Abstract
Cells require growth factors to support glucose metabolism for survival and growth. It is unclear, however, how noninsulin growth factors may regulate glucose uptake and glucose transporters. We show that the hematopoietic growth factor interleukin (IL)3, maintained the glucose transporter Glut1 on the cell surface and promoted Rab11a-dependent recycling of intracellular Glut1. IL3 required phosphatidylinositol-3 kinase activity to regulate Glut1 trafficking, and activated Akt was sufficient to maintain glucose uptake and surface Glut1 in the absence of IL3. To determine how Akt may regulate Glut1, we analyzed the role of Akt activation of mammalian target of rapamycin (mTOR)/regulatory associated protein of mTOR (RAPTOR) and inhibition of glycogen synthase kinase (GSK)3. Although Akt did not require mTOR/RAPTOR to maintain surface Glut1 levels, inhibition of mTOR/RAPTOR by rapamycin greatly diminished glucose uptake, suggesting Akt-stimulated mTOR/RAPTOR may promote Glut1 transporter activity. In contrast, inhibition of GSK3 did not affect Glut1 internalization but nevertheless maintained surface Glut1 levels in IL3-deprived cells, possibly via enhanced recycling of internalized Glut1. In addition, Akt attenuated Glut1 internalization through a GSK3-independent mechanism. These data demonstrate that intracellular trafficking of Glut1 is a regulated component of growth factor-stimulated glucose uptake and that Akt can promote Glut1 activity and recycling as well as prevent Glut1 internalization.
INTRODUCTION
Growth factors play critical roles to promote cell metabolism, sustain viability, and allow cell growth. When cells are deprived access to appropriate growth factors, a default pathway of cellular atrophy is activated in which glucose uptake and metabolism become sharply reduced, ultimately leading to cell death (Kan et al., 1994; Deshmukh et al., 1996; Rathmell et al., 2000, 2003; Edinger and Thompson, 2002; Ciofani and Zuniga-Pflucker, 2005). A variety of oncogenes, including Akt (Thompson and Thompson, 2004), BCR-Abl (Barnes et al., 2005), and c-Myc (Dang, 1999) also promote glucose metabolism. If glucose metabolism is not sustained by growth factors or oncogenes, developmental defects occur as rates of apoptosis increase (Moley and Mueckler, 2000; Heilig et al., 2003), immune responses fail as lymphocytes undergo apoptosis or produce inappropriate cytokines (Rathmell et al., 2000; Krauss et al., 2001; Cham and Gajewski, 2005), and cancer cells show enhanced apoptosis (Gottlob et al., 2001; Plas et al., 2001; Kansara and Berridge, 2004). The first and potentially critical regulatory step in glucose metabolism is glucose uptake. The mechanisms by which growth factors or oncogenes regulate glucose uptake, however, remain largely unresolved.
In hematopoietic cells, growth factors such as the cytokines interleukin (IL)3 or IL7 can provide critical signals to promote glucose uptake (Kan et al., 1994; Rathmell et al., 2001; Bentley et al., 2003). Glucose uptake is controlled via a family of facilitative glucose transporters that has been best characterized by the insulin-mediated regulation of Glut4 trafficking in muscle and adipose tissues (Watson et al., 2004). Hematopoietic cells instead rely on the ubiquitous glucose transporter Glut1 as a primary source for intracellular glucose (Rathmell et al., 2000). Glut1 synthesis is cytokine-dependent, with loss of Glut1 mRNA and surface protein levels when cells are cytokine-deprived (Rathmell et al., 2000; Vander Heiden et al., 2001; Bentley et al., 2003). Glut1 trafficking can also be regulated, because insulin signaling has been shown to promote Glut1 translocation to the cell surface (Piper et al., 1991). Although growth factor-stimulated synthesis of Glut1 has been shown to affect surface Glut1 levels in hematopoietic cells (Plas et al., 2002; Rathmell et al., 2003), a role for noninsulin growth factors in regulation of Glut1 activity and trafficking has not yet been directly determined.
