Abstract
During embryonic lymphatic development, a homeobox transcription factor Prox1 plays important roles in sprouting and migration of a subpopulation of blood vessel endothelial cells (BECs) toward VEGF-C–expressing cells. However, effects of Prox1 on endothelial cellular behavior remain to be elucidated. Here, we show that Prox1, via induction of integrin α9 expression, inhibits sheet formation and stimulates motility of endothelial cells. Prox1-expressing BECs preferentially migrated toward VEGF-C via up-regulation of the expression of integrin α9 and VEGF receptor 3 (VEGFR3). In mouse embryos, expression of VEGFR3 and integrin α9 is increased in Prox1-expressing lymphatic endothelial cells (LECs) compared with BECs. Knockdown of Prox1 expression in human LECs led to decrease in the expression of integrin α9 and VEGFR3, resulting in the decreased chemotaxes toward VEGF-C. These findings suggest that Prox1 plays important roles in conferring and maintaining the characteristics of LECs by modulating multiple signaling cascades and that integrin α9 may function as a key regulator of lymphangiogenesis acting downstream of Prox1.
INTRODUCTION
The major roles of the lymphatic vessels are to drain interstitial fluid that leaks out from the blood capillaries and to return it to the blood vessels. In addition, the lymphatic system performs an immune function by transporting immune cells that patrol the tissues to the lymphoid organs (Witte et al., 2001). Insufficiency or obstruction of the lymphatics results in lymphedema, characterized by disabling swelling of the affected tissues. In addition, in many types of cancer, the lymphatic vessels provide a major pathway for tumor metastasis, and regional lymph node metastasis has been shown to be correlated with cancer progression (Karpanen and Alitalo, 2001).
Despite the importance of lymphatic vessels in both normal and pathological conditions, progress in the study of lymphangiogenesis had been hampered by the lack of specific markers. Recent studies have revealed the various transcriptional and signaling components that play important roles in lymphatic development. Embryonic lymphatic endothelial cells (LECs) arise by sprouting from the jugular veins and migrate to form primary lymphatic plexus (Oliver, 2004). In E10 mouse embryos, the prospero-related transcription factor Prox1 is expressed in a subset of ECs of the cardinal vein, from which they sprout to form primary lymph sacs (Wigle and Oliver, 1999; Wigle et al., 2002). In Prox1-null mice, sprouting of LECs from the veins appears unaffected at embryonic day (E)10.5, but their migration is arrested at around E11.5–E12.0, leading to a complete absence of the lymphatic vasculature. Being a homeobox transcription factor, Prox1 has been shown to up-regulate the expression of lymphatic endothelial cell (LEC) markers, and to down-regulate blood vascular endothelial cell (BEC) markers in mature BECs (Hong et al., 2002; Petrova et al., 2002). These findings suggest that Prox1 regulates the program of differentiation of embryonic BECs to LECs by reprogramming the profiles of expression of specific markers of BECs and LECs. However, it is unclear which target genes elicit the functions of Prox1 during the process of lymphangiogenesis. Lymphangiogenesis is absent in the mice lacking some of Prox1 target genes including podoplanin and vascular endothelial growth factor receptor 3 (VEGFR3).
VEGFR3 serves as a receptor for lymphatic-specific VEGFs, VEGF-C and VEGF-D. VEGF-C is important for normal development of the lymphatic vessels, because deletion of Vegfc leads to complete absence of the lymphatic vasculature in mouse embryos (Karkkainen et al., 2004). In Vegfc-null mice, LECs initially differentiate in the cardinal veins but fail to migrate and to form primary lymph sacs, suggesting that VEGF-C is an essential chemotactic and survival factor during embryonic lymphangiogenesis. Vegfr3 deletion leads to defects in blood-vessel remodeling and embryonic death at midgestation, indicating its importance during early blood vascular development (Dumont et al., 1998).
Recently, integrin α9β1 was shown to function as a receptor for VEGF-C and VEGF-D (Vlahakis et al., 2005). Integrin α9-null mice die at 6–12 d of age from bilateral chylothorax, suggesting an underlying defect in lymphatic development (Huang et al., 2000). Furthermore, integrin α9 was shown to be a target gene of the signals mediated by hepatocyte growth factor (HGF), which induces neo-lymphangiogenesis during tissue repair and inflammation (Kajiya et al., 2005). Neo-lymphangiogenesis is also induced by two types of receptor tyrosine kinases, platelet-derived growth factor receptor β (PDGFRβ), which serves as one of receptors for PDGF-BB (Cao et al., 2004) and fibroblast growth factor receptor 3 (FGFR3), which serves as one of receptors for FGF-2 (Shin et al., 2006). Notably, Prox1 has recently been shown to induce FGFR3 expression in BECs (Shin et al., 2006).
Although various signaling cascades have been implicated in embryonic and/or adult lymphangiogenesis, their relationships with Prox1 remain largely unknown. Furthermore, although Prox1 has been shown to activate VEGF-C/VEGFR3 and FGF-2/FGFR3 signals, the direct effects of Prox1 on the behavior of ECs have not yet been elucidated. To address these questions, we expressed Prox1 in two types of ECs, mouse embryonic stem cell (ESC)-derived ECs and human umbilical venous endothelial cells (HUVECs). We found that Prox1 expression regulates the chemotaxis, sheet formation, and migration of ECs by modulating the expression of vascular and lymphatic signaling components and for the first time identified integrin α9 as a target gene of Prox1. Interestingly, our findings revealed that integrin α9 plays a pivotal role in sheet formation by and migration of LECs. These findings were confirmed in developing mouse embryos, suggesting their in vivo significance. Furthermore, knockdown of Prox1 expression in LECs resulted in decrease in the expression of VEGFR3 and integrin α9, leading to the decreased chemotaxes toward VEGF-C. These findings suggest that Prox1 alters the characteristics of BECs and maintains those of LECs by regulating multiple signaling cascades implicated in lymphangiogenesis.
