Abstract
Cholangiocytes are cellular components of the bile duct system of the liver, which originate from hepatoblasts during embryonic liver development. Although several transcription factors and signaling molecules have been implicated in bile duct development, its molecular mechanism has not been studied in detail. Here, we applied a three-dimensional (3D) culture technique to a liver progenitor cell line, HPPL, to establish an in vitro culture system in which HPPL acquire differentiated cholangiocyte characteristics. When HPPL were grown in a gel containing Matrigel, which contains extracellular matrix components of basement membrane, HPPL developed apicobasal polarity and formed cysts, which had luminal space inside. In the cysts, F-actin bundles and atypical protein kinase C were at the apical membrane, E-cadherin was localized at the lateral membrane, and β-catenin and integrin α6 were located at the basolateral membrane. HPPL in cysts expressed cholangiocyte markers, including cytokeratin 19, integrin β4, and aquaporin-1, but not a hepatocyte marker, albumin. Furthermore, HPPL transported rhodamine 123, a substrate for multidrug resistance gene products, from the basal side to the central lumen. These data indicate that HPPL develop cholangiocyte-type epithelial polarity in 3D culture. Phosphatidylinositol 3-kinase signaling was essential for proliferation and survival of HPPL in culture, whereas laminin-1 was a crucial component of Matrigel for inducing epithelial polarization of HPPL. Because HPPL cysts display structural and functional similarities with bile ducts, the 3D culture of HPPL recapitulates in vivo cholangiocyte differentiation and is useful to study the molecular mechanism of bile duct development in vitro.
INTRODUCTION
The liver consists of two types of endodermal epithelial cells, hepatocytes and cholangiocytes, which differentiate from hepatoblasts. Hepatocytes are liver parenchymal cells providing numerous metabolic functions, such as glycogenesis, gluconeogenesis, urea synthesis, and lipid synthesis. Cholangiocytes form bile ducts, which connect between the liver and the intestine to secrete bile, which is generated in hepatocytes, into the intestine. Cholangiocytes control the rate of bile flow and the pH of the bile by secreting water and bicarbonate ion, respectively, into luminal space (Fitz, 2002). To achieve these functions, both hepatocytes and cholangiocytes establish apicobasal epithelial polarity during liver organogenesis. Interestingly, these cells have different types of polarity; the basal surface of hepatocytes faces the space of Disse and the apical surface forms the bile canalicular space between neighboring cells. In contrast, the basal surface of cholangiocytes is associated with basement membrane, and the apical surface forms the luminal space surrounded by the monolayer of cholangiocytes.
Bile duct development can be divided into two steps, i.e., cell fate decision and morphogenesis. Cell fate decision occurs in midgestation, as cholangiocytes split from hepatoblasts around the portal veins. The process of morphogenesis has been deduced from histochemical analysis of developing liver (Tan et al., 1995; Lemaigre, 2003). Cholangiocytes form a single cell layer called the ductal plate, which turns into a double cell layer. Luminal spaces are generated between the cell layers in late gestation, and then the ductal plate is reorganized into bile duct tubules. Several mutant mice have abnormalities of bile duct development, including mice lacking hepatocyte nuclear factor (HNF) 1β, HNF6, or HES1, and mice harboring a mutation in the Notch signaling pathway (Clotman et al., 2002, 2005; Coffinier et al., 2002; Kodama et al., 2004; McCright et al., 2002). A human genetic disease also provides information about bile duct development; Alagille syndrome, which is caused by mutation of Jagged1, shows multiple phenotypes, including bile duct malformation (Li et al., 1997). We previously used primary culture of hepatoblasts and overexpressed the intracellular domain of Notch, a constitutively active form, in hepatoblasts, which resulted in blocking hepatic differentiation and altered the expression of liver enriched transcription factors toward cholangiocyte differentiation (Tanimizu and Miyajima, 2004). These studies have increased the number of molecules that are implicated in bile duct development. However, it has still been difficult to tell whether a molecule is involved in cell fate decision and/or in morphogenesis of bile ducts and how it regulates bile duct formation. Because primary culture of fetal liver cells has revealed the molecular mechanisms of hepatocyte differentiation (Kamiya et al., 1999; Ito et al., 2000; Matsui et al., 2002), a good culture system in which liver progenitors become differentiated cholangiocytes may greatly improve our understanding of bile duct development.
In previous work, we established a cell line named HPPL, which is derived from mouse hepatoblasts (Tanimizu et al., 2004). HPPL showed high proliferative capability and bidirectional differentiation potential as hepatoblasts. We applied a three-dimensional (3D) culture technique for HPPL, in which HPPL were embedded in a gel of extracellular matrix (ECM). HPPL showed tubule-like structures and expressed cytokeratin 19 (CK19), a conventional marker of cholangiocytes, in type I collagen. However, it turned out that these structures had no luminal space, indicating that they did not develop apicobasal polarity. Histochemical data that cholangiocytes are associated with basement membrane suggested that interaction between cholangiocytes and ECM components of the basement membrane is important for bile duct morphogenesis. Based on these data, we now added Matrigel, which has a similar composition of ECM proteins as basement membrane, to type I collagen gel to mimic the in vivo environment around developing bile ducts, and we examined the morphology of HPPL.
MATERIALS AND METHODS
Extracellular Matrix, Growth Factors, and Chemicals
Type I collagen was purchased from Cohesion Technologies (Palo Alto, CA). Growth factor reduced Matrigel, purified laminin-1, high concentration laminin-1/entactin complex, and type IV collagen were purchased from BD Biosciences (Bedford, MA). Mouse oncostatin M was purchased from R&D Systems (Minneapolis, MN). U0126, an inhibitor for mitogen-activated protein kinase kinase (MEK); SB431542, an inhibitor for transforming growth factor (TGF)β1/activin-like receptor kinase; LY294002, an inhibitor for phosphatidylinositol 3-kinase (PI3K); and BB94, an inhibitor for matrix metalloproteinases (MMPs) with a broad spectrum, were purchased, respectively, from Promega (Madison WI), Calbiochem (La Jolla, CA), Tocris Cookson (Ellisville, MO), and British Biotech (Oxford, United Kingdom).