Several mechanisms may promote cytokine-mediated regulation of Glut1 trafficking. First, phosphatidylinositol 3-kinase (PI3K) and its downstream effector kinase Akt/PKB have a well-established role in Glut4 vesicle trafficking to the cell membrane in response to insulin (Welsh et al., 2005). Likewise, the cytokines IL3 and IL7 can activate Akt in hematopoietic cells (Plas et al., 2002), and we have shown by immunofluorescence and biochemical fractionation that an activated and oncogenic form of Akt can promote accumulation of Glut1 on the surface of lymphoid cells even in the absence of cytokine (Plas et al., 2001; Rathmell et al., 2003). The mechanism of this regulation is unknown, but Akt may increase Glut1 protein synthesis (Barthel et al., 1999; Plas et al., 2001). Alternatively, Akt activation of mammalian target of rapamycin (mTOR)/regulatory associated protein of mTOR (RAPTOR) (mTOR/RAPTOR) has been shown to regulate trafficking of the transferrin receptor (TfR), low-density lipoprotein (LDL) receptor, and the amino acid transporter 4F2 (Edinger and Thompson, 2002). The Akt substrate glycogen synthase kinase (GSK3) may also regulate Glut1 trafficking, because it has been recently shown to control integrin recycling (Roberts et al., 2004).
To better understand how growth factor or oncogenic stimulation may promote glucose uptake, we have directly analyzed cytokine-mediated regulation of Glut1 in lymphoid/myeloid hematopoietic precursor cells. Here, we show that the hematopoietic growth factor IL3 promotes glucose uptake in part by promoting localization and retention of Glut1 on the cell surface. IL3 receptor engagement attenuated Glut1 internalization, and this activity required Rab11a to promote maximal surface levels, suggesting IL3 regulation of Glut1 endocytosis and recycling. IL3-induced activation of PI3K, and Akt was found to be necessary and sufficient to regulate Glut1 activity and trafficking. These data directly demonstrate that Glut1 activity, recycling, and internalization are regulated aspects of growth factor-stimulated glucose uptake in hematopoietic cells and implicate Akt as an important mediator of each of these processes.
MATERIALS AND METHODS
Plasmids
Exofacial-tagged rGlut1 was constructed by inserting a tandem repeat of a FLAG epitope tag (5′-GACTACAAAGACGATGACGACAAG-3′) into the first extracellular loop (between 55 and 56 amino acids) of rat Glut1 by pEF6/V5-His TOPO cloning (Invitrogen, Carlsbad, CA). An N-terminal green fluorescent protein (GFP)-tagged rGlut1 construct was made by GFP fusion with pCDNA3.1 NT-GFP TOPO cloning (Invitrogen). hBcl-xL was subcloned into pEF6/V5-His vector. The myristoylated Akt1 (myrAkt) plasmid was constructed by amplifying Akt1 from mouse cDNA and inserting the myristoylated sequence (5′-ATGGGGAGCAGCAAGAGCAAGCCCAAG-3′) to the 5′ end and cloning into pEF6/V5-His vector. The phospho-mimetic Akt1 mutant (AktDD) was made with S473D and T308D mutations in the pEF6/V5-His Akt1 vector. pEGFP-C2-Rab11aS25N and pEGFP-C2-Rme1G429 constructs were generously provided by Dr. Michael Ehlers (Duke University, Durham, NC). pCMV-FLAG GSK3β S9A was generously provided by Dr. Xiao-Fan Wang (Duke University).
Cells
The early hematopoietic myeloid/lymphoid cell line FL5.12 was cultured as described previously (Vander Heiden et al., 1997) with addition of recombinant murine IL3 (500 pg/ml; Peprotech, Rocky Hill, NJ). Inhibitors included the following: for protein synthesis, 10 μg/ml cycloheximide (CHX) (Sigma-Aldrich, St. Louis, MO); for PI3K, 10 μM LY294002 (Calbiochem, San Diego, CA); for mTOR/RAPTOR, 25 nM rapamycin (Calbiochem); and for GSK3, 10 μM SB216763, 20 μM SB415286, or 10 μM AR-A0144-18 (Sigma-Aldrich). Cells with inducible myrAkt have been described previously (Plas et al., 2001) or were generated by transient transfection of FL5.12 cells with myrAkt1 in pEF6.