MATERIALS AND METHODS
Cell Culture and Adenovirus Infection
Establishment of Tc-inducible ES cell lines from parental MGZ5TcH2 cells was as described (Masui et al., 2005). Maintenance, differentiation, culture, and cell sorting of MGZ5 ES cells were as described (Yamashita et al., 2000). VEGF-A (30 ng/ml), VEGF-C (300 ng/ml), PDGF-BB (10 ng/ml), and tetracycline (1 μg/ml) were used in each experiment unless otherwise described. HUVECs were obtained from Sanko Junyaku and cultured as described (Ota et al., 2002). Human dermal lymphatic endothelial cells (HDLECs) were obtained from Clonetics (San Diego, CA) and cultured in endothelial basal medium (EBM) containing 5% fetal bovine serum (FBS) and EC growth supplements (Clonetics). Recombinant adenoviruses with wild-type and mutant mouse Prox1 were generated and used as described (Fujii et al., 1999).
RNA Interference and Oligonucleotides
Small interfering RNAs (siRNAs) were introduced into cells as described previously (Koinuma et al., 2003). The target sequence for human Prox1 siRNA was 5′-CACCTTATTCGGGAAGTGCAA-3′. Control siRNAs were obtained from Ambion (Austin, TX).
Immunohistochemistry and Western Blot Analysis
Monoclonal antibodies to platelet-endothelial cell adhesion molecule 1 (PECAM1; Mec13.3) and α-smooth muscle actin (SMA; 1A4) for immunohistochemistry were purchased from BD PharMingen (San Diego, CA) and Sigma (St. Louis, MO), respectively. Staining of cultured cells was performed as described (Yamashita et al., 2000). Stained cells were photographed using a phase-contrast microscope (Model IX70; Olympus, Melville, NY) or a confocal microscope (Model LSM510 META; Carl Zeiss MicroImaging, Thornwood, NY). All images were imported into Adobe Photoshop (San Jose, CA) as JPEGs or TIFFs for contrast manipulation and figure assembly. Antibodies to FLAG and α-tubulin for Western blot analysis and immunohistochemistry were obtained from Sigma. Antibodies to mouse VEGFR3, podoplanin, human VEGFR3, and Prox1 for Western blot analysis and immunohistochemistry were obtained from eBioscience (San Diego, CA), RDI (Flanders, NJ), Santa Cruz Biotechnology (Santa Cruz, CA), and Chemicon (Temecula, CA), respectively. Western blot analysis was performed as described (Kawabata et al., 1998).
Fluorescence-activated Cell Sorting
To sort the LECs and BECs, we performed fluorescence-activated cell sorting (FACS) of mouse embryo cells with an FACS Vantage (Becton Dickinson, Mountain View, CA) as described previously (Morisada et al., 2005). Briefly, E14 mouse embryos were dissociated and subjected to antibody staining for CD45-peridinin chlorophyll protein (PerCP) cyanine 5.5 (Cy5.5) to sort CD45- nonhematopoietic cells for further analysis. Subsequently, the cells were incubated with biotinylated anti-LYVE-1 antibodies (ALY7) followed by allophycocyanin-conjugated streptavidin (PharMingen, San Diego, CA). For double or triple staining, the cells were stained with CD31-phycoerythrin (PE)/FITC, CD34-PE (PharMingen), and TEK4-PE.14.
RNA Isolation and RT-PCR
Total RNA was prepared with ISOGEN reagent (Nippongene, Tokyo, Japan) according to the manufacturer's instructions and reverse-transcribed by random priming and a Superscript first-strand synthesis kit (Invitrogen, Carlsbad, CA). Quantitative RT-PCR analysis was performed using the GeneAmp 5700 Sequence Detection System (Applied Biosystems, Tokyo, Japan). The primer sequences and expected sizes of PCR products are available online as indicated in Supplementary Table 1.
Migration Assay
Chemotaxis was determined using a Cell Culture Insert (8-μm pore size, BD Biosciences, San Jose, CA). A total of 5 × 104 cells were seeded in medium containing 0.5% serum in the upper chamber and migrated toward various growth factors as chemoattractants in the lower chamber for 4 h. When anti-integrin α9β1-neutralizing antibodies (Chemicon) were tested, cells were dissociated by trypsin/EDTA, incubated with neutralizing antibodies (30 μg/ml), and seeded in the upper chamber. Cells in the upper chamber were carefully removed using cotton buds, and cells at the bottom of the membrane were fixed and stained with crystal violet 0.2%/methanol 20%. Quantification was performed by counting the stained cells. Assays were performed in triplicate at least three times.
Video Time-lapse Microscopy
Time-lapse imaging of migrating cells was performed on a Leica DM IRB microscope (Deerfield, IL) equipped with a hardware-controlled motor stage over 24 h in serum-reduced (0.5%) medium at 37°C/5% CO2. Images were obtained with a Leica DC 350F CCD camera every 15 min and analyzed using Image J software (National Institutes of Health, Bethesda, MD). Migration of each cell was analyzed by measuring the distance traveled by a cell nucleus over the 24-h time period (Michl et al., 2005). Average migration speed was calculated by analyzing at least 10 cells per group.