Cell Culture
HPPL were kept in DMEM/F-12 (Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 1× insulin/transferrin/selenium (Invitrogen), 10 mM nicotinamide (Wako Pure Chemicals, Osaka, Japan), 0.1 μM dexamethasone (Sigma-Aldrich), 5 mM l-glutamine, and 5 ng/ml hepatocyte growth factor (HGF) and epidermal growth factor (EGF). HPPL were suspended in type I collagen gel or mixture of type I collagen and Matrigel at a density of 4 × 104 cells/ml. One hundred fifty microliters of the cell suspension was added to each 1-cm-diameter culture insert (Millipore, Billerica, MA). After incubation at 37°C for 2 h to solid the gel, 500 μl of DMEM/F-12 with or without growth factors was added on top and under the insert. All the inhibitors were dissolved in dimethyl sulfoxide and added to medium from the beginning of the 3D culture to give a final concentration of 10 μM.
Immunofluorescence Chemistry
Mouse embryonic and neonatal livers were fixed in phosphate-buffered saline containing 4% paraformaldehyde (PFA) at 4°C for ∼4 h. They were embedded in OCT compound and frozen and ultimately used for preparation of thin sections with a cryostat (Leica, St. Gallen, Switzerland). Samples of the 3D culture were treated with collagenase and fixed in PFA solution as reported previously (O'Brien et al., 2006). Frozen sections and culture samples were incubated with primary antibodies listed in Table 1. Signals were visualized with AlexaFluor-conjugated secondary antibodies (Molecular Probes, Eugene, OR) used at a dilution of 1:500. F-actin bundles were detected with AlexaFluor 546- or 633-conjugated phalloidin (Molecular Probes) at a dilution of 1:250. Nuclei were counterstained with Hoechst 34580. Samples were examined on a Zeiss Pascal or 510 confocal laser scanning fluorescence microscope.
Table 1.
Primary antibodies used in immunofluorescence chemistry
| Antigen | Host | Company or source | Working dilution | |
|---|---|---|---|---|
| Albumin | Mouse | Goat | Bethyl Laboratories (Montgomery, TX) | 1:500 |
| Aquaporin-1 | Rat | Rabbit | Chemicon International (Temecula, CA) | 1:500 |
| β-Catenin | Mouse | Mouse | BD Transduction Laboratories (Lexington, KY) | 1:2000 |
| Cleaved caspase3 | Human | Rabbit | Cell Signaling Technology (Beverly, MA) | 1:250 |
| Cytokeratin 19 | Mouse | Rabbit | Tanimizu et al., 2003 | 1:2000 |
| E-cadherin | Human | Mouse | BD Transduction Laboratories | 1:2000 |
| Entactin | Mouse | Rat | Chemicon International | 1:500 |
| Integrin α6 | Human | Rat | BD Biosciences PharMingen (San Diego, CA) | 1:500 |
| Integrin β4 | Mouse | Rat | BD Biosciences PharMingen | 1:500 |
| Ki67 | Mouse | Rat | Dako North America (Carpinteria, CA) | 1:100 |
| Protein kinase Cζ | Human | Rabbit | Santa Cruz Biotechnology (Santa Cruz, CA) | 1:200 |
| ZO-1 | Human | Rat | A gift from Dr. Bruce Stevenson (University of Alberta) | 1:2000 |
Assay for Transport of Fluorescence Dye
HPPL were cultured in a coverglass chamber (Nalge Nunc, Naperville, IL) for 6 d, and then they were incubated in phenol red- and serum-free DMEM/F-12 (Invitrogen) for overnight. Cells were incubated with fresh serum-free medium containing 100 μM rhodamine 123 (Sigma-Aldrich) for 5 min and washed with serum-free medium for three times. To show that transport of rhodamine 123 depends on activity of multidrug resistance gene products (mdr), HPPL were incubated with 10 μM R-(+)-verapamil (Sigma-Aldrich), an mdr inhibitor, for 30 min before adding rhodamine 123. The chamber was placed on a stage of the 510 confocal microscope, and images were taken every 2 min for 30 min. Temperature and CO2 concentration were kept at 37°C and 5%, respectively.
RESULTS
Bile Duct Morphogenesis in Mouse Liver
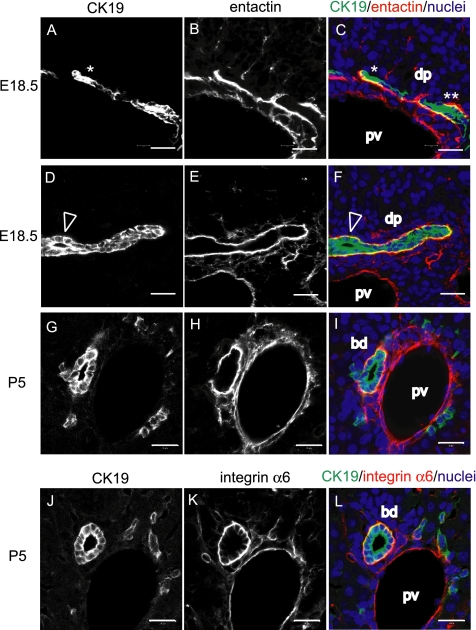
Hepatoblasts differentiate to cholangiocytes and form ductal plates around the portal veins in midgestation, whereas they proceed to tubular morphogenesis in late gestation and in neonatal days. In Figure 1, we analyzed bile duct morphogenesis in embryonic day 18.5 (E18.5) and postnatal day 5 (P5) livers by immunofluorescence staining of frozen sections. The morphogenesis is very dynamic in late gestation, because staining with anti-CK19 antibody (Figure 1, A and D, white, and C and F, green) demonstrated that ductal plates are either a single layer of CK19+ cholangiocytes (Figure 1, A and C, *), a double layer (Figure 1, A and C, **), or one with a tiny luminal space (Figure 1, D and F, arrowheads) in E18.5 liver. The transition from the ductal plates to bile duct tubules is mostly completed by P5, because staining with anti-CK19 antibody (Figure 1, G and J, white, and I and L, green) demonstrated that most of cholangiocytes form tubule structures at P5. As reported previously, staining with anti-entactin antibody showed that both ductal plates and bile ducts are laminated with the basement membrane containing entactin (Figure 1, B, E, and H, white, and C, F, and I, red) (Kikkawa et al., 2005). Integrin α6 was observed on the basolateral surface of cholangiocytes and in endothelial cells of the portal vein (Figure 1K, white, and L, red). The close association between cholangiocytes and basement membrane led to a hypothesis that interactions between cholangiocytes and ECM proteins are important for bile duct morphogenesis.
Figure 1.