2-Deoxy-d-glucose (2-DOG) Transport Assay
Glucose uptake was measured as described previously with small modifications (Bentley et al., 2003; Rathmell et al., 2003). Cells were washed and resuspensed in Krebs-Ringer-HEPES (KRH) (at pH 7.4, 136 mM NaCl, 4.7 mM KCl, 1.25 mM CaCl2, 1.25 mM MgSO4, and 10 mM HEPES). 2-Deoxy-d-[H3]glucose (2 μCi/reaction) was added for a period of either 1 or 5 min at 37°C. Reactions were quenched by addition of ice-cold 200 μM phloretin (Calbiochem) and centrifugation through an oil layer (1:1 Dow Corning 550 Silicon fluid [Motion Industries, Birmingham, AL] and dinonyl phthalate [Sigma-Aldrich]). The cell pellet was washed and solubilized in 1 M NaOH, and radioactivity was measured with scintillation counter.
Surface Glut1 Measurement and Flow Cytometry
Cells were analyzed by a FACscan (BD Biosciences, San Jose, CA) and FlowJo software (Tree Star, Inc., Ashland, OR). To determine FLAG surface expression, cells were washed once in phosphate-buffered saline (PBS)/2% fetal bovine serum (FBS). Cells were blocked with anti-FcγIII/II (BD Biosciences PharMingen, San Diego, CA) and incubated with 5% rat serum and rabbit anti-FLAG (Sigma-Aldrich) followed by R-Phycoerythrin donkey anti-rabbit (Jackson ImmunoResearch, West Grove, PA) for analysis. Surface FLAG-Glut1 levels were normalized to total FLAG expression by division of the mean fluorescence of surface FLAG by the average pixel density from respective immunoblot. To determine TfR (CD71) surface expression, cells were incubated with anti-mouse CD71-PE (BD Biosciences PharMingen). For pulse-chase assays, cells were blocked with anti-FcγIII/II (BD Biosciences PharMingen) for 5 min followed by a pulse with anti-FLAG antibody (Sigma-Aldrich) at room temperature for 10 min, washed, and cultured for various periods at 37°C before addition of secondary stain on ice. Mean fluorescence of cell surface Glut1 levels were normalized to the starting value.
Fluorescence Microscopy
Cells were transfected with GFP-Glut1 and fixed with 1% paraformaldehyde in PBS and viewed with a Zeiss LSM410 confocal microscope (Carl Zeiss, Thornwood, NY) and MetaMorph software (Molecular Devices, Sunnyvale, CA).
Immunoblotting
Cells were lysed for Western blotting for 1 h on ice in 1% Triton and 0.1% SDS in PBS with protease inhibitors (BD Biosciences PharMingen) and precleared by centrifugation. Equivalent protein concentrations were loaded on a 4–15% SDS-polyacrylamide gel electrophoresis gel (Bio-Rad, Hercules, CA). Primary antibodies used were anti-FLAG M2 peroxidase (Sigma-Aldrich), rabbit anti-Glut1 (Abcam, Cambridge, MA), mouse anti-Akt1 (Cell Signaling Technology, Beverly, MA), rabbit anti-phospho Akt ser473 (Cell Signaling Technology), rabbit anti-phospho-mTOR ser2448 (Cell Signaling Technology), rabbit anti-phospho GSK3 α/β (Cell Signaling Technology), rabbit anti-Bcl-2 (BD Biosciences PharMingen), and mouse anti-actin (Sigma-Aldrich). Secondary antibodies used were anti-rabbit horseradish peroxidase (HRP) (Cell Signaling Technology), anti-mouse HRP (BD Biosciences PharMingen), Alexa Fluor 680 anti-rabbit IgG (Invitrogen), and IR Dye 800 anti-mouse IgG (LI-COR Biosciences, Lincoln, NE). Secondary antibodies consisting of HRP were viewed by ECL-Plus (GE Healthcare, Little Chalfont,, Buckinghamshire, United Kingdom), and secondary antibodies conjugated with fluorophores were scanned with LI-COR Odyessy (LI-COR Biosciences).
