Abstract
Microglia are the main immune cells of the brain, and under some circumstances they can play an important role in removal of fibrillar Alzheimer amyloid β peptide (fAβ). Primary mouse microglia can internalize fAβ, but they do not degrade it efficiently. We compared the level of lysosomal proteases in microglia and J774 macrophages, which can degrade fAβ efficiently, and we found that microglia actually contain higher levels of many lysosomal proteases than macrophages. However, the microglial lysosomes are less acidic (average pH of ∼6), reducing the activity of lysosomal enzymes in the cells. Proinflammatory treatments with macrophage colony-stimulating factor (MCSF) or interleukin-6 acidify the lysosomes of microglia and enable them to degrade fAβ. After treatment with MCSF, the pH of microglial lysosomes is similar to J774 macrophages (pH of ∼5), and the MCSF-induced acidification can be partially reversed upon treatment with an inhibitor of protein kinase A or with an anion transport inhibitor. Microglia also degrade fAβ if lysosomes are acidified by an ammonia pulse-wash or by treatment with forskolin, which activates protein kinase A. Our results indicate that regulated lysosomal acidification can potentiate fAβ degradation by microglia.
INTRODUCTION
Extracellular senile plaques containing fibrillar forms of amyloid-β peptide (Aβ) are observed in brains of Alzheimer's disease (AD) patients (Selkoe, 2001). The Aβ peptide is a 39–42 amino acid fragment derived by proteolytic processing from a larger membrane-spanning glycoprotein called β-amyloid precursor protein (βAPP). Aβ peptides form fibrillar β-pleated sheet structures (fAβ) that are the major component of senile plaques. Although there may be multiple initial causes of AD, inherited mutations in βAPP that lead to excess Aβ production are strongly associated with familial early onset AD (Selkoe, 2001), indicating that excess Aβ can be causative for AD. Furthermore, treatments that lead to clearance of fAβ in mouse models of AD have been reported to be associated with some reversal of behavioral impairments (Morgan et al., 2000; Hartman et al., 2005).
Net accumulation of fAβ is related to the rate of production of Aβ and to its rate of degradation. As the main immune cells of the brain, microglia would be expected to play a role in clearing extracellular debris similar to a function carried out elsewhere by macrophages, a closely related cell type. Indeed, under some circumstances it has been shown that microglia can degrade fAβ. Immunotherapy against Aβ leads to a striking removal of amyloid plaques in βAPP transgenic mice (Schenk et al., 1999), and there is good evidence that microglia are responsible for plaque clearance (Bard et al., 2000). In humans, microglia also are frequently found in regions surrounding amyloid plaques in AD brains, and Aβ was found in microglia in a human patient immunized against Aβ (Nicoll et al., 2003). The regions surrounding such microglia were devoid of extracellular plaques, suggesting that the microglia had removed plaque material.
Primary mouse microglia in tissue culture do not degrade fAβ even though they internalize it rapidly by receptor-mediated uptake and deliver it to lysosomes that can degrade other endocytosed proteins in the same organelles (Paresce et al., 1997; Chung et al., 1999). Furthermore, primary microglia in tissue culture do not degrade fAβ when it is coated with anti-Aβ antibodies with or without the addition of complement components (Brazil et al., 2000), although microglia in brain slices could digest some plaques (Bard et al., 2000). These findings indicate that binding via immune receptors is not sufficient to trigger degradation of internalized fAβ, but a more complex activation mechanism is needed to facilitate degradation. This is also supported by studies in APP transgenic mice in which immunotherapy effectively reduced fAβ deposits even in an FcRγ knockout background (Das et al., 2003).
Primary mouse microglia in tissue culture actually release macromolecular fAβ that had been internalized (Chung et al., 1999). This suggests that microglia in a nonactivated state would be unable to clear fAβ from the brain, which may account, in part, for the accumulation of fAβ. Consistent with the release observed in tissue culture, reports based on postmortem examination of the relationship between microglia and plaques have indicated that microglia could actually contribute to plaque formation (Wegiel et al., 2001; Wegiel et al., 2003; Townsend et al., 2005).
In the current study, we examined the effect of activation of microglia on fAβ degradation. We find that some inflammatory mediators, which cause the microglia to take on some macrophage-like properties, enable them to degrade fAβ in the absence of antibodies. We also found that unactivated microglia have weakly acidic lysosomes and that treatments that increase the ability to degrade fAβ also cause acidification of lysosomes. Preliminary characterization of the mechanism for increasing the lysosomal acidity indicates that it requires activation of protein kinase A and the activity of chloride channels. This lysosomal acidification during activation of microglia is required for fAβ degradation by microglia.
MATERIALS AND METHODS
Materials
Lipopolysaccharide (LPS), interleukin (IL)-6, macrophage colony-stimulating factor (MCSF), forskolin, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), and KT 5720 were purchased from Sigma-Aldrich (St. Louis, MO).
Cells
Primary microglial cultures were isolated and maintained as described previously (Paresce et al., 1997) J774. A1 mouse macrophage-like cells (American Type Culture Collection, Manassas, VA) were maintained as described previously (Majumdar et al., 2007).
Treatment of Microglial Cells
Microglial cells were plated 2 d before an experiment in 35-mm coverslip-bottom dishes in DMEM supplemented with 10% serum and 1% antibiotic penicillin-streptomycin. For LPS and IL-6 treatments, 200 ng/ml LPS or 200 ng/ml IL-6 was added to the culture media 16 h before an experiment. After 16 h, cells were thoroughly washed with growth medium, and they were maintained in growth medium throughout the experiment. For the MCSF treatment of microglial cells, 25 ng/ml MCSF was included in the culture medium during the isolation of microglia from mixed glial cultures, and cells were incubated for 5 d in 25 ng/ml MCSF before the start of the experiment and throughout the experiment.