RESULTS
Prox1 Expression in ESC-derived ECs Induces Morphological Changes and Inhibits Sheet Formation
To examine the effects of Prox1 expression on embryonic ECs, we used an in vitro vascular differentiation system from mouse ESCs (Yamashita et al., 2000). This system allows us to induce both endothelial and mural cells derived from common progenitors expressing VEGF receptor 2 (VEGFR2, Flk1). Because we wanted to induce the expression of Prox1 in differentiated ECs instead of undifferentiated ESCs, we established ESC lines carrying a tetracycline (Tc)-regulatable Prox1 transgene (Tc-Prox1) or no transgene (Tc-Empty; Supplementary Figure 1A; Masui et al., 2005). Removal of Tc from culture of undifferentiated Tc-Prox1 cells, but not that of Tc-Empty cells, induced the expression of the FLAG epitope-tagged Prox1 gene (Supplementary Figure 1B).
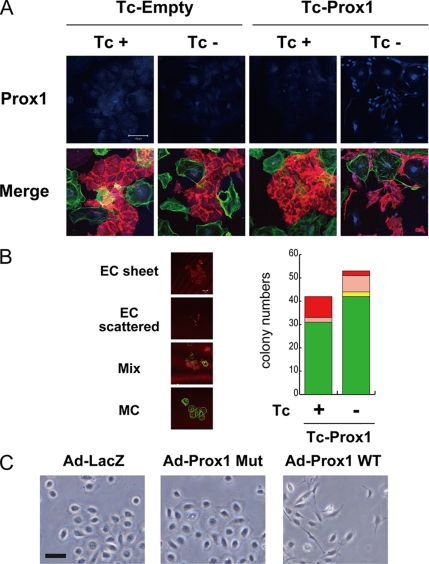
To examine the effects of Prox1 expression on vascular development, we differentiated the Tc-Empty and Tc-Prox1 ES cells into Flk1-expressing (Flk1+) vascular progenitor cells in the presence of Tc, so that no transgene expression is induced. Flk1+ cells were sorted using anti-Flk1 antibodies and were redifferentiated in the presence or absence of Tc. As shown in Figure 1A, Prox1 transgene expression was induced in the vascular cells derived from Tc-Prox1 ES cells only in the absence of Tc. The level of Prox1 transgene expression in ESC-derived vascular cells was approximately twice as high as that of endogenous expression in the LECs derived from E14 mouse embryos (Supplementary Figure S1C). ESC-derived ECs formed a fine cobblestone-like structure of endothelial sheets when Prox1 was not expressed (Figure 1A). However, when Prox1 was expressed, ECs exhibited spindle shapes and failed to form sheet structures.
Figure 1.
Effect of Tc-regulated Prox1 expression on the morphology and sheet formation of ESC-derived ECs and HUVECs. (A) Flk1+ endothelial progenitor cells were sorted from the differentiated ESCs carrying a Tc-regulated transgene encoding FLAG-epitope-tagged mouse Prox1 (Tc-Prox1) or control transgene (Tc-Empty) and redifferentiated in the presence (+) or absence (−) of Tc to obtain PECAM-1–positive ECs (bottom, red) and smooth muscle α-actin (SMA)-positive mural cells (bottom, green). Expression of FLAG-Prox1 (top, blue) and the morpholoby and sheet formation of ECs (bottom, red) were examined. Scale bars, 100 μm. (B) Quantitation of colony formation, EC and mural cell production, and endothelial sheet formation. Flk1+ cells derived from Tc-Prox1 ESCs were cultured sparsely with 10% fetal calf serum in the absence or presence of Tc for 4 d and stained for PECAM1 (red) and SMA (green). Numbers of different types of colonies per well were counted to determine the effects of Prox1 on colony formation of Flk1+ cells. Four colony types were observed: pure ECs forming sheet structures (EC-sheet, red); pure scattered ECs (EC-scattered, pink); pure mural cells (MC, green); and mixed colonies consisting of endothelial and mural cells (Mix, yellow). Experiments were repeated at least three times with essentially the same results. Bars, 50 μm. (C) Morphology of HUVECs infected with adenoviruses encoding LacZ, DNA-binding mutant (Mut), or wild-type (WT) Prox1. Bars, 100 μm.
To further dissect the roles of Prox1 in endothelial sheet formation, we performed quantitative colony formation assays. When Flk1+ cells were plated at a lower density in the presence of VEGF-A, they formed four types of colonies emerging from single Flk1+ cells (Yamashita et al., 2000; Watabe et al., 2003): PECAM1 (CD31)+ pure ECs with or without sheet structure (EC-sheet and EC-scattered, respectively), pure mural cells (MC), and mixtures of both (Mix; Figure 1B). Although the frequencies of pure EC colonies (EC-scattered and -sheet) were ∼25% in the absence and presence of Prox1 expression, formation of endothelial sheets was significantly affected by Prox1 (Figure 1B). The frequency of sheet formation among pure endothelial colonies was 82% when single Flk1+ cells were cultured in the absence of Prox1. When Prox1 was expressed, most endothelial colonies exhibited scattered phenotypes (with a frequency of sheet formation of 22%). Furthermore, 95% of sheet-forming ECs derived from Tc-Prox1 ESCs failed to express Prox1 even in the absence of Tc (unpublished data), further suggesting that Prox1 expression in ESC-derived ECs inhibits sheet formation.