Cholangiocytes form ductal plates and bile ducts that are associated with basement membrane. (A–F) At E18.5, CK19+ cholangiocytes form ductal plates (dp) around the portal vein (pv) (A and D). Entactin, a component of basement membrane, laminates the ductal plate (B and E). Images of CK19 and entactin staining are merged in C and F, in which CK19 and entactin expression are shown in green and red, respectively. The ductal plate in A and C has one (*) or two cell layers (**), whereas the ductal plate in D and F has a tiny lumen (arrowhead). (G–L) At P5, CK19+ cholangiocytes form bile duct tubules (bd) (G and J). Entactin are evident around bile ducts and also around the portal vein (H). Cholangiocytes express integrin α6 (K) in the basolateral domains. Integrin α6 is also expressed in endothelial cells of the portal vein. G and H are merged in I, in which CK19 and entactin expression are shown in green and red, respectively. J and K are merged in L, in which CK19 and integrin α6 expression are shown in green and red, respectively. dp, ductal plate; bd, bile duct; and pv, portal vein. Bars, 20 μm.
Three-Dimensional Culture of HPPL
The 3D culture systems, in which cells are grown in a gel consisting of ECM proteins, have been used to investigate the molecular mechanisms underlying polarization and tubulogenesis of epithelial cells (Zegers et al., 2003; Mostov et al., 2005). We applied a 3D culture methodology to a liver progenitor cell line, HPPL, because ECM proteins surround the cells in the culture, similar to the way ECM surrounds cholangiocytes in developing liver. It has been established that Madin-Darby canine kidney (MDCK) cells develop apicobasal polarity in type I collagen gel and form cysts that have a central lumen inside. The apical domains of polarized MDCK cells surround the central lumen (O'Brien et al., 2001; Yu et al., 2003, 2005). Thus, we searched for conditions in which HPPL form cysts that have luminal space inside to show that they acquire apicobasal polarity as differentiated cholangiocytes. For this purpose, staining with phalloidin, which binds F-actin bundles, was used to identify the formation of the apical lumen.
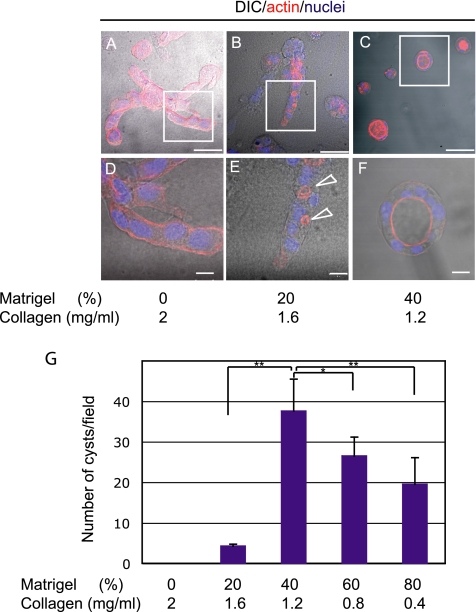
First, we used type I collagen alone as a component of the ECM gel. HPPL grew in the gel and formed extended structures (Figure 2A). F-actin was localized all around the cell cortex (Figure 2D). Next, Matrigel, which contains ECM proteins of basement membrane, was mixed with type I collagen gel to better mimic the environment around developing bile ducts. When Matrigel was added to type I collagen up to 20% of the total volume of gel, HPPL formed extended structures and cell aggregates (Figure 2B). Importantly, F-actin localization dramatically changed compared with that in pure type I collagen gel, i.e., F-actin relocated from cell–ECM contacts and now surrounded small lumens at cell–cell boundaries (Figure 2E, arrows). When Matrigel was increased to 40% of the total volume, HPPL formed round shape cysts instead of extended structures (Figure 2C), which had the central luminal space, surrounded by a monolayer of cells (Figure 2F). Further increase of Matrigel concentration did not affect the efficiency of cyst formation (Figure 2G). That HPPL developed cysts with the central lumen in 40% Matrigel suggested that they might have acquired apicobasal polarity.
Figure 2.
Matrigel induces the formation of luminal space. (A–F) HPPL were cultured in gel containing different concentration of Matrigel for 7 d with EGF and HGF. F-actin and nuclei were visualized with AlexaFluor 546-conjugated phalloidin (red) and Hoechst 34580 (blue), respectively. Merged images of differential interference contrast microscopy (DIC) and fluorescence staining are shown. In type I collagen gel, HPPL formed extended structures (A), in which F-actin localizes along the cell cortex (D). In 20% Matrigel, HPPL formed extended structures (B) and generated tiny luminal space at cell–cell contacts (E, arrowheads). In 40% Matrigel, HPPL formed round cysts (C) with the central lumen (F). Boxes in A, B, and C are magnified, respectively, in D, E, and F. Bars in A–C and those in D–F represent 50 and 20 μm, respectively. (G) The number of cysts observed in each condition is shown. The culture was done three times independently, and five areas in each sample were randomly selected for counting cysts. Values on y-axis represent the sum of the number of cysts in five fields. Error bars refer standard deviations. Student's t test was performed using Microsoft Excel (Microsoft, Redmond, WA). Asterisks (*) and double asterisks (**) represent p < 0.05 and p < 0.01, respectively.
HPPL Develop Epithelial Polarity and Acquire Cholangiocyte Characteristics
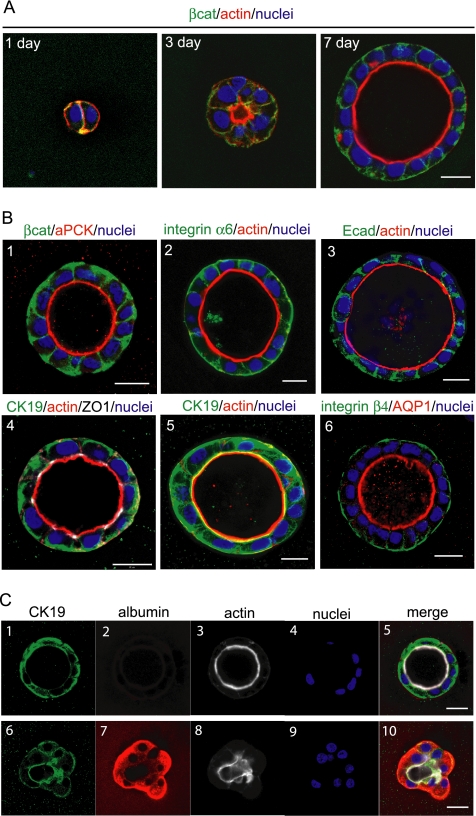
We further analyzed structures of HPPL in 40% Matrigel. First, we used F-actin and β-catenin as markers for the apical and the basolateral domains, respectively, to follow the time course of HPPL morphogenesis in the culture (Figure 3A). At day 1 of culture, HPPL seemed not to have developed apicobasal polarity yet, because F-actin (red) was uniformly distributed along the cell cortex. Lumens were not detected at this point. At day 3 of the culture, tiny luminal spaces surrounded by F-actin bundles (red) became evident. Actin was concentrated around the lumen. By day 7 of culture, HPPL formed round cysts with luminal spaces inside, and they showed clear basolateral localization of β-catenin (green), indicating that HPPL had acquired apicobasal polarity.