RESULTS
IL3 Promotes Glucose Uptake and Trafficking of Glut1 to the Cell Surface
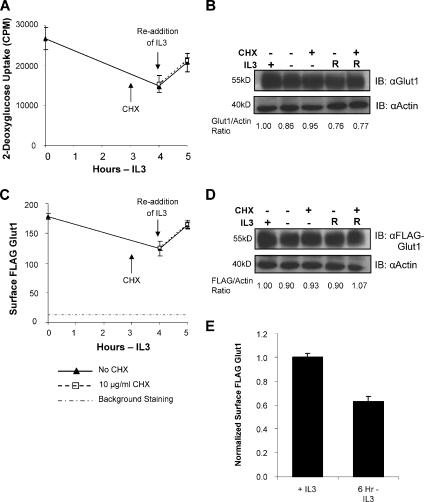
The cytokine IL3 regulates both total cellular expression and cell surface levels of Glut1 in the lymphoid cell line FL5.12 (Rathmell et al., 2003). It was unclear, however, whether cell surface Glut1 levels were regulated strictly via protein synthesis and total Glut1 levels or whether IL3 could also regulate the intracellular trafficking of Glut1. To address this question, we analyzed the ability of IL3 to regulate glucose uptake and Glut1 trafficking. Uptake of the radiolabeled glucose analogue 2-DOG was measured in the presence of IL3, after a 4-h withdrawal from IL3 and after 1 h with IL3 returned (Figure 1A). On withdrawal from IL3, glucose uptake decreased and readdition of IL3 after 4 h rapidly restored glucose uptake. Rescue of glucose uptake by readdition of IL3 did not seem to be due to the synthesis of new glucose transporters or associated proteins. Addition of the protein synthesis inhibitor CHX 1 h before readdition of IL3 failed to affect the restoration of glucose uptake by IL3. Endogenous Glut1 levels remain unchanged during the withdrawal and readdition of IL3, further lending support for glucose uptake regulation independent of changes in total levels of Glut1 (Figure 1B).
Figure 1.
IL3 regulates intracellular trafficking of Glut1 to the cell surface independent of new protein synthesis. (A) Glucose uptake was measured in FL5.12 cells in IL3 or cultured in IL3-free media for 4 h followed by 1 h with IL3 restored. CHX at 10 μg/ml was added to some samples at 3 h. (B) Total endogenous Glut1 expression was measured by immunoblot with actin as a loading control (R, IL3 restored). (C) FL5.12 cells expressing exofacially FLAG-tagged Glut1 were cultured as described in A. Mean cell surface fluorescence for FLAG-Glut1 was determined at indicated times. (D) Total FLAG-Glut1 levels were analyzed by immunoblot with actin as a loading control (R, IL3 restored). (E) Cell surface FLAG-Glut1 was normalized to total FLAG expression. Standard deviations of triplicate samples are shown. Total levels of Glut1 and FLAG were normalized to actin and are shown below the respective immunoblots. Representative results are shown for three or more repeated experiments.
Protein synthesis-independent IL3 regulation of glucose uptake may occur via increased transport activity of surface transporters (Levine et al., 1998) or trafficking of intracellular Glut1 proteins to the cell surface. To distinguish between transport activity and Glut1 intracellular trafficking, we constructed an exofacially tandem FLAG epitope-tagged Glut1 for direct analysis of cell surface Glut1 protein levels independently of transporter activity. This tagged Glut1 protein was functional (data not shown) and could be readily detected and quantified on the surface of live cells by flow cytometry. Cells expressing exofacial FLAG-tagged Glut1 were cultured in IL3 or withdrawn for 4 h followed by readdition of IL3 (Figure 1C). Similar to regulation of glucose uptake, IL3 was capable of regulating Glut1 cell surface levels independently of new protein synthesis. Total levels of stably expressed FLAG-Glut1 remained unchanged when cells were withdrawn from IL3 followed by readdition of the cytokine (Figure 1D), suggesting that changes in Glut1 surface levels were not due to loss of Glut1 expression. Normalizing surface FLAG-Glut1 levels to total cellular FLAG-Glut1 expression demonstrated a true drop in the proportion of surface FLAG-Glut1 after a 6-h growth factor-withdrawal (Figure 1E). These data indicate that in addition to regulating Glut1 synthesis (Vander Heiden et al., 2001; Rathmell et al., 2003), cytokines, such as IL3, can promote Glut1 trafficking to the cell surface in lymphoid cells.