Prox1 Induces Morphological Changes and Inhibits Sheet Formation in HUVECs
We next examined whether Prox1 transgene expression also modulates the morphology and sheet formation of HUVECs, which are mature venous ECs. We used adenoviruses encoding wild-type Prox1 (Ad-Prox1WT), a Prox1 mutant containing two amino acid substitutions in its DNA-binding domain (Ad-Prox1Mut) (Petrova et al., 2002), and LacZ (Ad-LacZ) as controls. Levels of expression of wild-type and mutant Prox1 were shown to be comparable at moi 100 (Supplementary Figure 2A) when > 90% of HUVECs were infected (Supplementary Figure 2B). The level of Prox1 transgene expression was shown to be approximately three times as high as that of endogenous Prox1 expression in HDLECs (Supplementary Figure S2, B–D).
The morphology of and sheet formation by HUVECs were also affected by Prox1 (Figure 1C). Although HUVECs infected with adenoviruses encoding LacZ or mutant Prox1 formed a flat cobblestone-like structure, Prox1-expressing HUVECs were spindle-shaped and did not form sheet structures.
Prox1 Expression Increases Motility of ECs
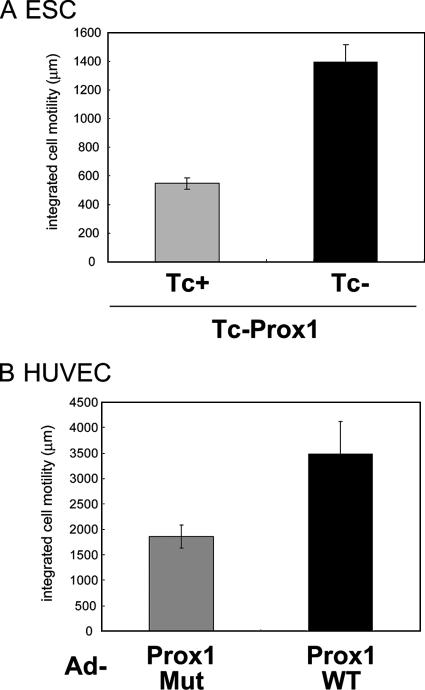
Present findings that Prox1-expressing cells lose a cobblestone-like structure prompted us to examine the effects of Prox1 on the motility of ECs. Tracking single ECs using video time-lapse microscopy showed that Prox1 expression significantly increased the motility of ESC-derived ECs (Supplementary Videos 1 and 2 and Figure 2A) and HUVECs (Supplementary Videos 3 and 4 and Figure 2B). These findings suggest that Prox1 expression results in morphological changes of ECs, inhibition of sheet formation, and induction of EC motility, all of which may be critical phenomena for the progression of embryonic lymphangiogenesis.
Prox1 Increases Endothelial Motility via Induction of Integrin α9 Expression
We next examined the molecular mechanisms by which Prox1 regulates morphological changes of BECs. Petrova et al. (2002) reported that adenovirus-mediated Prox1 expression in human dermal microvascular endothelial cells (HDMECs) resulted in the down-regulation of BEC marker expression and up-regulation of LEC marker expression. We also found that the levels of transcripts for BEC markers (VE-cadherin and VEGFR2) and those for LEC markers (podoplanin and VEGFR3) were down- and up-regulated, respectively, only by Ad-Prox1WT infection in HUVECs, (Supplementary Figure 2), suggesting that Prox1 reprograms the vascular and lymphatic gene expression in HUVECs.
We then examined whether the activation of VEGFR3 signals by Prox1 mediates Prox1-induced morphological changes. However, inhibition of VEGFR3 signals in Prox1-expressing HUVECs by dominant-negative VEGFR3 mutants (Karkkainen et al., 2000) failed to induce reversion of the Prox1-mediated phenotypes (Supplementary Figure 3), suggesting that VEGFR3 signaling is not directly involved in the induction of morphological changes of ECs by Prox1.
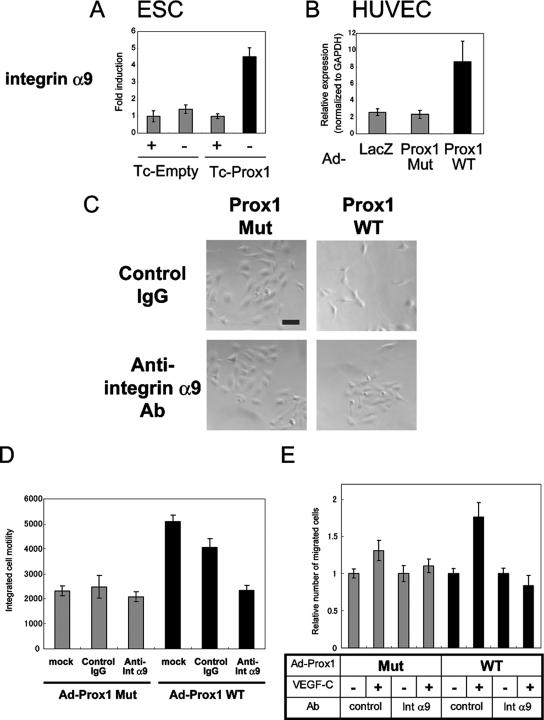
Recently, integrin α9, a member of the integrin family that is preferentially expressed in LECs (Petrova et al., 2002), was shown to function as a receptor for VEGF-C and VEGF-D (Vlahakis et al., 2005). Furthermore, integrin α9-null mice exhibit defects in lymphatic systems (Huang et al., 2000). Therefore, in order to examine the roles of integrin α9 in the Prox1-mediated morphological changes, we, for the first time, studied the roles of Prox1 in the regulation of integrin α9 expression. As shown in Figure 3, A and B, Prox1 expression increased the expression of integrin α9 in both ESC-derived ECs and HUVECs.