Cellular Degradation of 125I-fAβ and Cy3-fAβ
Degradation of 125I-fAβ and Cy3-fAβ in J774 macrophages and microglia was measured as described previously (Chung et al., 1999). For Cy3-fAβ degradation, the cells were imaged using a Leica epifluorescence microscope with a cooled charge-coupled device (CCD) camera (Micromax 521BFT; Princeton Scientific Instruments, Monmouth Junction, NJ, and Roper Scientific, Trenton, NJ), and the Cy3 fluorescence power was quantified using MetaMorph Imaging System software (Molecular Devices, Sunnyvale, CA).
Cellular Degradation of Cy3-fAβ Associated with Forskolin Treatment
To study the effect of activation of protein kinase A (PKA) by forskolin, Cy3-fAβ loaded microglia were exposed to 45-min pulses of 200 μM forskolin during the 1st, 6th, 24th, and 48th h of a 72-h chase period. After the chase periods, the cells were imaged as in other degradation experiments.
Immunofluorescence of Treated and Untreated Microglia
To measure the expression of cell surface markers on microglial cells, the cells were first treated with LPS, IL-6, or MCSF as described above. Cells were then incubated in culture media for 72 h. After 72-h incubation, microglia were fixed with 3% paraformaldehyde in phosphate-buffered saline (PBS) and then permeabilized for 15 min with 0.01% saponin in PBS. Fixed and permeabilized cells were incubated in 3% bovine serum albumin for 60 min at room temperature followed by addition of primary antibody (1:100 dilution). Primary antibodies, anti-mouse CD11b monoclonal (eBiosciences, San Diego, CA), anti-mouse major histocompatibility complex (MHC) II monoclonal (eBiosciences), and anti-mouse CD 45 monoclonal (eBiosciences) were incubated for 60 min at 4°C. Cells were washed three times with PBS, and then they were incubated with A546-tagged anti-rat IgG secondary antibodies (Invitrogen, Carlsbad, CA) for 60 min at 4°C. After secondary antibody incubation, cells were washed and imaged. For each marker, the level of expression was measured by measuring the fluorescence power/cell.
In Vitro Protease Activity Assay
Lysosomal proteases were assayed as described previously (Tyynela et al., 2000; Awano et al., 2006).
Lysosomal pH Measurement
The measurement of lysosomal/endosomal pH by confocal microscopy (Presley et al., 1993; Dunn et al., 1994) is based on the use of the ratio of the pH-sensitive fluorescein fluorescence to pH-insensitive rhodamine fluorescence. Cells were incubated for 16 h with 5 mg/ml dextran conjugated to both fluorescein and rhodamine (70,000 mol. wt.; Invitrogen) in complete growth media. Cells were washed thoroughly in complete media, and then they were incubated for another 4 h to chase the dextran to lysosomes. The cells were then washed with medium 2 (150 mM NaCl, 20 mM HEPES, pH 7.4, 1 mM CaCl2, 5 mM KCl, and 1 mM MgCl2) and kept in medium 2 for 20 min at 37°C in air. Cells were then taken for confocal imaging at 37°C. Confocal images were collected on an LSM510 laser scanning confocal unit (Carl Zeiss, Thornwood, NY) attached to an Axiovert 100 M inverted microscope (Carl Zeiss) with a 63× numerical aperture 1.4 plan Apochromat objective (Carl Zeiss). Excitation on the LSM510 laser confocal microscope was with 25-mW Argon laser emitting 488 nm and a 1.0-mW helium/neon laser emitting at 543 nm. Emissions were collected using a 505- to 530-nm band pass filter to collect fluorescein emission and a 560- to 615-nm band pass filter to collect rhodamine emission. Typically, 0.3- to 0.5-μm vertical steps were used with axial resolution <1.0 μm. Using image processing techniques developed previously (Dunn et al., 1994), lysosome fluorescence was quantified, and the ratio of fluorescein to rhodamine fluorescence was determined for each individual lysosome. Images were analyzed using MetaMorph image processing software (Molecular Devices). For all experimental sets, cross-talk of the fluorophores was negligible. Calibration curves were generated after fixing and equilibrating the fluorescein-rhodamine-dextran–loaded cells to a range of pH buffers. Although the fluorescein/rhodamine ratio was sensitive to differential photobleaching of fluorescein and rhodamine fluorescence, the effects of differential photobleaching were minimized by 1) attenuating the laser intensity, 2) minimizing the cells exposure to the lasers, and 3) rigidly adhering to a standard protocol for image collection for both living cells and pH-standardized fixed cells. Consequently, the effect of photobleaching on fluorescein-rhodamine fluorescence was negligible and consistent for all the images.
Degradation of Cy3-fAβ by Microglia after Artificial Lysosomal Acidification
For artificial lysosomal acidification, we used an ammonium chloride pulse-wash technique (Ohkuma and Poole, 1978). Cells were washed with medium 2, and then they were incubated for 30 min in medium 2 containing 25 mM ammonium chloride. Cells were washed thoroughly and incubated in medium 2 for another 60 min. After this incubation, cells were transferred to complete culture medium. For Cy3-fAβ degradation with artificial acidification, the cells were first loaded with Cy3-fAβ, and then they were subjected to ammonium chloride pulse-wash at the 1st, 6th, 24th, and 48th h during the 72-h chase period. After the chase period, the cells were imaged using a Leica epifluorescence microscope with a cooled CCD camera (Micromax 521BFT; Princeton Scientific Instruments and Roper Scientific), and the Cy3 fluorescence power was quantified using MetaMorph Imaging System software (Molecular Devices).
RESULTS
In Vitro Assay of Lysosomal Proteases
From tissue culture studies, we know that J774 macrophages can degrade the same fAβ that is not digested by microglia (Majumdar et al., 2007). To understand the reason for this difference, we compared properties of microglia and macrophages. First, we compared the levels of key lysosomal proteases in microglia and in J774 cells. As shown in Table 1, most of the lysosomal proteases tested actually showed a higher specific activity in microglial extracts than in extracts from macrophages. Thus, lack of proteases is not the primary cause of poor lysosomal hydrolysis of fAβ by microglia.