Glut1 Internalization and Recycling Are Growth Factor Regulated
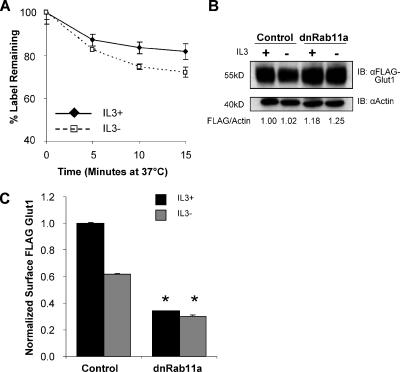
In addition to synthesis of new Glut1 protein, IL3 may regulate Glut1 surface levels through effects on Glut1 internalization or recycling of internalized Glut1 back to the cell surface. To begin to dissect how IL3 may regulate Glut1 surface levels, we performed a pulse-chase assay to directly measure the internalization of labeled Glut1 proteins by flow cytometry. Cells stably expressing FLAG-Glut1 were cultured in the presence or absence of IL3, stained with anti-FLAG antibody, and washed free of unbound antibody. Remaining surface Glut1 staining was then measured by addition of secondary antibody after various times in culture. Cell surface FLAG-Glut1 staining at each time point was normalized to the level of staining at the starting time point to determine the percentage of labeled Glut1 protein that was retained on the cell surface over time (Figure 2A). Surface Glut1 decreased more rapidly in cytokine-deprived cells, suggesting that IL3 regulated Glut1 internalization in addition to possible effects on Glut1 efflux to the cell surface.
Figure 2.
Growth factor attenuates Glut1 internalization and promotes Rab11a-dependent recycling of intracellular Glut1. (A) FLAG-Glut1 cells were cultured in IL3 or withdrawn from IL3 for 6 h. Cells were stained with anti-FLAG antibody, washed, and cultured at 37°C for indicated times before staining with fluorescent secondary antibody. (B and C) FLAG-Glut1 cells were transiently transfected with control or dominant-negative Rab11a (Rab11aS25N/dnRab11a) vector and cultured in IL3 or in the absence of IL3 for 6 h. (B) Total FLAG-Glut1 expression levels were measured by immunoblot with actin as a loading control. Total levels of FLAG were normalized to actin and are shown below the immunoblot. (C) Surface FLAG-Glut1 levels were measured by flow cytometry and normalized to total FLAG expression. Mean and SD of triplicate samples are shown. Asterisk (*) indicates p < 0.005 within the experiment.
Rab GTPases are key regulators of intracellular vesicle trafficking (Maxfield and McGraw, 2004), and Rab11a has been specifically implicated in endosomal recycling (Ullrich et al., 1996). To determine whether recycling of Glut1 was also growth factor regulated, dominant-negative Rab11a (dnRab11a; Rab11a S25N) was expressed, and total cellular FLAG-Glut1 levels were measured by immunoblot (Figure 2B). Blockade of Rab11a function did not result in FLAG-Glut1 degradation; rather, FLAG-Glut1 levels were modestly increased. Despite this increase in Glut1 protein levels, dominant-negative Rab11a lead to decreased surface FLAG-Glut1 both in the presence and absence of IL3 (Figure 2C), suggesting that Glut1 was accumulating intracellularly. Consistent with this finding, expression of a dominant-negative Rme1 (Rme1G429R), also implicated in endosomal recycling (Lin et al., 2001), decreased surface Glut1 levels similar to those observed after expression of dnRab11a (data not shown). Recycling of internalized Glut1 seems, therefore, to play a key role for IL3 to maintain maximal surface Glut1 levels. Together, these results indicate that the presence of IL3 both attenuates Glut1 internalization and promotes trafficking of internalized Glut1 back to the cell surface in a Rab11a-dependent manner.