Figure 3.
Roles of integrin α9 in the migration of HUVECs. (A and B) Expression of transcripts for integrin α9 was determined by quantitative real-time PCR analysis in ESC-derived ECs (A) and HUVECs (B). (C and D) HUVECs were infected with adenoviruses encoding DNA-binding mutant (Mut) or wild-type (WT) Prox1 and were subjected to videomicroscopy for 24 h in the presence of control IgG or anti-integrin α9 (int α9)-neutralizing antibodies. The final images of HUVECs (C) and integrated cell motility (D) are shown. Bars, 100 μm. Each value represents the mean of 10 determinations; bars, SD (E) Cell migration was measured by Boyden chamber assay. HUVECs were infected with adenoviruses (Ad) encoding DNA-binding mutant (Mut) or wild-type (WT) and plated on Boyden chambers in the presence of control IgG or anti-integrin α9 (int α9)-neutralizing antibodies with VEGF-C placed in lower wells. Results are expressed as the ratio of number of migrated cells normalized to the control (no attractant). Each value represents the mean of triplicate determinations; bars, SD.
To elucidate the roles played by integrin α9 in the induction of phenotypic changes by Prox1, we used anti-human integrin α9β1 neutralizing (function-blocking) antibodies. Reversion of the morphological changes and decreased sheet formation of HUVECs induced by Prox1 was observed with the addition of anti-integrin α9-neutralizing antibodies to culture (Figure 3C). Furthermore, the increase in motility of HUVECs by Prox1 was lowered to basal level by anti-integrin α9 antibodies (Supplementary Videos 5–8 and Figure 3D). These findings suggest that the phenotypic changes of HUVECs induced by Prox1 are due to increased integrin α9 expression.
Prox1 Increases Chemotaxis to VEGF-C via Induction of Integrin α9 Expression
Because it was recently reported that VEGF-C and VEGF-D are ligands for integrin α9β1 (Vlahakis et al., 2005), we examined whether the up-regulation of integrin α9 expression by Prox1 contributed to the Prox1-induced increase in migration of ECs toward VEGF-C. Chamber migration assays showed that HUVECs expressing wild-type Prox1 migrated toward VEGF-C, and this migration was abrogated by anti-α9β1 neutralizing antibody (Figure 3E), suggesting that Prox1 induces the migration of ECs toward VEGF-C by regulating the expression of integrin α9.
Prox1 Expression Inhibits Chemotaxis of BECs to VEGF-A and Promotes that to VEGF-C via Modulation of their Receptors
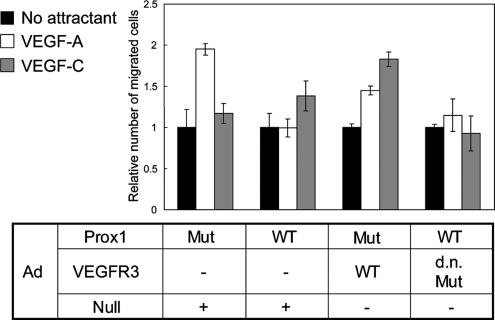
Although our findings suggest that signaling from VEGFR3 is not directly involved in Prox1-induced morphological changes (Supplementary Figure 3), it may play important roles in other changes induced by Prox1 in ECs. BECs expressing VEGFR2 and LECs expressing VEGFR3 migrate preferentially toward their ligands VEGF-A and VEGF-C, respectively (Makinen et al., 2001). The alteration of expression of VEGFRs in HUVECs by Prox1 prompted us to study their chemoattraction toward VEGFs. Chamber migration assays showed that VEGF-A, but not VEGF-C, stimulated chemotaxis of the HUVECs expressing mutant Prox1 (Figure 4). In contrast, VEGF-C, but not VEGF-A, induced motility of those expressing wild-type Prox1. To examine whether Prox1 induces chemotaxis of HUVECs toward VEGF-C via up-regulation of VEGFR3 expression, we used adenoviruses encoding wild-type and dominant-negative forms of VEGFR3. Expression of wild-type VEGFR3 enhanced chemotaxis of HUVECs toward VEGF-C without altering their migration toward VEGF-A. In addition, inhibition of VEGFR3 signals by the dominant-negative VEGFR3 significantly decreased their chemotaxis toward VEGF-C, which was induced by Prox1. These findings suggest that Prox1 modulates endothelial chemotaxis toward VEGFs via its regulation of VEGFRs expression in addition to that of integrin α9.
Figure 4.
Effect of Prox1 on the migration of HUVECs stimulated with VEGF-A and VEGF-C. Cell migration was measured by Boyden chamber assay as described in Materials and Methods. HUVECs were infected with adenoviruses (Ad) encoding DNA-binding mutant (Mut) or wild-type (WT) Prox1 in combination with those encoding wild-type (WT), dominant-negative mutant form (d.n. Mut) of VEGFR3, or Null (encoding no transcripts) and plated on Boyden chambers with indicated chemoattractants placed in lower wells. Ad-Null was used in order to infect HUVECs with the same quantities of adenoviruses for all of samples. Results are expressed as the ratio of number of migrated cells normalized to control (no attractant). Each value represents the mean of triplicate determinations; bars, SD.