Table 1.
Lysosomal enzyme activity in cell lysates
| Enzyme | nmol/h/mg cell protein |
Microglia activity/macrophage activity | |
|---|---|---|---|
| Macrophage activity | Microglia activity | ||
| Tripeptidyl peptidase (TPP) I | 50 | 238 | 4.74 |
| Cathepsin B | 56 | 80 | 1.44 |
| Dipeptidyl peptidase (DPP) I | 1915 | 7242 | 3.78 |
| Cathepsin H | 563 | 472 | 0.84 |
| DPP II | 16 | 79 | 5.05 |
| Cathepsin D | 51 | 147 | 2.88 |
The assay media were buffered as follows: cathepsin D, pH 3.5; TPP I, pH 4.5; cathepsin B, DPP I and DPP II, pH 5.5; and cathepsin H, pH 6.8.
Measurement of Lysosomal pH in Live Cells
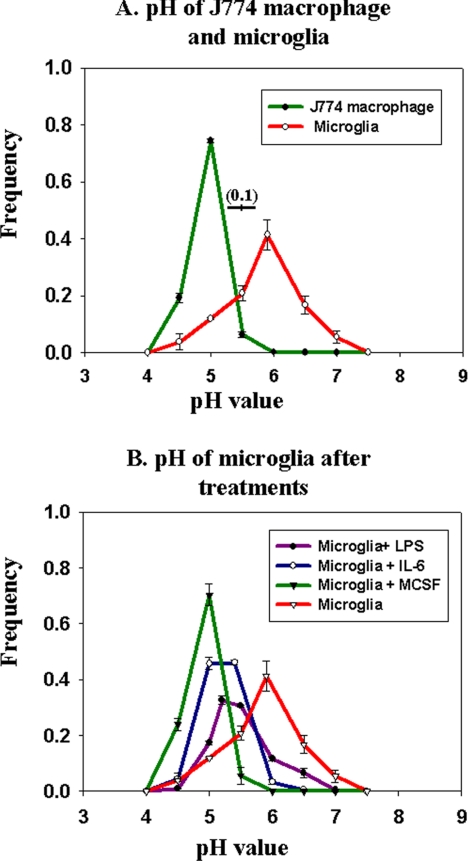
The in vitro assays of lysosomal enzyme activity were carried out at acidic pH. In cells, the activity of lysosomal proteases is also dependent upon the pH of the lysosomes. Most lysosomal proteases have acidic pH optima, and many are converted from proenzymes to their active form in the acidic lumen of lysosomes. Thus, the pH of lysosomes can greatly influence their proteolytic power. We compared the lysosomal pH of microglia and macrophages by using fluorescence ratio imaging of late endosomes and lysosomes containing endocytosed fluorescein-rhodamine-dextran (Dunn and Maxfield, 2003). Because fluorescein fluorescence decreases at acidic pH, whereas rhodamine fluorescence is pH independent, the fluorescein/rhodamine ratio is pH dependent (Dunn and Maxfield, 2003). As shown in Figure 1A, macrophages had an average lysosomal pH close to 5.0, whereas the average microglial lysosomal pH was ∼5.9. This difference in lysosomal pH could contribute significantly to the inability of microglia to degrade fAβ.
Figure 1.
Lysosomal pH measurements in live cells. J774 macrophages and microglia were loaded with fluorescein-rhodamine-dextran for 16 h followed by a 4-h chase. Fluorescein/rhodamine ratio values were measured for individual lysosomes and compared with calibration curves generated from fixed cells in various pH buffers. (A) Frequency distribution of lysosomal pH in macrophages (green curve) and microglia (red curve). (B) Frequency distribution of lysosomal pH in microglia after treatment with 200 ng/ml LPS for 16 h, 200 ng/ml IL-6 for 16 h, or 25 ng/ml MCSF for 5 d. Data for lysosomal pH measurements for each experiment and for each condition were obtained from at least 750 lysosomes from four different fields, and the data shown here are pH values obtained from four different experiments done on four different days. Error bars represent the SEM. The horizontal bar on the figure shows the SD (±0.1 pH unit) for the measurement of pH values in cells fixed at pH 5.5.
Degradation of fAβ by Activated Macrophage-like Microglia
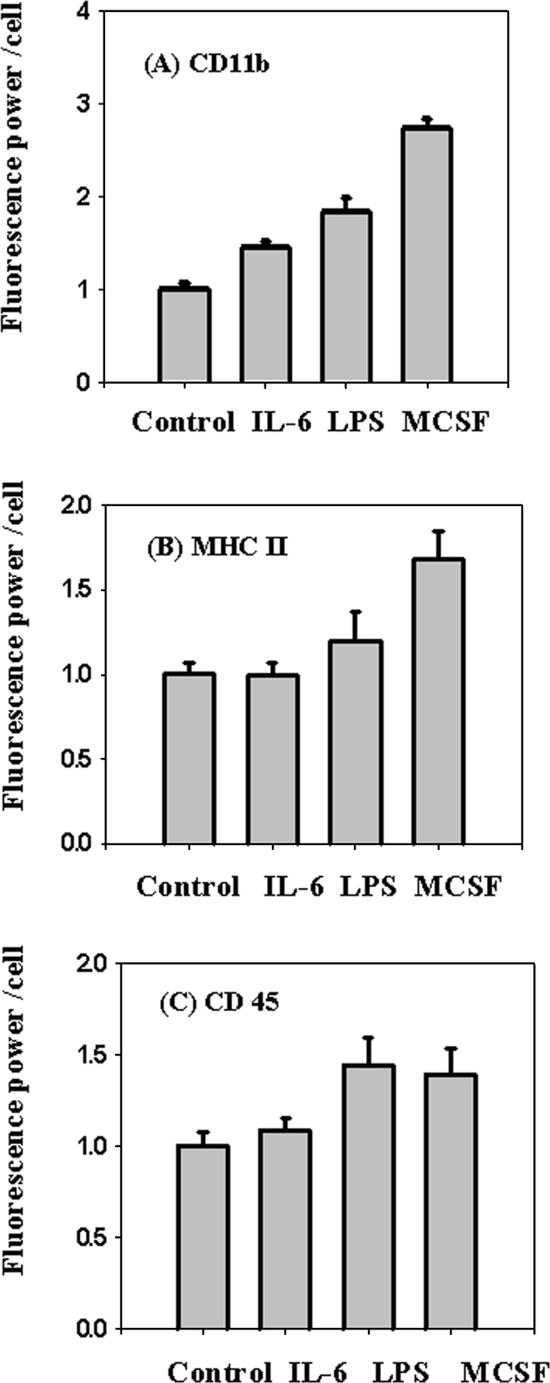
Because microglia can degrade fAβ under some conditions, we examined the effects of microglial activation by proinflammatory treatments. It has been suggested that there is a continuum of microglial activation rather than a single activated state (Town et al., 2005), so we tested a variety of agents that might increase macrophage-like properties of microglia. MCSF and IL-6 are proinflammatory molecules that are increased in various mouse models of AD (Morgan et al., 2005), and they can differentiate microglia into activated cells that resemble macrophages in the expression of various cell surface proteins (Santambrogio et al., 2001; Monsonego and Weiner, 2003). IL-6 also plays a role in differentiating monocytes toward the macrophage lineage (Chomarat et al., 2000). LPS was tested because it is a strong mediator of inflammation that has been reported to reduce plaque burden in APP transgenic mice (Herber et al., 2004). We monitored the cell surface expression of CD11b and MHC II in primary microglia as an index of activation toward a macrophage-like state (Santambrogio et al., 2001). We also monitored the cell surface expression of CD45 as an index of microglial activation (Herber et al., 2006). As shown in Figure 2, treatment with proinflammatory reagents led to increases in the surface expression of CD11b, MHC II, and CD45, which indicates differentiation of activated microglia toward macrophage-like properties.
