to the role of the ERp29 dimer in viral infection, our findings also revealed that dimerization of ERp29 is essential during its normal cellular function. ERp29 was shown previously to bind to the secretory protein Tg in the ER (Sargsyan et al., 2002) and facilitate its secretion (Baryshev et al., 2006). However, the detailed mechanism underlying this function remains unclear. We found that ERp29 maintains its interaction with Tg once Tg is secreted, suggesting that ERp29 aids Tg secretion by serving as an escort factor. This finding is consistent with the previously shown cosecretion of ERp29 with Tg despite the presence of the ER-retrieval signal in its C-terminus that normally facilitates recycling of ER-resident proteins back to the ER (Sargsyan et al., 2002). Other ER proteins, such as SCAP (Brown et al., 2002) or RAP (Bu, 2001), have also been shown to act as escort factors in guiding the transport of proteins further along the secretory pathway. Importantly, the dimer of ERp29 was found to be crucial for this activity as overexpression of wild-type ERp29, but not the D42A mutant, stimulated Tg secretion. In fact, D42A overexpression led to a decrease in Tg secretion. Our evidence suggests that the inhibitory action of the mutant ERp29 is attributed to its inability to escort the bound Tg out of the ER. This is consistent with the notion that the ER contains quality control machineries that can recognize misassembled proteins (Ellgaard and Helenius, 2003), preventing them from exiting this compartment. In this context, we observed a ∼25-kDa protein that coimmunoprecipitated with mutant but not wild-type ERp29 (Figure 6B). This protein may represent either an additional secretory substrate similar to Tg trapped by the defective D42A ERp29 in the ER or another helper protein involved along with ERp29 in the escort of Tg.
It was shown previously, using a gel filtration approach, that a His-tagged, purified mutant Wind protein, in which the aspartic acid residue at position 31 was changed to asparagine (D31N), is essentially monomeric (Ma et al., 2003). The D31N Wind protein was shown to be active, as assessed by its ability to transport Pipe, a substrate of Wind, from the ER to the Golgi in COS cells (Ma et al., 2003). In a subsequent chemical crosslinking study, the D31N mutant expressed in COS cells was impaired in dimerization when compared to the wild-type protein, although a low level of dimer can be observed (Barnewitz et al., 2004). In this report, the D31N mutant displayed activity similar to the wild-type protein, consistent with the authors' previous observations (Ma et al., 2003). As suggested by the authors, the residual level of dimer in the D31N protein may be sufficient for its activity. However, we found that the D42A ERp29 single mutant (corresponding to D31N Wind) failed to dimerize and was functionally compromised in both the Py infection and Tg secretion processes. The reason for this apparent discrepancy remains unclear, but may be due to differences in the chosen mutations (i.e. D mutated to A versus N), subtle structural differences in the ERp29 and Wind dimers, or differences in substrate concentrations in the ER (i.e. Py and Tg versus Pipe).
When a second site mutation (R41S) was introduced to the D31N Wind single mutant, the D31N/R41S double mutant was reported to exhibit an even more severe dimerization defect than the D31N mutant (Barnewitz et al., 2004). Importantly, this double mutant was shown to be inactive in transporting Pipe to the Golgi. The authors reported that a high expression level of the Wind double mutant in the ER caused the defect in Pipe transport. Although this raises the possibility that the high concentration of Wind mutant, but not the absence of Wind dimerization per se, prevented Pipe transport, these findings suggest that dimerization of Wind plays a role in regulating its function, consistent with our finding that dimerization regulates the activities of ERp29.
PDI and ERp29 have been shown to oligomerize under certain conditions (Pace and Dixon, 1979; Yu et al., 1994; Mkrtchian et al., 1998b). In contrast, other studies indicated that PDI (Solovyov and Gilbert, 2004; Li et al., 2006) and ERp57 (Frickel et al., 2004) exist as monomers. The reported discrepancy in PDI's dimerization state may reflect a difference in the purification procedures. It is also possible that the high-concentration storage of PDI induced oligomerization in vitro. Further studies aimed at determining the oligomerization state of PDI (if any), as well as other PDI-family members, in live cells may help to elucidate the oligomerization properties of these proteins. Of note, as PDI was shown previously to participate in Py infection (Gilbert et al., 2006) and in cholera toxin intoxication (Tsai et al., 2001; Forster et al., 2006), it is unclear whether oligomerization of PDI, should it occur, regulates any of these processes.
How do PDI-like proteins possess such tremendous flexibility in selecting a wide myriad of substrates, ranging from endogenous secretory proteins to foreign pathogens? Perhaps the process of oligomerization, which increases the number of conformations that can be adopted by these proteins, provides a mechanism by which they may engage their numerous substrates. Alternatively, interactions with other ER-resident factors may enable the PDI-like proteins to act on a specific substrate. In this regard, identifying the binding partners of the PDI-like proteins would help to clarify whether this interaction regulates the substrate selection process.