Cytokine-mediated Activation of the PI3K Pathway Is Necessary to Maintain Maximal Cell Surface Levels of Glut1
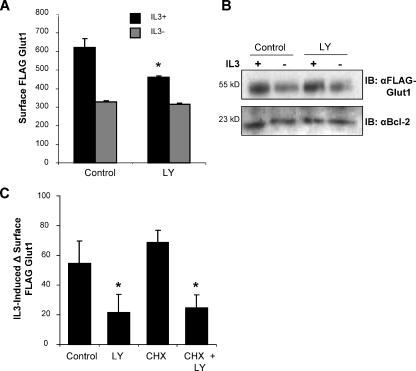
IL3 may regulate Glut1 trafficking through a number of signaling pathways (Fox et al., 2003; Rathmell et al., 2003). Among these, activation of the PI3K pathway and its downstream effector kinase Akt have been shown to promote cell surface trafficking of Glut4 (Welsh et al., 2005) and to increase expression and cell surface levels of Glut1 (Piper et al., 1991) in response to insulin stimulation. We found that endogenous PI3K pathway activity was necessary for growth factor to stimulate maximal cell surface Glut1, because treatment of FLAG-Glut1–expressing cells with the PI3K inhibitor LY294002 reduced surface Glut1 levels in the presence of IL3 (Figure 3A). No difference in surface FLAG-Glut1 levels was observed when LY294002 was added at the time of IL3-withdrawal, an occasion when endogenous PI3K was inactive. Importantly, LY294002 had no effect on total levels of FLAG-Glut1 protein at these time points, suggesting that LY294002 was not altering surface Glut1 levels via changes in Glut1 synthesis or degradation (Figure 3B). Rather, inhibition of PI3K seemed to lead to a failure of IL3 to promote Glut1 protein localization to the cell surface.
Figure 3.
PI3K activity is required to promote Glut1 cell surface localization. FLAG-Glut1–expressing cells were cultured in the presence or absence of IL3 for 8 h with a vehicle control or 10 μM LY294002. (A) Cells were stained with a FLAG antibody, and mean surface levels were analyzed with flow cytometry. (B) Total FLAG expression was measured by immunoblot and Bcl-2 expression was measured for loading control. (C) FLAG-Glut1 cells were cultured in the absence of IL3 for 3 h. Vehicle control, 10 μM LY294002, and 10 μg/ml CHX were added 1 h before readdition of IL3. After an additional hour, cells were stained with FLAG antibody, and surface levels of FLAG-Glut1 were compared before and after IL3 add-back by flow cytometry. The differences in mean fluorescence of surface FLAG-Glut1 before and after IL3 readdition are shown. Mean fluorescence and SD of triplicate samples are shown. Asterisk (*) indicates p value <0.05 within the experiment. Representative results are shown for three or more repeated experiments.
PI3K and Akt are important regulators of protein synthesis (Kandel and Hay, 1999) that may affect glucose uptake through regulation of Glut1 protein levels and/or trafficking. To further characterize protein synthesis-independent regulation of FLAG-Glut1 surface levels via PI3K, we analyzed the rescue of surface FLAG-Glut1 levels in growth factor-withdrawn cells after readdition of IL3 either in the presence or absence of LY294002 and CHX. Inhibition of PI3K decreased the amount of FLAG-Glut1 that was returned to the surface compared with the vehicle control 1 h after readdition of IL3 (Figure 3C). This effect was not due to the inhibition of new Glut1 protein synthesis, because treatment of cells with CHX had no effect on either the rescue of surface Glut1 by IL3 or the ability of LY294002 to prevent this rescue (Figure 3C). Together, these data directly demonstrate that the PI3K pathway is critical in IL3-stimulated trafficking of Glut1 to the cell surface independent of new protein synthesis.