Expression of VEGFR3 and Integrin α9 Is Increased in Prox1-expressing LECs from Mouse Embryos
To examine the in vivo significance of our finding that Prox1 induces the expression of VEGFR3 and integrin α9 in ESC-derived ECs, we compared their expression in LECs and BECs derived from E14 mouse embryos. We previously raised monoclonal antibodies against a LEC marker, LYVE-1, and found that sorted CD45-CD31+CD34-lowLYVE-1+ cells derived from E14 mouse embryos represent LECs and that CD45-CD31+CD34+LYVE-1− cells represent BECs (Morisada et al., 2005). We confirmed that expression of LYVE-1 and Prox1 was detected only in sorted LECs (Figure 5). We further examined the expression of Prox1 target genes in LECs and BECs. Expression of VEGFR3 and integrin α9 was detected in the cells in both the LEC and BEC fractions, and their levels of expression in LEC were higher than those in BECs. These findings together suggest that the present in vitro induction by Prox1 of expression of VEGFR3 and integrin α9 may mimic the process of embryonic lymphangiogenesis.
Figure 5.
Expression of BEC and LEC markers in Prox1-expressing LECs derived from mouse embryos. E14 mouse embryos were dissected, and embryonic liver and spleen were removed. Other tissues were dissociated and subject to FACS sorting with anti-CD45, LYVE1 (ALY7), CD31, and CD34 antibodies (see Materials and Methods). CD45−; CD31+; CD34+; LYVE1− BEC fractions and CD45−; CD31+; CD34−; LYVE1+ LEC fractions were analyzed for the expression of transcripts for LYVE1 (A), Prox1 (B), VEGFR3 (C), and integrin α9 (D) by quantitative real-time PCR analysis. Each value represents the mean of triplicate determinations; bars, SD.
Prox1 Knockdown in LECs Modulates Expression of Lymphatic Endothelial Markers and their Cellular Behavior
Prox1 expression is initiated during embryonic lymphangiogenesis and is maintained in mature LECs, prompting us to examine whether knockdown of Prox1 expression in mature LEC affects their characteristics.
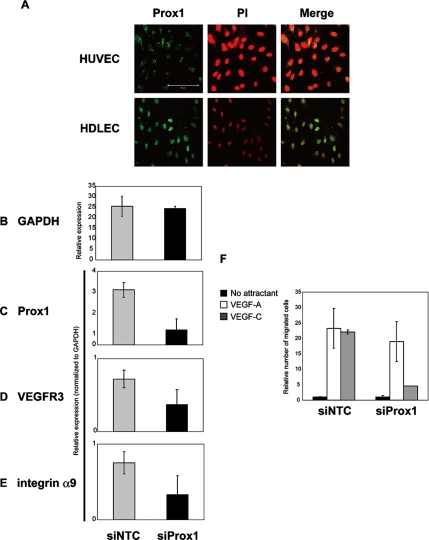
We detected endogenous Prox1 protein in the nuclei of HDLECs, whereas no Prox1 protein was detected in HUVECs (Figure 6A). Expression of transcripts for Prox1, VEGFR3, and integrin α9 was significantly higher in HDLECs than in HUVECs (unpublished data). We next decreased Prox1 levels with siRNAs (Figure 6, B and C). siRNA-mediated decrease of Prox1 led to decrease in the expression of various target genes of Prox1, such as VEGFR3, and integrin α9 (Figure 6, D and E), whereas the expression of most genes including GAPDH was not affected, suggesting that Prox1 maintains the expression of LEC markers in HDLECs.
Figure 6.
Roles of endogenous Prox1 in HDLECs. (A) Expression of endogenous Prox1 (left; green) was examined by specific antibodies in HUVECs (top) and HDLECs (bottom), with counterstaining for nuclei with propidium iodide (PI, middle; red; and right, merge). Bars, 100 μm. (B–E) Effects of Prox1 knockdown on expression of GAPDH (B), Prox1 (C), VEGFR3 (D), and integrin α9 (E) were examined by quantitative real-time PCR analysis. A scrambled siRNA sequence (Ambion) was used as a negative control siRNA (siNTC). Each value represents the mean of triplicate determinations; bars, SD. (F) Effects of Prox1 knockdown on the chemotaxes of HDLECs toward VEGF-A (50 ng/ml) and VEGF-C (50 ng/ml). Cell migration was measured by Boyden chamber assay as described in Materials and Methods. HDLECs were transfected with scrambled siRNAs (siNTC) or Prox1 siRNAs and plated on Boyden chambers with indicated chemoattractants placed in the lower wells. Results are expressed as the ratio of number of migrated cells normalized to control (no attractant). Each value represents the mean of triplicate determinations; bars, SD.
Mature LECs have been reported to migrate toward VEGF-C (Makinen et al., 2001). To study the effects of Prox1 knockdown on the behavior of HDLECs, we examined their chemotaxis toward VEGFs (Figure 6F). Although HDLECs migrate toward VEGF-A and VEGF-C, Prox1 knockdown caused a specific decrease in the chemotaxis toward VEGF-C (Figure 6F). These results suggest that Prox1 maintains the characteristics of LECs by sustaining the expression of LEC markers.