ACKNOWLEDGMENTS
We thank Diane Fingar for critical review of the manuscript. E.K.R. is supported by a Graduate Research Fellowship from the National Science Foundation. S.M. is supported by the Swedish Medical Research Council and the Swedish Society of Medicine. B.T. is a Biological Scholar at the University of Michigan and a Burroughs Wellcome Fund Investigator in Pathogenesis of Infectious Disease. B.T. is supported by the National Institutes of Health (R01-AI064296).
Abbreviations used:
- PDI
protein disulfide isomerase
- ER
endoplasmic reticulum
- Py
polyomavirus
- NTD
N-terminal domain
- CTD
C-terminal domain
- LE
lumenal extract
- Tg
thyroglobulin.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-11-1004) on January 31, 2007.
REFERENCES
- Bader M. W., Hiniker A., Regeimbal J., Goldstone D., Haebel P. W., Riemer J., Metcalf P., Bardwell J.C.A. Turning a disulfide isomerase into an oxidase: DsbC mutants that imitate DsbA. EMBO J. 2001;20:1555–1562. doi: 10.1093/emboj/20.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnewitz K., Guo C., Sevvana M., Ma Q., Sheldrick G. M., Söling H. D., Ferrari D. M. Mapping of a substrate binding site in the protein disulfide isomerase-related chaperone Wind based on protein function and crystal structure. J. Biol. Chem. 2004;279:39829–39837. doi: 10.1074/jbc.M406839200. [DOI] [PubMed] [Google Scholar]
- Baryshev M., Sargsyan E., Mkrtchian S. ERp29 is an essential endoplasmic reticulum factor regulating secretion of thyroglobulin. Biochem. Biophys. Res. Commun. 2006;340:617–624. doi: 10.1016/j.bbrc.2005.12.052. [DOI] [PubMed] [Google Scholar]
- Brown A. J., Sun L., Feramisco J. D., Brown M. S., Goldstein J. L. Cholesterol addition to ER membranes alters conformation of SCAP, the SREBP escort protein that regulates cholesterol metabolism. Mol. Cell. 2002;10:237–245. doi: 10.1016/s1097-2765(02)00591-9. [DOI] [PubMed] [Google Scholar]
- Bu G. The roles of receptor-associated protein (RAP) as a molecular chaperone for members of the LDL receptor family. Int. Rev. Cytol. 2001;209:79–116. doi: 10.1016/s0074-7696(01)09011-8. [DOI] [PubMed] [Google Scholar]
- Ellgaard L., Helenius A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- Ellgaard L., Ruddock L. W. The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep. 2005;6:28–32. doi: 10.1038/sj.embor.7400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D. M., Nguyen Van P., Kratzin H. D., Soling H. D. ERp28, a human endoplasmic-reticulum-lumenal protein, is a member of the protein disulfide isomerase family but lacks a CXXC thioredoxin-box motif. Eur. J. Biochem. 1998;255:570–579. doi: 10.1046/j.1432-1327.1998.2550570.x. [DOI] [PubMed] [Google Scholar]
- Forster M. L., Sivick K., Park Y. N., Arvan P., Lencer W. I., Tsai B. Protein disulfide isomerase-like proteins play opposing roles during retrotranslocation. J. Cell Biol. 2006;173:853–859. doi: 10.1083/jcb.200602046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frickel E. M., Frei P., Bouvier M., Stafford W. F., Helenius A., Glockshuber R., Ellgaard L. ERp57 is a multifunctional thiol-disulfide oxidoreductase. J. Biol. Chem. 2004;279:18277–18287. doi: 10.1074/jbc.M314089200. [DOI] [PubMed] [Google Scholar]
- Gilbert J., Benjamin T. Uptake pathway of polyomavirus via ganglioside GD1a. J. Virol. 2004;78:12259–12267. doi: 10.1128/JVI.78.22.12259-12267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J., Ou W., Silver J., Benjamin T. Downregulation of protein disulfide isomerase inhibits infection by the mouse polyoma virus. J. Virol. 2006;80:10868–10870. doi: 10.1128/JVI.01117-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber U. F., Kasamatsu H. Nuclear targeting of SV40 and adenovirus. Trends Cell Biol. 1996;6:189–195. doi: 10.1016/0962-8924(96)10016-7. [DOI] [PubMed] [Google Scholar]
- Hubbard S. R., Till J. H. Protein tyrosine kinase structure and function. Annu. Rev. Biochem. 2000;69:373–398. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- Li S. J., Hong X. G., Shi Y. Y., Li H., Wang C. C. Annular arrangement and collaborative actions of four domains of protein-disulfide isomerase: a small angle X-ray scattering study in solution. J. Biol. Chem. 2006;281:6581–6588. doi: 10.1074/jbc.M508422200. [DOI] [PubMed] [Google Scholar]