Figure 2.
Expression of cell surface markers on microglia. Microglia were treated with 200 ng/ml IL-6 for 16 h, 200 ng/ml LPS for 16 h, or 25 ng/ml MCSF for 5 d, and expression of cell surface markers CD11b, MHC II, and CD45 were measured using digital imaging of immunofluorescence. The figure shows the fluorescence power (arbitrary units) per cell for the treated and untreated cells. CD 11b (A), MHC II (B), and CD 45 (C). Error bars are SEM from six different experiments done on six different days.
Along with the expression of cell surface markers, these proinflammatory stimuli decreased the pH of microglial lysosomes (Figure 1B). The greatest change was seen with MCSF treatment, which brought the lysosomal pH to about the same value as seen in J774 macrophages.
If poor lysosomal acidification is the basis for inefficient degradation of fAβ, then this should be overcome by these treatments. We used an assay for fAβ degradation in which microglia take up fAβ labeled with the fluorescent dye Cy3 and deliver it to late endosomes and lysosomes (Chung et al., 1999). When intracellular Cy3-fAβ is degraded, the fluorescence is released from the cells, and the degradation kinetics measured by this loss of fluorescence is very similar to degradation measured using radiolabeled fAβ. We measured the degradation of Cy3-fAβ by microglia with or without various proinflammatory treatments. As shown in Figure 3, all of these proinflammatory treatments increased the ability of microglia to degrade fAβ, and MCSF had the greatest effect. The MCSF-treated microglia were almost as effective in degrading fAβ as J774 macrophage-like cells were found to be in a similar assay (Majumdar et al., 2007). To confirm that the loss of cell-associated florescence was due to degradation as opposed to release of macromolecular fAβ, we examined the properties of material released from the cells. We had shown previously (Majumdar et al., 2007) that after 125I-fAβ was taken up by J774 cells the radiolabeled material was released mainly as trichloroacetic acid (TCA)-soluble (i.e., low-molecular-weight) degradation products. We verified that >70% of the radiolabeled material released from MCSF-treated microglia after uptake of 125I-fAβ was also TCA soluble (data not shown). In vitro measurements of lysosomal enzyme activity of the same six proteases measured in Table 1 did not show a large increase in protease specific activity after MCSF simulation (data not shown).
Figure 3.
Degradation of Cy3 fAβ in treated and untreated microglia. Microglia were treated with 200 ng/ml LPS for 16 h, 200 ng/ml IL-6 for 16 h, or 25 ng/ml MCSF for 5 d. After the treatment, cells were incubated for 60 min with 5 μg/ml Cy3-fAβ, rinsed, and chased for 72 h. After the chase period, the cells were rinsed, fixed, and imaged by digital fluorescence microscopy. The integrated Cy3 fluorescence power per cell before and after the chase was quantified. The figure shows the percentage of the Cy3 signal retained in the cells after the 72-h chase period with respect to the Cy3 signal at a 0-h chase. The data presented here are the average from six different experiments done on different days. Error bars represent the SEM.