DISCUSSION
Recent studies have revealed that lymphangiogenesis is regulated by various signaling cascades mediated by VEGFs/VEGFRs (Dumont et al., 1998; Karkkainen et al., 2000; Suzuki et al., 2005), and integrin α9β1 (Huang et al., 2000). The present study showed that expression of Prox1 in ECs regulates the expression of various signaling components, including integrin α9, VEGFR2, and VEGFR3, leading to alteration of chemotaxis, sheet formation, and migration of ECs. We also observed that Prox1 modulates the signaling pathways mediated by angiopoietins/Tie2 (Morisada et al., 2005) and FGF/FGFR3 (Shin et al., 2006) in both ESC-derived ECs and HUVECs (unpublished data). In addition, because integrin α9 has been implicated in modulation of signaling cascades mediated by HGF (Kajiya et al., 2005), Prox1 may indirectly modulate HGF signaling. These findings, together with those of previous studies, suggest that Prox1 is a master transcription factor that induces the differentiation of ECs into LECs via regulation of multiple signaling cascades that play important roles in lymphangiogenesis.
Interestingly, we have found that Prox1 induces growth of Flk1+ ECs derived from ESCs, but inhibits the growth of undifferentiated ESCs (unpublished data). These findings suggest that Prox1 requires cell-type–specific modulators for its transcriptional activities and further imply the importance of choosing appropriate endothelial cell types in the identification of Prox1 target genes during embryonic lymphatic differentiation. Although previous studies used mature human dermal ECs to identify target genes of Prox1 (Hong et al., 2002; Petrova et al., 2002), it will be of great interest to use ESC-derived ECs for this purpose.
Alteration of endothelial signaling cascades by Prox1 resulted in decrease in sheet formation, increased motility, and down- and up-regulation of chemotaxis toward VEGF-A and VEGF-C, respectively. An important question is whether these changes mimic the differentiation from BECs to LECs. Blood vascular endothelium and lymphatic endothelium differ in certain specific morphological characteristics. For example, the lymphatic capillaries are larger than the blood capillaries and have an irregular or collapsed lumen, a discontinuous basal lamina, overlapping intercellular junctional complexes, and anchoring filaments that connect the LECs to the extracellular matrix (Witte et al., 2001). The results of morphological observation of in vitro cultured BECs and LECs are controversial. Makinen et al. (2001) reported that LECs sorted from human dermal microvascular cells using anti-VEGFR3 antibodies exhibit elongated cell shapes in the presence of VEGF-C compared with the BECs sorted from the same source. However, Kriehuber et al. (2001) reported that LECs sorted from dermal cell suspensions using anti-podoplanin antibodies were not morphologically distinguishable from the BECs sorted from the same source. These differences in findings may be explained by the differences in methods used to isolate and culture LECs and BECs.
During embryonic lymphangiogenesis, Prox1-expressing ECs sprout from cardinal veins and migrate toward VEGF-C–expressing mesenchymal cells (Oliver, 2004). These observations suggest that Prox1-expressing cells need to be mobile to form primary lymphatic sacs. In addition, Prox1 induces proliferation of ESC-derived ECs and HUVECs (unpublished data). However, such cells become stabilized in the VEGF-C–expressing region and form lymphatic capillaries. Furthermore, present work showed that the morphological changes of ECs induced by Prox1 is not dependent on VEGFR3 (Supplementary Figure S3), but on integrin α9 (Figure 3) and that the enhanced chemotaxis toward VEGF-C requires VEGFR3 and integrin α9 (Figures 3 and 4). These findings suggest the hypothesis that Prox1 activates ECs by inducing integrin α9 expression, which results in chemotaxis toward VEGF-C in collaboration with VEGFR3, expression of which is also induced by Prox1, and stabilizes and induces maturation of them via signals mediated mainly by VEGF-C/VEGFR3. This hypothesis is supported by the observation that survival of mature LECs was inhibited by Prox1 knockdown (unpublished data). Prox1 thus appears to function as a master transcription factor for lymphangiogenesis, playing key roles in most of the critical steps during the development of lymphatic sacs, including mobilization, migration, and proliferation of LECs, as well as stabilization of lymphatic capillaries, through modulation of multiple signaling cascades (Figure 7).
Figure 7.
Schematic representation of the roles of Prox1 during the differentiation of blood vessel ECs (BECs) to lymphatic endothelial progenitor cells (LEPCs). See text for details.
The lymphatic vasculature plays important roles in the pathogenesis of various conditions and diseases such as lymphedema and cancer metastasis. The findings of the present study suggest the possibility that expression of Prox1 in BECs and/or endothelial progenitor cells derived from patients may be useful as a therapeutic strategy in regenerative medical treatment of lymphedema. Alternatively, knockdown of Prox1 in cancer patients may inhibit tumor lymphangiogenesis and prevent metastasis.
Supplementary Material
Figure 2.
Effects of Prox1 on the migration of ESC-derived ECs and HUVECs. Cell migration was measured by video time-lapse microscopy as described in Materials and Methods. (A) ECs derived from Tc-Prox1 ESCs were subjected to video microscopy for 24 h (Supplementary Videos 1 and 2). (B) HUVECs were infected with adenoviruses (Ad) encoding DNA-binding mutant (Mut) or wild-type (WT) Prox1 and subjected to videomicroscopy for 24 h (Supplementary Videos 3 and 4). Results are expressed as the integrated cell motility over 24 h. Each value represents the mean of 10 determinations; bars, SD.