Figure 3.
HPPL localize markers for epithelial polarization and cholangiocyte markers in cysts. (A) Localization of β-catenin (green) and F-actin (red) was examined at day 1, 3, or 7 of the culture. The luminal space surrounded by F-actin bundles was formed at day 3 of the culture and expanded by day 7. Bar, 20 μm. (B) HPPL cysts at day 7 of the culture were examined for localization of epithelial polarity and cholangiocyte markers. (1–4) Localization of epithelial polarity markers is shown. aPKC (red) and β-catenin (green) are localized in the apical and the basolateral membrane, respectively (1). F-actin bundles (red) and integrin α6 (green) are localized in the apical and the basolateral membrane, respectively (2). F-actin bundles (red) and E-cadherin (green) are localized at the apical and the lateral membrane, respectively (3). ZO-1 (white) is localized at the apical tip of the lateral membrane (4), in which expression of CK19 and F-actin are shown in green and red, respectively. (5 and 6) Localization of cholangiocyte markers is shown. CK19 (green) is both in cytosol and along the cell cortex (5) in which F-actin bundles are shown in red. AQP1 and integrin β4 are localized in the apical and the basolateral membrane, respectively (6). Bars, 20 μm. (C) Expression of CK19 and albumin were examined in the 3D culture. HPPL positive for CK19 (1) but negative for albumin (2) formed cysts with the apical lumen surrounded by F-actin bundles (3). In contrast, HPPL weakly positive for CK19 (6) and strongly positive for albumin (7) did not have clear lumen (8). Nuclei were counterstained with Hoechst 34580 (4 and 9). Images 1–4 and 6–9 are merged in 5 and 10, respectively. Bars, 20 μm.
Next, we checked the localization of apical, lateral, and basal proteins in the cysts (Figure 3B). To detect atypical protein kinase C (aPKC), we used a rabbit polyclonal anti-PKCζ antibody, which actually recognizes both λ and ζ isoforms. In addition to F-actin (Figure 3B2–5, red), aPKC was localized at the apical membrane (Figure 3B1, red). Besides β-catenin (Figure 3B1, green), integrin α6 (Figure 3B2, green) was observed at the basolateral membrane. E-cadherin was localized at cell–cell contacts (Figure 3B3, green). Zonula occludens 1 (ZO-1) was detected at the apical tip of the lateral membrane (Figure 3B4, white), where tight junctions are formed. These data further demonstrated that HPPL developed apicobasal epithelial polarity in gels containing 40% Matrigel.
We also examined expression of the lineage markers for liver epithelial cells. CK19, a conventional marker of cholangiocytes, was detected both along plasma membranes and in the cytosol (Figure 3B4 and 5, green), whereas integrin β4 and aquaporin 1 (AQP1) were localized at the basolateral and the apical membrane, respectively (Figure 3B6, green and red, respectively). These data indicated that HPPL localized cholangiocyte markers properly. Acquisition of cholangiocyte characteristics could be further demonstrated by down-regulation of albumin, because it is expressed in liver progenitors and hepatocytes but not in mature cholangiocytes. We found that HPPL in cysts, which were positive for CK19 (Figure 3C1) and formed lumens (Figure 3C3), did not express albumin (Figure 3C2). In contrast, HPPL, which failed to form a clear luminal space (Figure 3C8) and were weakly positive for CK19 (Figure 3C6), strongly expressed albumin (Figure 3C7). These data support that HPPL in cysts differentiate along the cholangiocyte lineage.
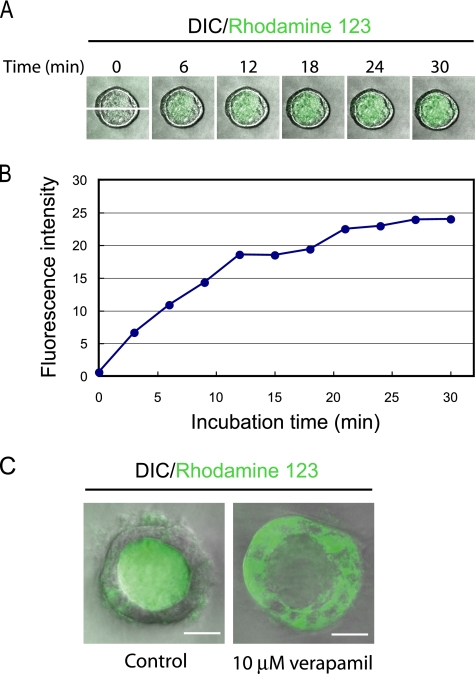
A physiological function of cholangiocytes is secretion of small substances, including water and bicarbonate ions, to modulate bile composition. The secretion function is attributed to transmembrane channel proteins such as cystic fibrosis transmembrane regulator chloride channels and mdr P-glycoprotein 170 gene products (Fitz, 2002). A recent article demonstrated that mdr1, which has been well characterized at the apical membrane of hepatocytes, is also functionally expressed in cholangiocytes (Gigliozzi et al., 2000), showing functionality of mdr1 by accumulation of rhodamine 123, an mdr substrate, in the apical luminal space of bile duct units isolated from rat. To determine whether HPPL express functional mdr, HPPL cysts were incubated in the presence of rhodamine 123, and time-lapse images were taken every 2 min by checking fluorescence signal in the central lumen. We found that the fluorescence intensity gradually increased inside the luminal space, indicating that HPPL cysts transported rhodamine 123 from the basal side to the apical luminal space (Figure 4A). The transport of rhodamine 123 almost reached a plateau after 20 min of incubation (Figure 4B). Furthermore, rhodamine 123 was trapped inside cells but not transported into the central lumen in the presence of 10 μM verapamil, an mdr inhibitor, at 40 min of incubation (Figure 4C, right), indicating that the transport of rhodamine 123 depends on functional mdr in the apical domain. Thus, HPPL in cysts acquired not only structural but also functional characteristics of differentiated cholangiocytes.
Figure 4.