Degradation of fAβ by Microglia after Artificial Lysosomal Acidification
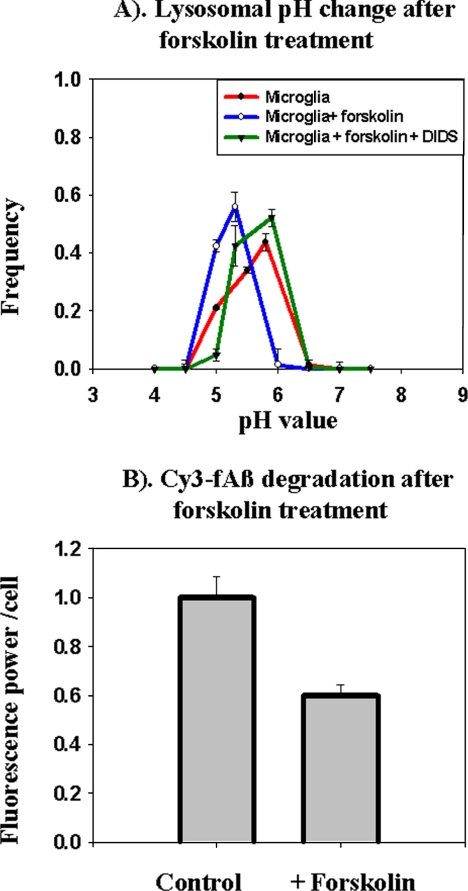
The high lysosomal pH in inactivated microglia and the decrease in pH that was associated with increased ability to degrade fAβ after inflammatory stimuli are consistent with a hypothesis that lysosomal pH is a determining factor for fAβ degradation. However, proinflammatory stimuli activate many signal transduction pathways that could affect degradation by another mechanism. To isolate the role of lysosomal acidity, we used an ammonia pulse-wash method to hyperacidify acidic organelles (Ohkuma and Poole, 1978). Cells were incubated for 30 min in buffer containing 25 mM ammonium chloride, which causes a partial neutralization of the pH of lysosomes and an accumulation of ammonia in acidic organelles (Ohkuma and Poole, 1978). When ammonium chloride is removed from the medium, the efflux of ammonia transiently acidifies the lysosomes (Figure 4A). The lysosomal pH dropped from 5.9 to 5.3 ∼15 min after the removal of ammonium chloride, and lysosomes returned to their normal pH values after ∼1 h.
Figure 4.
Artificial lysosomal acidification causes degradation of Cy3-fAβ by microglia. (A) Lysosomal pH measurements. Microglia were incubated with fluorescein-rhodamine dextran for 16 h followed by a 4-h chase. The cells were pulsed with 25 mM NH4Cl for 30 min, followed by rinsing and incubation in medium without NH4Cl for 15, 45, or 60 min. At each time, lysosomal pH measurements were obtained from at least 750 lysosomes from four different fields, and the pH values from four different experiments done on four different days are shown. Error bars represent the SEM. (B) Degradation of Cy3-fAβ. Microglia were incubated for 60 min with 5 μg/ml Cy3-fAβ and chased for 72 h. During the chase period, lysosomes were acidified by ammonium pulse-wash Cy3 fluorescence power after 72 h normalized to the fluorescence power before the chase. The data represent the average of the normalized intensity values from four different experiments done on four different days. Error bars represent the SEM.
To determine whether lysosomal acidification was sufficient to enable microglia to degrade fAβ, cells were subjected to ammonia pulse-wash treatments at the 1st, 6th, 24th, and 48th h of the 72-h chase period after uptake of Cy3-fAβ. As shown in Figure 4B, the microglia treated in this way were able to degrade a significant amount of fAβ. This shows that lysosomal acidification can make the microglia capable of digesting fAβ in the absence of inflammatory stimulation. To further establish the importance of lysosomal acidification for fAβ degradation by activated microglia, we measured the degradation of Cy3-fAβ in MCSF-treated microglia in the presence of the vacuolar type (V)-ATPase inhibitor drug bafilomycin A1 at 0.25 μM (Presley et al., 1997). We found that after 24 h of chase the MCSF-treated microglia degraded 30% of the Cy3-fAβ, whereas the cells treated with MCSF and bafilomycin A1 failed to show any degradation of Cy3-fAβ (data not shown).
Mechanism of Lysosomal Acidification in Activated Microglia
The mechanisms for regulation of endosome and lysosome pH are complex and not fully understood (Pillay et al., 2002). Among the factors that can alter the pH of an organelle are the number of electrogenic H+-pumping V-ATPases and the detailed properties of these V-ATPase complexes (Nishi and Forgac, 2002). We compared the abundance of the B and E subunits of the V-ATPase in macrophages and microglia by immunoblotting, and we found that microglia expressed higher levels of these V-ATPase subunits than macrophages (data not shown). This suggests that abundance of the V-ATPase is not the major limiting factor in lysosomal acidification in microglia.
Cl− channels can reduce the inside positive electrical potential that is generated by the V-ATPase, allowing greater acidification of organelles. The form of the Cl− channel that is on the lysosomes of microglia is not known, but chloride channels are found on the lysosomes of many cell types, and they are known to regulate the pH of endosomes and lysosomes (Jentsch et al., 2004).
Several types of chloride channel can be activated by phosphorylation by PKA (Bae and Verkman, 1990), so we examined the effects of activators of PKA and inhibitors of anion channels on lysosomal pH in microglia. When cellular cAMP levels were elevated by treatment of cells with forskolin, a significant acidification of lysosomes was seen within 45 min, and this acidification was blocked by the anion transport inhibitor DIDS (Figure 5A). We tested whether forskolin treatment could increase the degradation of fAβ by microglial cells. Treatment of microglia with forskolin for the entire 72-h degradation assay caused a loss of cells, so we used a protocol in which cells were treated with forskolin for 45 min during the 1st, 6th, 24th, and 48th h of a 72-h degradation assay. As shown in Figure 5B, forskolin-treated microglia degraded fAβ more effectively than untreated cells. Long-term treatment with DIDS was also toxic to the cells, so we could not test the effect of treatment with DIDS on degradation of fAβ.
Figure 5.
Forskolin treatment causes lysosome acidification and degradation of Cy3f-Aβ by microglia. (A) Lysosomal pH was measured 45 min after addition of 200 μM forskolin. Where indicated, 1 mM DIDS was added 15 min before forskolin addition. Lysosomal pH measurements were obtained from at least 750 lysosomes from four different fields. The average pH values from four different experiments from four different days are shown. Error bars represent the SEM. (B) Microglia were incubated for 60 min with 7 μg/ml Cy3-fAβ and chased for 72 h. During the chase period, the cells were treated with 45-min pulses of 200 μM forskolin to activate PKA during the 1st, 6th, 24th, and 48th h. Cy3 fluorescence power after 72 h normalized to the fluorescence power at the 0-h time point. The data shown here represent average of the normalized intensity values from four different experiments done on four different days. Error bars represent the SEM.