- Liepinsh E., Baryshev M., Sharipo A., Ingelman-Sundberg M., Otting G., Mkrtchian S. Thioredoxin fold as homodimerization module in the putative chaperone ERp 29, NMR structures of the domains and experimental model of the 51 kDa dimer. Structure. 2001;9:457–471. doi: 10.1016/s0969-2126(01)00607-4. [DOI] [PubMed] [Google Scholar]
- Lippert U., Diao D., Barak N. N., Ferrari D. M. Conserved structural and functional properties of D-domain containing redox active and redox inactive protein disulfide isomerase related protein chaperones. J. Biol. Chem. 2007 doi: 10.1074/jbc.M604440200. in press. [DOI] [PubMed] [Google Scholar]
- Ma Q., Guo C., Barnewitz K., Sheldrick G. M., Söling H. D., Usón I., Ferrari D. M. Crystal structure and functional analysis of Drosophila Wind, a protein-disulfide isomerase-related protein. J. Biol. Chem. 2003;278:44600–44607. doi: 10.1074/jbc.M307966200. [DOI] [PubMed] [Google Scholar]
- Magnuson B., Rainey E. K., Benjamin T., Baryshev M., Mkrtchian S., Tsai B. ERp29 triggers a conformational change in Polyomavirus to stimulate membrane binding. Mol. Cell. 2005;20:289–300. doi: 10.1016/j.molcel.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Mkrtchian S., Fang C., Hellman U., Ingelman-Sundberg M. A stress-inducible rat liver endoplasmic reticulum protein, ERp29. Eur. J. Biochem. 1998a;251:304–313. doi: 10.1046/j.1432-1327.1998.2510304.x. [DOI] [PubMed] [Google Scholar]
- Mkrtchian S., Baryshev M., Matvijenko O., Sharipo A., Sandalova T., Schneider G., Ingelman-Sundberg M. Oligomerization properties of ERp29, an endoplasmic reticulum stress protein. FEBS Lett. 1998b;431:322–326. doi: 10.1016/s0014-5793(98)00786-8. [DOI] [PubMed] [Google Scholar]
- Pace M., Dixon J. E. The nature of the multiple forms of bovine thiol:protein disulfide oxidoreductase. Intl. J. Peptide Protein Res. 1979;14:409–413. doi: 10.1111/j.1399-3011.1979.tb01952.x. [DOI] [PubMed] [Google Scholar]
- Park S., You K. H., Shong M., Goo T. W., Yun E. Y., Kang S. W., Kwon O. Y. Overexpression of ERp29 in the thyrocytes of FRTL-5 cells. Mol. Biol. Rep. 2005;32:7–13. doi: 10.1007/s11033-004-3069-3. [DOI] [PubMed] [Google Scholar]
- Pearl L. H., Prodromou C. Structure and mechanism of the HSP90 molecular chaperone machinery. Annu. Rev. Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- Rizvi S. M., Mancino L., Thammavongsa V., Cantley R. L., Raghavan M. A polypeptide binding conformation of calreticulin is induced by heat shock, calcium depletion, or by deletion of the C-terminal acidic region. Mol. Cell. 2004;15:913–923. doi: 10.1016/j.molcel.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Sargsyan E., Baryshev M., Szekely L., Sharipo A., Mkrtchian S. Identification of ERp29, an endoplasmic reticulum lumenal protein, as a new member of the thyroglobulin folding complex. J. Biol. Chem. 2002;277:17009–17015. doi: 10.1074/jbc.M200539200. [DOI] [PubMed] [Google Scholar]
- Sevvana M., Biadene M., Ma Q., Guo C., Söling H., Sheldrick G. M., Ferrari D. M. Structural elucidation of the PDI-related chaperone Wind with the help of mutants. Acta Cryst. 2006;D62:589–594. doi: 10.1107/S0907444906010456. [DOI] [PubMed] [Google Scholar]
- Solovyov A., Gilbert H. F. Zinc-dependent dimerization of the folding catalyst, protein disulfide isomerase. Protein Sci. 2004;13:1902–1907. doi: 10.1110/ps.04716104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle T., Yan Y., Benjamin T. L., Harrison S. C. Structure of murine polyomavirus complexed with an oligosaccharide receptor fragment. Nature. 1994;369:160–163. doi: 10.1038/369160a0. [DOI] [PubMed] [Google Scholar]
- Tsai B., Rodighiero C., Lencer W. I., Rapoport T. A. Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell. 2001;104:937–948. doi: 10.1016/s0092-8674(01)00289-6. [DOI] [PubMed] [Google Scholar]
- Tsai B., Gilbert J. M., Stehle T., Lencer W., Benjamin T. L., Rapoport T. A. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 2003;22:4346–4355. doi: 10.1093/emboj/cdg439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. L., O'Shea J. J. JAKs and STATs: biological implications. Annu. Rev. Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- Yu X. C., Wang C. C., Tsou C. L. Association and dissociation of protein disulfide isomerase. Biochim. Biophys. Acta. 1994;1207:109–113. doi: 10.1016/0167-4838(94)90058-2. [DOI] [PubMed] [Google Scholar]