HPPL cysts transport rhodamine 123 from the basal side to the apical. (A) HPPL cysts accumulated rhodamine 123 in the central lumen. HPPL cysts formed in 40% Matrigel were incubated with rhodamine 123, an mdr substrate, for 5 min. After washing the culture, time-lapse images were taken by a confocal microscope. (B) The time course of transport of rhodamine 123. HPPL transported rhodamine 123 efficiently during the first 15 min into the central lumen, and then the fluorescence intensity inside the lumen almost reached a plateau after 20 min. The fluorescence intensity values along the x-y axis shown in the picture at 0 min in A were displayed using Carl Zeiss LSM software (Carl Zeiss, Jena, Germany) and summed up on Microsoft Excel. The total values at each time point were plotted in the graph. (C) Verapamil, an mdr inhibitor, blocked luminal accumulation of rhodamine 123. Rhodamine 123 was transported into the central lumen of a cyst in the control (left), whereas it was trapped inside cells in the presence of verapamil (right). Cysts were incubated with 10 μM verapamil (right) or without it (left) for 30 min before adding rhodamine 123 in culture. After 40 min of incubation, pictures were taken by a confocal microscope. Bars, 20 μm.
Effect of Growth Factors and Cytokines on Cyst Formation
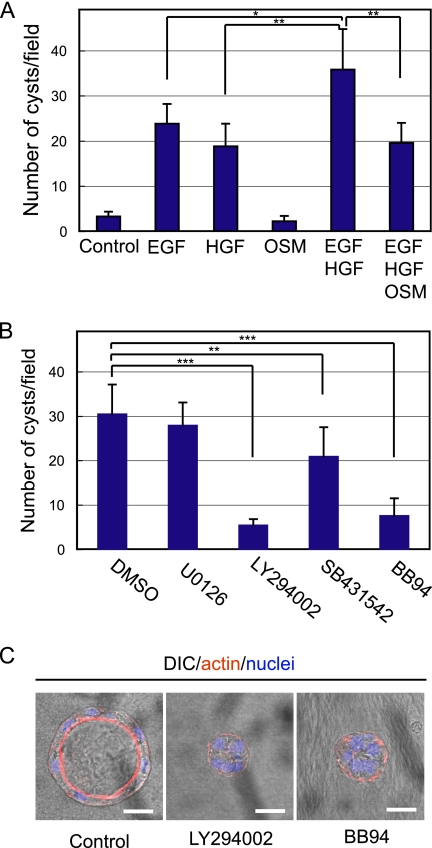
Because HPPL had been maintained in culture with EGF and HGF (Tanimizu et al., 2004), we used the same conditions for 3D culture. To verify that these factors were important for HPPL also in 3D culture, we examined the effect of EGF and HGF on cyst formation (Figure 5A). Although serum and Matrigel supplied numerous growth factors, HPPL did not grow and form cysts in gels efficiently without additional growth factors. EGF or HGF alone induced cyst formation, and the combination of EGF and HGF was more efficient than either EGF or HGF. Thus, we used both EGF and HGF in the following experiments.
Figure 5.
Effect of cytokines, growth factors, and inhibitors for intracellular signaling pathways on cyst formation. (A) HPPL were grown in gel containing 40% Matrigel with cytokines and growth factors as shown in the graph. EGF and HGF, but not OSM, promoted cyst formation. The combination of EGF and HGF was more efficient than either alone, whereas OSM reduced the number of cysts in the presence of EGF and HGF. (B) HPPL were grown for 7 d in the presence of 10 μM either U0126 (MEK inhibitor), LY294002 (PI3K inhibitor), SB431542 (TGFβI/activin receptor-like kinase inhibitor), or BB94 (MMP inhibitor). EGF and HGF were supplied in all the culture. LY294002 and BB94 dramatically reduced the number of cysts, whereas SB431542 slightly reduced the number. U0126 did not significantly affect cyst formation. (C) HPPL mostly formed small cell aggregates in the presence of 10 μM LY294002 or BB94 in contrast to forming cysts in the control. HPPL were grown in the presence of 10 μM LY294002 or BB94 for 7 d. Merged images of DIC and fluorescence staining are shown. Bars, 20 μm. HPPL were stained with AlexaFluor 546 phalloidin (red) and Hoechst34580 (blue). For A and B, five areas were randomly selected in each sample, and the number of cysts with the central lumen was counted under a fluorescence microscope. Experiments were repeated three or four times, and average values are shown in the graphs. Error bars refer to standard deviations. Asterisks (*), double asterisks (**), and triple asterisks (***) represent p < 0.05, p < 0.01, and p < 0.005, respectively.
The data that HPPL cysts express cholangiocyte markers but not a hepatocyte marker suggested that HPPL differentiate along the cholangiocyte lineage and develop epithelial polarity in 3D culture. Thus, we hypothesized that promoting hepatocyte differentiation might block cyst formation by HPPL. In support of this hypothesis, we found that oncostatin M (OSM), which has been used to induce hepatocyte differentiation in vitro (Kamiya et al., 1999) and activated signal transducer and activator of transcription 3 in 3D culture (Supplemental Figure 1A), showed no positive effect on cyst formation; rather, it inhibited the effect of EGF and HGF (Figure 5A). However, OSM did not increase expression of albumin or reduce CK19 (our unpublished data), suggesting that OSM is unlikely to control the lineage decision of HPPL in 3D culture
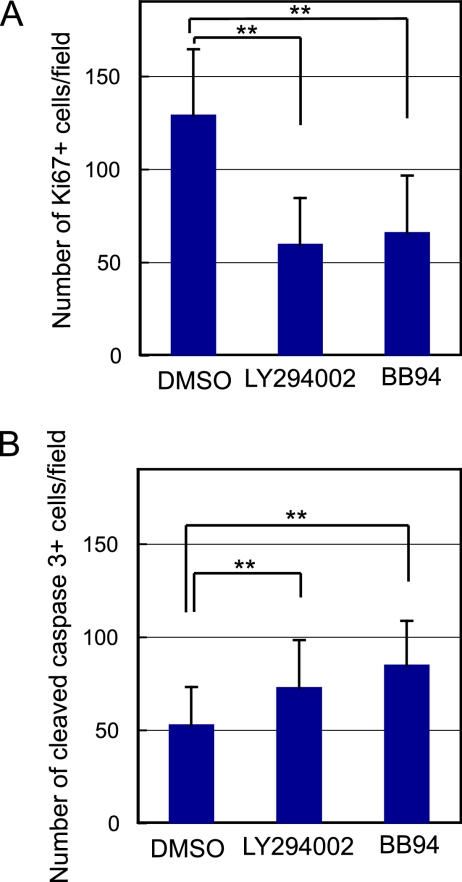
EGF and HGF activate many intracellular signaling pathways, including MEK/ERK and PI3K/Akt pathways. To identify intracellular signals important for cyst formation, we added an MEK inhibitor, U0126, or a PI3K inhibitor, LY294002, to the culture. We counted the number of cysts at day 7 of the culture, and we found that U0126, which reduced phosphorylation of ERK (Supplemental Figure 1B), did not significantly affect cyst formation of HPPL, whereas LY294002 dramatically reduced the number of cysts (Figure 5B). Most of multicellular structures were small aggregates in the presence of LY294002 (Figure 5C). We also added inhibitors against p38 and c-Jun NH2-terminal kinase (JNK). SB202190, a p38 inhibitor, did not affect cyst formation, whereas SP600125, a JNK inhibitor, blocked cyst formation (Supplemental Figure 2). At day 2 of the culture, LY294002 significantly reduced Ki67+ cells (Figure 6A) and slightly increased cleaved caspase 3+ cells (Figure 6B), indicating that EGF and HGF use the PI3K/Akt pathway to promote proliferation and survival of HPPL during cyst formation.