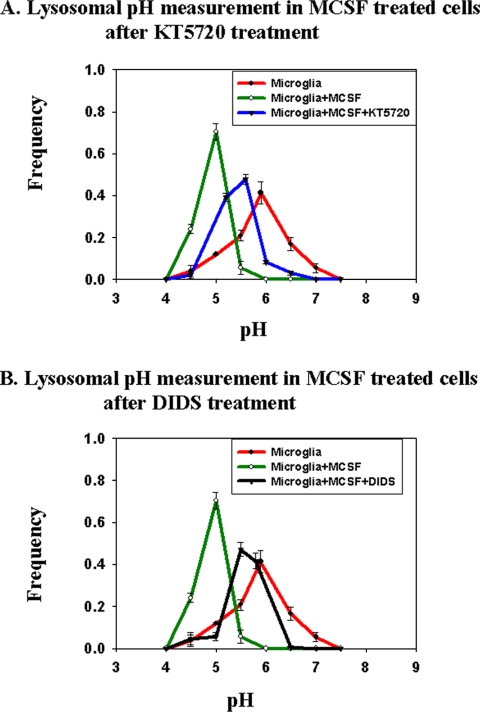
The signal transduction pathways activated by MCSF in microglia are not well characterized, but in monocytes it is known that increased cAMP is associated with the MCSF-mediated differentiation of monocytes toward macrophages (Wilson et al., 2005). It is also known that treatment of macrophages with inflammatory cytokines can raise cAMP levels (Aronoff et al., 2005). We tested the effects of DIDS and inhibitors of PKA on the lysosomal pH of MCSF-treated cells. The cells were pretreated with MCSF for 5 d as in the other experiments reported here. As shown in Figure 6A, a 45-min treatment of cells with KT5720, an inhibitor of PKA, significantly increased the lysosomal pH in MCSF-treated cells. Treatment of MCSF-treated cells with DIDS for 1 h caused a similar increase in the lysosomal pH (Figure 6B). These results suggest that activation of PKA and chloride channel activity play an important role in microglial lysosomal pH regulation under inflammatory stimulation.
Figure 6.
Lysosomal pH measurements in activated microglia in presence of PKA inhibitor and chloride channel blocker. Lysosomal pH was measured in treated and untreated microglia. For measurements with PKA inhibitor, KT 5720 at 10 μM was added to MCSF-treated microglia, and pH was measured 45 min after addition. For measurements in the presence of the chloride channel blocker DIDS at 1 mM was added to MCSF-treated microglia, and lysosomal pH was measured 60 min after the addition of DIDS. (A) Frequency distribution of lysosomal pH in MCSF-treated microglia (green curve), untreated microglia (red curve), and KT 5720-treated activated microglia (blue curve). (B) Frequency distribution of lysosomal pH in MCSF-treated activated microglia (green curve), untreated microglia (red curve), and DIDS-treated activated microglia (black curve). Lysosomal pH measurements were obtained from at least 750 lysosomes from three different fields. The average pH values from three different experiments from three different days are shown. Error bars represent the SEM.
DISCUSSION
Immunotherapy against Aβ leads to a striking removal of amyloid plaques in APP transgenic mice (Schenk et al., 1999), and there is good evidence that microglia play an important role in this clearance (Bard et al., 2000). However, the mechanisms by which immunization promotes clearance and degradation by microglia are uncertain. Recent studies have shown that activation of microglia in the absence of antibodies can also lead to the clearance of Aβ plaques in a mouse model of AD (Frenkel et al., 2005). This indicates that antibodies to Aβ are not necessary for clearance of plaques, but it has been unclear how microglial activation or inflammatory stimuli can lead to improved clearance.
The data presented herein show that increased acidification of lysosomes may be the key factor that determines the ability of microglia to degrade fAβ. Microglia constitutively express a scavenger receptor, SRA, that can mediate the binding and uptake of fAβ (El Khoury et al., 1996; Paresce et al., 1996). As shown here, microglia have sufficient levels of lysosomal proteases to degrade proteins effectively; moreover, the levels of most proteases are higher in microglia than in J774 cells, which can digest fAβ efficiently. However, microglia have weakly acidic lysosomes, which would decrease the activity of many lysosomal proteases and could lead to the inability to degrade fAβ effectively. Treatment with inflammatory agents acidifies microglial lysosomes and makes them able to degrade fAβ. Although treatment with inflammatory agents can cause many changes in microglia, it is striking that a pulse-wash treatment with a weak base that transiently acidifies lysosomes also facilitates significant degradation of fAβ. This suggests that lysosomal acidification is the key event that is required for fAβ degradation by microglia. This phenomenon of increased proteolytic power as a consequence of lysosomal acidification was also described in dendritic cells during their maturation (Trombetta et al., 2003).
In the assays of fAβ degradation with the ammonia pulse-wash or the forskolin treatment, we could only transiently acidify the lysosomes. In our experiments, the lysosomes were acidified four times for about an hour each time during a 72-h degradation assay. Nevertheless, we were able to observe ∼40% degradation of fAβ as a consequence of these treatments. We typically observe <40% degradation in a 4-h chase even with macrophage cells (data not shown). It is possible that some key proteolytic enzymes are converted from the proenzyme to the active enzyme during the periods of high acidity and that these enzymes remain active during periods of reduced acidity in the microglial lysosomes.
We explored the mechanisms of lysosomal acidification in microglia. Several signal transduction pathways are activated in cells in response to MCSF treatment (Pixley and Stanley, 2004). Activation of PKA by forskolin caused a drop in lysosomal pH similar to that seen in MCSF-treated cells. The acidification caused by forskolin was inhibited by treatment with DIDS, suggesting that anion transport was required for the acidification. These results would be consistent with activation of chloride channels downstream of PKA activation as a mechanism to lower the lysosomal pH in microglia. The lysosomal acidification in MCSF-treated microglia was partially reversed by inhibition of PKA or by treatment with DIDS, again indicating that PKA-dependent activation of chloride channels is important for the regulation of lysosomal acidification of microglia during inflammatory activation. Further work will be required to analyze the signaling pathways involved and to identify the regulated chloride channels on lysosomes of microglia.