ACKNOWLEDGMENTS
We thank Drs. J. Yamashita, H. Miki, S. Nishikawa, and members of Department of Molecular Pathology of the University of Tokyo for discussion. This research was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
Abbreviations used:
- EC
endothelial cell
- ESC
embryonic stem cell
- PECAM1
platelet-endothelial cell adhesion molecule 1
- SMA
α- smooth muscle actin
- VEGF
vascular endothelial growth factor
- BEC
blood vascular endothelial cell
- LEC
lymphatic endothelial cell.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0780) on February 7, 2007.

 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Cao R., et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Dumont D. J., Jussila L., Taipale J., Lymboussaki A., Mustonen T., Pajusola K., Breitman M., Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- Fujii M., Takeda K., Imamura T., Aoki H., Sampath T. K., Enomoto S., Kawabata M., Kato M., Ichijo H., Miyazono K. Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Mol. Biol. Cell. 1999;10:3801–3813. doi: 10.1091/mbc.10.11.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y. K., Harvey N., Noh Y. H., Schacht V., Hirakawa S., Detmar M., Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- Huang X. Z., Wu J. F., Ferrando R., Lee J. H., Wang Y. L., Farese R. V., Jr., Sheppard D. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol. Cell. Biol. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiya K., Hirakawa S., Ma B., Drinnenberg I., Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen M. J., Ferrell R. E., Lawrence E. C., Kimak M. A., Levinson K. L., McTigue M. A., Alitalo K., Finegold D. N. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat. Genet. 2000;25:153–159. doi: 10.1038/75997. [DOI] [PubMed] [Google Scholar]
- Karkkainen M. J., et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Karpanen T., Alitalo K. Lymphatic vessels as targets of tumor therapy? J. Exp. Med. 2001;194:F37–F42. doi: 10.1084/jem.194.6.f37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata M., Inoue H., Hanyu A., Imamura T., Miyazono K. Smad proteins exist as monomers in vivo and undergo homo- and hetero-oligomerization upon activation by serine/threonine kinase receptors. EMBO J. 1998;17:4056–4065. doi: 10.1093/emboj/17.14.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriehuber E., Breiteneder-Geleff S., Groeger M., Soleiman A., Schoppmann S. F., Stingl G., Kerjaschki D., Maurer D. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J. Exp. Med. 2001;194:797–808. doi: 10.1084/jem.194.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koinuma D., et al. Arkadia amplifies TGF-β superfamily signalling through degradation of Smad7. EMBO J. 2003;22:6458–6470. doi: 10.1093/emboj/cdg632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen T., et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui S., Shimosato D., Toyooka Y., Yagi R., Takahashi K., Niwa H. An efficient system to establish multiple embryonic stem cell lines carrying an inducible expression unit. Nucleic Acids Res. 2005;33:e43. doi: 10.1093/nar/gni043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl P., et al. CUTL1 is a target of TGFβ signaling that enhances cancer cell motility and invasiveness. Cancer Cell. 2005;7:521–532. doi: 10.1016/j.ccr.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Morisada T., et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- Oliver G. Lymphatic vasculature development. Nat. Rev. Immunol. 2004;4:35–45. doi: 10.1038/nri1258. [DOI] [PubMed] [Google Scholar]
- Ota T., Fujii M., Sugizaki T., Ishii M., Miyazawa K., Aburatani H., Miyazono K. Targets of transcriptional regulation by two distinct type I receptors for transforming growth factor-β in human umbilical vein endothelial cells. J. Cell. Physiol. 2002;193:299–318. doi: 10.1002/jcp.10170. [DOI] [PubMed] [Google Scholar]
- Petrova T. V., Makinen T., Makela T. P., Saarela J., Virtanen I., Ferrell R. E., Finegold D. N., Kerjaschki D., Yla-Herttuala S., Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J. W., Min M., Larrieu-Lahargue F., Canron X., Kunstfeld R., Nguyen L., Henderson J. E., Bikfalvi A., Detmar M., Hong Y. K. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: a role for FGF signaling in lymphangiogenesis. Mol. Biol. Cell. 2006;17:576–584. doi: 10.1091/mbc.E05-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Watabe T., Kato M., Miyazawa K., Miyazono K. Roles of vascular endothelial growth factor receptor 3 signaling in differentiation of mouse embryonic stem cell-derived vascular progenitor cells into endothelial cells. Blood. 2005;105:2372–2379. doi: 10.1182/blood-2004-07-2547. [DOI] [PubMed] [Google Scholar]
- Vlahakis N. E., Young B. A., Atakilit A., Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin α9β1. J. Biol. Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe T., Nishihara A., Mishima K., Yamashita J., Shimizu K., Miyazawa K., Nishikawa S., Miyazono K. TGF-β receptor kinase inhibitor enhances growth and integrity of embryonic stem cell-derived endothelial cells. J. Cell Biol. 2003;163:1303–1311. doi: 10.1083/jcb.200305147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle J. T., Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Wigle J. T., Harvey N., Detmar M., Lagutina I., Grosveld G., Gunn M. D., Jackson D. G., Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte M. H., Bernas M. J., Martin C. P., Witte C. L. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc. Res. Tech. 2001;55:122–145. doi: 10.1002/jemt.1163. [DOI] [PubMed] [Google Scholar]
- Yamashita J., Itoh H., Hirashima M., Ogawa M., Nishikawa S., Yurugi T., Naito M., Nakao K., Nishikawa S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.