Figure 6.
Inhibition of PI3K or MMP activity suppresses proliferation and increases apoptotic death of HPPL. HPPL were grown in the presence of 10 μM LY294002 or BB94 for 2 d. Inhibition of PI3K or MMP activity significantly reduced Ki67+ cells (A) and increased cleaved caspase 3+ cells (B). HPPL were stained with anti-Ki67 and anti-cleaved caspase 3 antibodies. Five areas were randomly selected in each sample and the number of Ki67+ and cleaved caspase 3+ cells was counted under a fluorescence microscope. Experiments were repeated three or four times, and average values are shown in graphs. Error bars refer to standard deviations. Double asterisks (**) represent p < 0.01.
Matrigel contains many growth factors, including EGF, insulin-like growth factor-I, and TGFβ, as well as ECM proteins. Because EGF and insulin were included in the medium, TGFβ is a soluble factor that is supplied by Matrigel and might affect HPPL morphogenesis. To check this possibility, we used SB431542, an inhibitor of the TGFβRI/activin receptor-like kinase, to block the TGFβ signaling pathway, and we found that the number of cysts decreased with the presence of SB431542 (Figure 5B). Although TGFβ alone unlikely induces cyst morphogenesis of HPPL as Matrigel alone did not induce cyst formation, it may facilitate the function of EGF and HGF.
Because cells are surrounded by ECM proteins in 3D culture, MMPs may be important for morphogenesis of HPPL. Indeed, BB94, an MMP inhibitor with broad specificity, dramatically reduced the number of cysts (Figure 5B). Most multicellular structures were small aggregates in the presence of BB94 (Figure 5C). At day 2 of the culture, BB94 significantly reduced Ki67+ cells (Figure 6A), suggesting that degradation of ECM may give HPPL some space, which allows them to proliferate in gel and undergo cyst morphogenesis. The inhibition of ECM degradation also affected cell survival, because cleaved caspase 3+ cells increased (Figure 6B), which may be another reason for that the number of cysts were reduced in the presence of BB94.
Laminin-1 Is Important for Cyst Formation
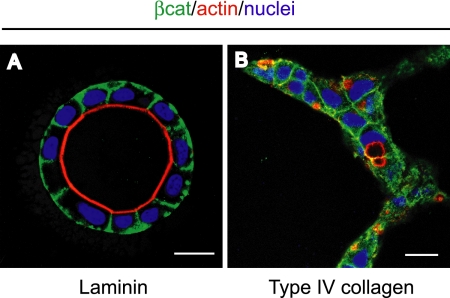
Matrigel contains basement membrane components, including laminin-1, type IV collagen, and entactin. Because laminin has been implicated in the formation of epithelial polarity (O'Brien et al., 2001; Yu et al., 2005), we hypothesized that it could replace Matrigel to induce cyst formation of HPPL. To test this, we mixed high concentration laminin-1/entactin complex with type I collagen, and we examined morphology of HPPL. When laminin-1 was added to type I collagen up to 40% of the total volume, HPPL developed apicobasal polarity and formed cysts with the central lumen (Figure 7A). In contrast, when type IV collagen, another major ECM protein in Matrigel, was added to type I collagen, HPPL still formed extended structures, although tiny lumens were generated (Figure 7B). These data demonstrated that laminin-1 is a major ECM protein contained in Matrigel that induces cyst formation of HPPL.
Figure 7.
Laminin-1 but not type IV collagen induces cyst formation of HPPL. HPPL grown in gel containing 6 mg/ml laminin-1/entactin complex formed cysts with the central lumen (A), whereas HPPL grown in gel containing 0.35 mg/ml type IV collagen formed extended structures (B). After 7 d of culture, samples were fixed and stained with anti-β-catenin antibody followed by AlexaFluor 488 anti-mouse IgG, AlexaFluor 546 phalloidin, and Hoechst 34580. Bars, 20 μm.
DISCUSSION
Studies using primary culture of fetal liver cells have identified molecular pathways governing hepatocyte differentiation (Kinoshita and Miyajima, 2002), whereas we do not know detailed mechanisms for cholangiocyte differentiation due to lack of a good culture system. Expression of metabolic genes has been effectively used to demonstrate hepatocyte differentiation, because this analysis is specific for differentiated hepatocytes and correlated with hepatocyte function (Kamiya et al., 1999). In contrast, it is hard to determine cholangiocyte differentiation only by analyzing gene expression, because only a few markers are available and they are not closely related with cholangiocyte function. Besides functional differences, hepatocytes and cholangiocytes display different types of epithelial polarity, which might be useful to distinguish cholangiocyte differentiation from hepatocyte in vitro. Although several reports have shown that liver progenitors form bile duct-like structures (Spagnoli et al., 1998; Strick-Marchand and Weiss, 2002; Ader et al., 2006), neither the spatial localization of epithelial polarity markers nor functional differentiation as cholangiocytes was studied in detail in those structures. Here, we demonstrated that HPPL, a liver progenitor cell line, formed cysts in gel containing Matrigel. HPPL in cysts localized epithelial polarity markers on distinct plasma membrane domains similarly to cholangiocytes that form bile ducts in vivo. They expressed CK19, a cholangiocyte marker, but not albumin, a hepatocyte marker that was expressed in HPPL before 3D culture. Furthermore, HPPL in cysts acquired the capability of transporting an mdr substrate from the basal side to the apical luminal space. Taken together, we concluded that HPPL develop cholangiocyte-type epithelial polarity in the 3D culture.