There is evidence that immunization of AD model mice against Aβ leads to some reversal of behavioral impairments in addition to clearing amyloid deposits (Morgan et al., 2000; Hartman et al., 2005), and this suggests that this type of therapy might be beneficial. A major limitation is that inflammatory responses to immunization may be difficult to control, and a human trial of Aβ immunization had to be discontinued due to development of meningoencephalitis in some patients (Nicoll et al., 2003).
It is possible that treatments that cause a specific type of microglial activation, leading to increased lysosomal acidification, may be especially beneficial in promoting clearance of plaques. It should be noted that not all types of microglial activation are equally effective. We found that treatment with MCSF, which causes microglia to express macrophage-like surface proteins, was especially effective at lowering lysosomal pH and activating the degradation of fAβ. In contrast, it has been reported that interaction with CD40 ligand can induce microglia to become more like antigen-presenting cells with decreased ability to phagocytose Aβ (Townsend et al., 2005). Another report showed that activation of microglia via the IL-1 pathway might not be important for plaque clearance because IL-1 receptor knockout mouse cleared fAβ deposits after receiving immunotherapy (Das et al., 2006). Other factors may also modulate the ability of microglia to internalize and degrade amyloid plaques. A recent study found that only bone marrow derived microglia and not resident microglia were capable of clearing fAβ deposits from the brains of AD model mice (Simard et al., 2006).
In summary, we show that unactivated microglia do not degrade fAβ because they have weakly acidic lysosomes, and lysosomal acidification is a key downstream event leading to fAβ degradation by activated microglia. This may also be a key to the effectiveness of therapies to promote clearance of amyloid plaques by microglia, and treatments that acidify microglial lysosomes while minimizing other inflammatory reactions may be particularly beneficial.
ACKNOWLEDGMENTS
We thank Dr. Lori Tortorella for critically reading the manuscript. This work was supported by National Institutes of Health grants NS-34761 (to F.R.M.), DK-27083 (to F.R.M.), and DK-54317 (to P.L.).
Abbreviations used:
- DIDS
4,4′-diisothiocyanato-stilbene-2,2′-disulfonic acid
- fAβ
fibrillar Alzheimer amyloid β peptide
- IL
interleukin
- LPS
lipopolysaccharide
- MCSF
macrophage colony-stimulating factor
- PKA
protein kinase A.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-10-0975) on February 21, 2007.
REFERENCES
- Aronoff D. M., Canetti C., Serezani C. H., Luo M., Peters-Golden M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J. Immunol. 2005;174:595–599. doi: 10.4049/jimmunol.174.2.595. [DOI] [PubMed] [Google Scholar]
- Awano T., Katz M. L., O'Brien D. P., Sohar I., Lobel P., Coates J. R., Khan S., Johnson G. C., Giger U., Johnson G. S. A frame shift mutation in canine TPP1 (the ortholog of human CLN2) in a juvenile Dachshund with neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 2006;89:254–260. doi: 10.1016/j.ymgme.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Bae H. R., Verkman A. S. Protein kinase A regulates chloride conductance in endocytic vesicles from proximal tubule. Nature. 1990;348:637–639. doi: 10.1038/348637a0. [DOI] [PubMed] [Google Scholar]
- Bard F., et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Brazil M. I., Chung H., Maxfield F. R. Effects of incorporation of immunoglobulin G and complement component C1q on uptake and degradation of Alzheimer's disease amyloid fibrils by microglia. J. Biol. Chem. 2000;275:16941–16947. doi: 10.1074/jbc.M000937200. [DOI] [PubMed] [Google Scholar]
- Chomarat P., Banchereau J., Davoust J., Palucka A. K. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- Chung H., Brazil M. I., Soe T. T., Maxfield F. R. Uptake, degradation, and release of fibrillar and soluble forms of Alzheimer's amyloid beta-peptide by microglial cells. J. Biol. Chem. 1999;274:32301–32308. doi: 10.1074/jbc.274.45.32301. [DOI] [PubMed] [Google Scholar]
- Das P., Howard V., Loosbrock N., Dickson D., Murphy M. P., Golde T. E. Amyloid-beta immunization effectively reduces amyloid deposition in FcRgamma−/− knock-out mice. J. Neurosci. 2003;23:8532–8538. doi: 10.1523/JNEUROSCI.23-24-08532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P., Smithson L. A., Price R. W., Holloway V. M., Levites Y., Chakrabarty P., Golde T. E. Interleukin-1 receptor 1 knockout has no effect on amyloid deposition in Tg2576 mice and does not alter efficacy following Abeta immunotherapy. J. Neuroinflammation. 2006;3:17. doi: 10.1186/1742-2094-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K., Maxfield F. R. Ratio imaging instrumentation. Methods Cell Biol. 2003;72:389–413. doi: 10.1016/s0091-679x(03)72019-6. [DOI] [PubMed] [Google Scholar]
- Dunn K. W., Park J., Semrad C. E., Gelman D. L., Shevell T., McGraw T. E. Regulation of endocytic trafficking and acidification are independent of the cystic fibrosis transmembrane regulator. J. Biol. Chem. 1994;269:5336–5345. [PubMed] [Google Scholar]
- El Khoury J., Hickman S. E., Thomas C. A., Cao L., Silverstein S. C., Loike J. D. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- Frenkel D., Maron R., Burt D. S., Weiner H. L. Nasal vaccination with a proteosome-based adjuvant and glatiramer acetate clears beta-amyloid in a mouse model of Alzheimer disease. J. Clin. Investig. 2005;115:2423–2433. doi: 10.1172/JCI23241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman R. E., Izumi Y., Bales K. R., Paul S. M., Wozniak D. F., Holtzman D. M. Treatment with an amyloid-beta antibody ameliorates plaque load, learning deficits, and hippocampal long-term potentiation in a mouse model of Alzheimer's disease. J. Neurosci. 2005;25:6213–6220. doi: 10.1523/JNEUROSCI.0664-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herber D. L., Maloney J. L., Roth L. M., Freeman M. J., Morgan D., Gordon M. N. Diverse microglial responses after intrahippocampal administration of lipopolysaccharide. Glia. 2006;53:382–391. doi: 10.1002/glia.20272. [DOI] [PubMed] [Google Scholar]