EGF and HGF are important factors for HPPL cyst formation. EGF family ligands have been implicated in proliferation of cholangiocytes; TGFα and EGF receptor were detected in cholangiocytes of intrahepatic bile ducts (Terada et al., 1994). Cholangiocytes isolated from polycystic kidney (PCK) rats, which suffer from cystic liver, overexpressed MEK5 and showed hyperresponsiveness to EGF, leading to abnormal proliferation (Sato et al., 2005). Here, we demonstrated that PI3K activated by EGF in combination with HGF promoted proliferation of HPPL during cyst morphogenesis, suggesting that the PI3K pathway in addition to MEK5/ERK5 could be important for cholangiocyte proliferation in vivo. Thus, future studies on the roles of PI3K in HPPL morphogenesis may reveal the molecular mechanisms governing cholangiocyte proliferation both in bile duct development and disease.
Studies of PCK rats indicate that overproliferation of cholangiocytes results in expansion of bile ducts, leading to cyst formation in PCK rats. This suggests that size of the apical lumen of bile ducts may be determined by proliferative capability of cholangiocytes. However, more proliferation did not change the size of the central lumen of HPPL cysts in 3D culture (Supplemental Figure 3). The combination of EGF and HGF increased the number of Ki67+ cells compared with either EGF or HGF alone, although the size of the central lumen was not increased. In contrast, cysts with a large central lumen were not efficiently formed in cultures without EGF and HGF, in which HPPL did not proliferate. These results suggest that proliferation is necessary for forming cysts with a large central lumen but that it is not sufficient for increasing the size of the lumen.
OSM inhibited cyst formation induced by EGF and HGF without affecting expression of CK19 and albumin. OSM is known to regulate expression of numerous genes, including tissue inhibitors of metalloproteinase (TIMPs) (Bugno et al., 1995; Nakamura et al., 2004; Weiss et al., 2005). Moreover, mRNA levels of TIMP1 and -2 at day 7 increased, respectively, by 13- and 4-fold (our unpublished data). Considering that BB94, an inhibitor for MMPs, dramatically reduced the number of cysts, the reduction of MMP activity by the increase of TIMPs in the presence of OSM might inhibit cyst formation.
Matrigel, which has a similar ECM composition with basement membrane, was necessary for HPPL to develop cholangiocyte-type epithelial polarity in the culture. Because laminin-1 induced cyst formation in the absence of Matrigel, it is one of the major components of Matrigel crucial for polarization of HPPL. In contrast, type IV collagen did not induced cyst formation. However, the concentration of type IV collagen was lower than laminin-1 in these experiments, because a higher concentration type IV collagen was not available. So, we cannot rule out that a higher concentration of type IV collagen might help cyst formation. We considered the possibility that HPPL do not produce ECM proteins and therefore that laminin-1, at least, must be supplied in the culture. Unexpectedly, we found that HPPL produced ECM proteins, including laminin α5, β1, β2, γ1, γ2 subunits, as detected by reverse transcription-polymerase chain reaction (our unpublished data). However, they were unlikely to be involved in formation of apicobasal polarity, because most of cysts were not associated with a laminin α5 layer at day 7 of the culture (our unpublished data). Thus, laminin-1 supplied in the culture probably mimics the role of in vivo basement membrane and induces apicobasal polarity of HPPL. Later, ECM proteins produced by HPPL might assemble to the basement membrane and contribute to the maintenance of the polarity. In contrast, because BB94 significantly inhibited cyst formation, the degradation of ECM proteins by MMPs is an important step for cyst formation of HPPL. MMP activities are probably important also for in vivo bile duct formation, because several MMPs were detected around bile ducts in developing liver (Terada et al., 1995). Thus, both the integrity of basement membrane and its degradation are important for cholangiocyte morphogenesis both in vitro and in vivo. The 3D culture system is useful to examine the relationship between production/assembly and degradation of ECM proteins during bile duct morphogenesis.
Our data demonstrate that the cyst formation by HPPL recapitulates several aspects of in vivo cholangiocyte differentiation. It has been difficult to approach detailed mechanisms of bile duct development only using mutant mice and organ culture. Thus, it is worth examining roles of molecules such as TGFβ, HNF1β, and Notch in cyst formation to know their contributions to cholangiocyte differentiation and morphogenesis. Besides these known molecules, we can now explore novel molecular pathways controlling cholangiocyte differentiation by disrupting genes in the 3D culture and characterizing the effects on morphology of HPPL. We expect that this novel culture system will help us to understand the molecular mechanism governing bile duct development and its disruption in disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank the members of the K.E.M. laboratory for helpful discussion. N.T. is supported by a fellowship from Japan Society for the Promotion of Science and by a Pilot/Feasibility funding from the Liver Center of University of California San Francisco. This work was supported by grants from the National Institutes of Health to K.E.M.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0848) on February 21, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Ader T., Norel R., Levoci L., Rogler L. E. Transcriptional profiling implicates TGFbeta/BMP and Notch signaling pathways in ductular differentiation of fetal murine hepatoblasts. Mech. Dev. 2006;123:177–194. doi: 10.1016/j.mod.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Bugno M., Graeve L., Gatsios P., Koj A., Heinrich P. C., Travis J., Kordula T. Identification of the interleukin-6/oncostatin M response element in the rat tissue inhibitor of metalloproteinases-1 (TIMP-1) promoter. Nucleic Acids Res. 1995;23:5041–5047. doi: 10.1093/nar/23.24.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clotman F., Jacquemin P., Plumb-Rudewiez N., Pierreux C. E., Van der Smissen P., Dietz H. C., Courtoy P. J., Rousseau G. G., Lemaigre F. P. Control of liver cell fate decision by a gradient of TGFbeta signaling modulated by onecut transcription factors. Genes Dev. 2005;19:1849–1854. doi: 10.1101/gad.340305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clotman F., Lannoy V. J., Reber M., Cereghini S., Cassiman D., Jacquemin P., Roskams T., Rousseau G. G., Lemaigre F. P. The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development. 2002;129:1819–1828. doi: 10.1242/dev.129.8.1819. [DOI] [PubMed] [Google Scholar]