- Herber D. L., Roth L. M., Wilson D., Wilson N., Mason J. E., Morgan D., Gordon M. N. Time-dependent reduction in Abeta levels after intracranial LPS administration in APP transgenic mice. Exp. Neurol. 2004;190:245–253. doi: 10.1016/j.expneurol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Jentsch T. J., Hubner C. A., Fuhrmann J. C. Ion channels: function unravelled by dysfunction. Nat. Cell Biol. 2004;6:1039–1047. doi: 10.1038/ncb1104-1039. [DOI] [PubMed] [Google Scholar]
- Majumdar A., Chung H., Dolios G., Wang R., Asamoah N., Lobel P., Maxfield F. R. Degradation of fibrillar forms of Alzheimer's amyloid beta-peptide by macrophages. Neurobiol. Aging. 2007 doi: 10.1016/j.neurobiolaging.2006.12.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsonego A., Weiner H. L. Immunotherapeutic approaches to Alzheimer's disease. Science. 2003;302:834–838. doi: 10.1126/science.1088469. [DOI] [PubMed] [Google Scholar]
- Morgan D., et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Morgan D., Gordon M. N., Tan J., Wilcock D., Rojiani A. M. Dynamic complexity of the microglial activation response in transgenic models of amyloid deposition: implications for Alzheimer therapeutics. J. Neuropathol. Exp. Neurol. 2005;64:743–753. doi: 10.1097/01.jnen.0000178444.33972.e0. [DOI] [PubMed] [Google Scholar]
- Nicoll J. A., Wilkinson D., Holmes C., Steart P., Markham H., Weller R. O. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat. Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Nishi T., Forgac M. The vacuolar (H+)-ATPases—nature's most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci. USA. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paresce D. M., Chung H., Maxfield F. R. Slow degradation of aggregates of the Alzheimer's disease amyloid β-protein by microglial cells. J. Biol. Chem. 1997;272:29390–29397. doi: 10.1074/jbc.272.46.29390. [DOI] [PubMed] [Google Scholar]
- Paresce D. M., Ghosh R., Maxfield F. R. Microglial cells internalize aggregates of the Alzheimer's Disease amyloid β-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Pillay C. S., Elliott E., Dennison C. Endolysosomal proteolysis and its regulation. Biochem. J. 2002;363:417–429. doi: 10.1042/0264-6021:3630417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pixley F. J., Stanley E. R. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Presley J. F., Mayor S., Dunn K. W., Johnson L. S., McGraw T. E., Maxfield F. R. The End2 mutation in CHO cells slows the exit of transferrin receptors from the recycling compartment but bulk membrane recycling is unaffected. J. Cell Biol. 1993;122:1231–1241. doi: 10.1083/jcb.122.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley J. F., Mayor S., McGraw T. E., Dunn K. W., Maxfield F. R. Bafilomycin A1 treatment retards transferrin receptor recycling more than bulk membrane recycling. J. Biol. Chem. 1997;272:13929–13936. doi: 10.1074/jbc.272.21.13929. [DOI] [PubMed] [Google Scholar]
- Santambrogio L., Belyanskaya S. L., Fischer F. R., Cipriani B., Brosnan C. F., Ricciardi-Castagnoli P., Stern L. J., Strominger J. L., Riese R. Developmental plasticity of CNS microglia. Proc. Natl. Acad. Sci. USA. 2001;98:6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D., et al. Immunization with amyloid-β attenuates Alzheimer disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Simard A. R., Soulet D., Gowing G., Julien J. P., Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Town T., Nikolic V., Tan J. The microglial “activation” continuum: from innate to adaptive responses. J. Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend K. P., Town T., Mori T., Lue L. F., Shytle D., Sanberg P. R., Morgan D., Fernandez F., Flavell R. A., Tan J. CD40 signaling regulates innate and adaptive activation of microglia in response to amyloid beta-peptide. Eur. J. Immunol. 2005;35:901–910. doi: 10.1002/eji.200425585. [DOI] [PubMed] [Google Scholar]
- Trombetta E. S., Ebersold M., Garrett W., Pypaert M., Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400–1403. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- Tyynela J., Sohar I., Sleat D. E., Gin R. M., Donnelly R. J., Baumann M., Haltia M., Lobel P. A mutation in the ovine cathepsin D gene causes a congenital lysosomal storage disease with profound neurodegeneration. EMBO J. 2000;19:2786–2792. doi: 10.1093/emboj/19.12.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J., Imaki H., Wang K. C., Wronska A., Osuchowski M., Rubenstein R. Origin and turnover of microglial cells in fibrillar plaques of APPsw transgenic mice. Acta Neuropathol. 2003;105:393–402. doi: 10.1007/s00401-002-0660-3. [DOI] [PubMed] [Google Scholar]
- Wegiel J., Wang K. C., Imaki H., Rubenstein R., Wronska A., Osuchowski M., Lipinski W. J., Walker L. C., LeVine H. The role of microglial cells and astrocytes in fibrillar plaque evolution in transgenic APP(SW) mice. Neurobiol. Aging. 2001;22:49–61. doi: 10.1016/s0197-4580(00)00181-0. [DOI] [PubMed] [Google Scholar]
- Wilson N. J., Cross M., Nguyen T., Hamilton J. A. cAMP inhibits CSF-1-stimulated tyrosine phosphorylation but augments CSF-1R-mediated macrophage differentiation and ERK activation. FEBS J. 2005;272:4141–4152. doi: 10.1111/j.1742-4658.2005.04826.x. [DOI] [PubMed] [Google Scholar]