- Coffinier C., Gresh L., Fiette L., Tronche F., Schutz G., Babinet C., Pontoglio M., Yaniv M., Barra J. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1β. Development. 2002;129:1829–1838. doi: 10.1242/dev.129.8.1829. [DOI] [PubMed] [Google Scholar]
- Fitz J. G. Regulation of cholangiocyte secretion. Semin. Liver Dis. 2002;22:241–249. doi: 10.1055/s-2002-34502. [DOI] [PubMed] [Google Scholar]
- Gigliozzi A., Fraioli F., Sundaram P., Lee J., Mennone A., Alvaro D., Boyer J. L. Molecular identification and functional characterization of Mdr1a in rat cholangiocytes. Gastroenterology. 2000;119:1113–1122. doi: 10.1053/gast.2000.18156. [DOI] [PubMed] [Google Scholar]
- Ito Y., Matsui T., Kamiya A., Kinoshita T., Miyajima A. Retroviral gene transfer of signaling molecules into murine fetal hepatocytes defines distinct roles for the STAT3 and ras pathways during hepatic development. Hepatology. 2000;32:1370–1376. doi: 10.1053/jhep.2000.19815. [DOI] [PubMed] [Google Scholar]
- Kamiya A., et al. Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J. 1999;18:2127–2136. doi: 10.1093/emboj/18.8.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa Y., Mochizuki Y., Miner J. H., Mitaka T. Transient expression of laminin alpha1 chain in regenerating murine liver: restricted localization of laminin chains and nidogen-1. Exp. Cell Res. 2005;305:99–109. doi: 10.1016/j.yexcr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Miyajima A. Cytokine regulation of liver development. Biochim. Biophys. Acta. 2002;1592:303–312. doi: 10.1016/s0167-4889(02)00323-3. [DOI] [PubMed] [Google Scholar]
- Kodama Y., Hijikata M., Kageyama R., Shimotohno K., Chiba T. The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology. 2004;127:1775–1786. doi: 10.1053/j.gastro.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Lemaigre F. P. Development of the biliary tract. Mech. Dev. 2003;120:81–87. doi: 10.1016/s0925-4773(02)00334-9. [DOI] [PubMed] [Google Scholar]
- Li L., et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat. Genet. 1997;16:243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- Matsui T., Kinoshita T., Morikawa Y., Tohya K., Katsuki M., Ito Y., Kamiya A., Miyajima A. K-Ras mediates cytokine-induced formation of E-cadherin-based adherens junctions during liver development. EMBO J. 2002;21:1021–1030. doi: 10.1093/emboj/21.5.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCright B., Lozier J., Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development. 2002;129:1075–1082. doi: 10.1242/dev.129.4.1075. [DOI] [PubMed] [Google Scholar]
- Mostov K., et al. Formation of multicellular epithelial structures. Novartis Found Symp. 2005;269:193–200. discussion 200–205, 223–230. [PubMed] [Google Scholar]
- Nakamura K., Nonaka H., Saito H., Tanaka M., Miyajima A. Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice. Hepatology. 2004;39:635–644. doi: 10.1002/hep.20086. [DOI] [PubMed] [Google Scholar]
- O'Brien L. E., Jou T. S., Pollack A. L., Zhang Q., Hansen S. H., Yurchenco P., Mostov K. E. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- O'Brien L. E., Yu W., Tang K., Jou T. S., Zegers M. M., Mostov K. E. Morphological and biochemical analysis of Rac1 in three-dimensional epithelial cell cultures. Methods Enzymol. 2006;406:676–691. doi: 10.1016/S0076-6879(06)06053-8. [DOI] [PubMed] [Google Scholar]
- Sato Y., Harada K., Kizawa K., Sanzen T., Furubo S., Yasoshima M., Ozaki S., Ishibashi M., Nakanuma Y. Activation of the MEK5/ERK5 cascade is responsible for biliary dysgenesis in a rat model of Caroli's disease. Am. J. Pathol. 2005;166:49–60. doi: 10.1016/S0002-9440(10)62231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnoli F. M., Amicone L., Tripodi M., Weiss M. C. Identification of a bipotential precursor cell in hepatic cell lines derived from transgenic mice expressing cyto-Met in the liver. J. Cell Biol. 1998;143:1101–1112. doi: 10.1083/jcb.143.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick-Marchand H., Weiss M. C. Inducible differentiation and morphogenesis of bipotential liver cell lines from wild-type mouse embryos. Hepatology. 2002;36:794–804. doi: 10.1053/jhep.2002.36123. [DOI] [PubMed] [Google Scholar]
- Tan C. E., Chan V. S., Yong R. Y., Vijayan V., Tan W. L., Fook Chong S. M., Ho J. M., Cheng H. H. Distortion in TGF beta 1 peptide immunolocalization in biliary atresia: comparison with the normal pattern in the developing human intrahepatic bile duct system. Pathol. Int. 1995;45:815–824. doi: 10.1111/j.1440-1827.1995.tb03401.x. [DOI] [PubMed] [Google Scholar]
- Tanimizu N., Miyajima A. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J. Cell Sci. 2004;117:3165–3174. doi: 10.1242/jcs.01169. [DOI] [PubMed] [Google Scholar]
- Tanimizu N., Saito H., Mostov K., Miyajima A. Long-term culture of hepatic progenitors derived from mouse Dlk+ hepatoblasts. J. Cell Sci. 2004;117:6425–6434. doi: 10.1242/jcs.01572. [DOI] [PubMed] [Google Scholar]
- Terada T., Ohta T., Nakanuma Y. Expression of transforming growth factor-alpha and its receptor during human liver development and maturation. Virchows Arch. 1994;424:669–675. doi: 10.1007/BF00195783. [DOI] [PubMed] [Google Scholar]
- Terada T., Okada Y., Nakanuma Y. Expression of matrix proteinases during human intrahepatic bile duct development. A possible role in biliary cell migration. Am. J. Pathol. 1995;147:1207–1213. [PMC free article] [PubMed] [Google Scholar]
- Weiss T. W., Kvakan H., Kaun C., Zorn G., Speidl W. S., Pfaffenberger S., Maurer G., Huber K., Wojta J. The gp130 ligand oncostatin M regulates tissue inhibitor of metalloproteinases-1 through ERK1/2 and p38 in human adult cardiac myocytes and in human adult cardiac fibroblasts: a possible role for the gp130/gp130 ligand system in the modulation of extracellular matrix degradation in the human heart. J. Mol. Cell Cardiol. 2005;39:545–551. doi: 10.1016/j.yjmcc.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Yu W., O'Brien L. E., Wang F., Bourne H., Mostov K. E., Zegers M. M. Hepatocyte growth factor switches orientation of polarity and mode of movement during morphogenesis of multicellular epithelial structures. Mol. Biol. Cell. 2003;14:748–763. doi: 10.1091/mbc.E02-06-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Datta A., Leroy P., O'Brien L. E., Mak G., Jou T. S., Matlin K. S., Mostov K. E., Zegers M. M. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol. Biol. Cell. 2005;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers M. M., O'Brien L. E., Yu W., Datta A., Mostov K. E. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol. 2003;13:169–176. doi: 10.1016/s0962-8924(03)00036-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.