Activated Akt Promotes Glucose Uptake and Maintains Surface Glut1 upon Growth Factor Withdrawal
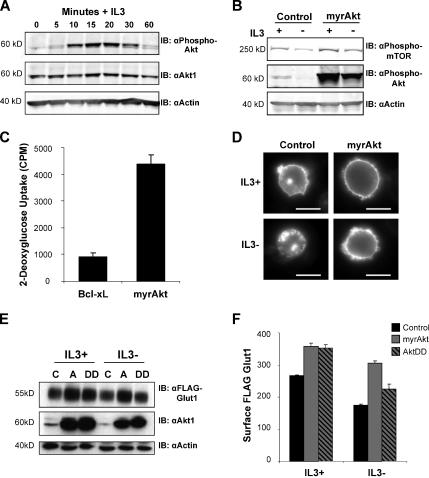
Akt is a major mediator of many PI3K-induced signals that can promote both glucose metabolism and Glut1 expression (Barthel et al., 1999; Plas et al., 2001). We next sought to characterize IL3 activation of Akt and to determine whether Akt could also regulate glucose uptake in hematopoietic cells. Stimulation of growth factor-deprived FL5.12 cells with IL3 induced a robust initial phosphorylation of endogenous Akt followed by a lower chronic level of phospho-Akt that became apparent between 30 and 60 min after IL3 addition (Figure 4A). Transient transfection of FL5.12 cells with a myristoylated and constitutively active oncogenic form of Akt (myrAkt) mimicked initial high levels of IL3-induced Akt phosphorylation both in the presence and absence of IL3 (Figure 4B). Phospho-Akt levels were indicative of Akt activity, because mTOR phosphorylation was enhanced in the presence of IL3 and expression of myrAkt was able to maintain this phosphorylation even in the absence of IL3.
Figure 4.
Activated Akt regulates Glut1 trafficking to maintain surface Glut1 levels after IL3 withdrawal. (A) FL5.12 cells were withdrawn from IL3 for 6 h, restimulated with IL3 for various times, and immunoblotted for phospho-Akt, total Akt1 and actin. (B) Cells were transiently transfected with control or myrAkt vectors and cultured in the presence or absence of IL3 for 6 h. Phosphorylation levels of both mTOR and Akt were analyzed by immunoblot with actin as a loading control. (C) FL5.12 cells were transfected with either Bcl-xL or myrAkt expression vectors and cultured in the presence of IL3 for 18 h followed by a growth factor-withdrawal for 24 h and glucose uptake was measured. (D) Control or myrAkt-expressing cells were transfected with GFP-Glut1, and then they were cultured in the presence or absence of IL3 for 8 h, and GFP was visualized by fluorescence microscopy. Bar, 10 μm. (E and F) FLAG-Glut1 cells were transiently transfected with control vector (C), myrAkt (A), or a phospho-mimetic aspartate mutant of Akt (DD). Cells were cultured in the presence and absence of IL3 for 6 h. (E) Total FLAG-Glut1 and Akt1 levels were analyzed by immunoblot with actin as a loading control. (F) Surface levels of FLAG-Glut1 were measured by flow cytometry. Standard deviations of triplicate samples are shown. Representative results are shown for three or more repeated experiments.
Activated Akt maintained glucose uptake of IL3-withdrawn cells (Figure 4C) and may have done so by regulation of Glut1 trafficking. To determine whether activated Akt was sufficient to regulate Glut1 trafficking in the absence of other cytokine-initiated signal transduction activity, we observed Glut1 localization in control cells or cells expressing myrAkt. Glut1 with an N-terminal GFP tag was expressed in control and myrAkt-expressing cells, and GFP-Glut1 localization was observed by fluorescence microscopy in the presence of IL3 or 8 h after IL3 withdrawal (Figure 4D). In control cells, GFP-Glut1 seemed to be on the cell surface in the presence of IL3, but it was predominantly intracellular upon IL3 withdrawal. The myrAkt-expressing cells, however, seemed to maintain high levels of surface GFP-Glut1 in both the presence and absence of IL3. To directly measure how Akt may impact levels of cell surface Glut1, cells stably expressing FLAG-Glut1 were transfected with constitutively active myristoylated (myrAkt) or phospho-mimetic (S473D/T308D; AktDD) forms of Akt1. These forms of Akt have been shown previously to regulate Glut4 trafficking (Cong et al., 1997; Foran et al., 1999). Expression of Akt1 and FLAG-Glut1 were shown by immunoblot (Figure 4E), and flow cytometric analyses showed that both active Akt proteins could increase total surface Glut1 levels in the presence and absence of IL3 (Figure 4F). Consistent with the more potent ability of myrAkt to prevent cell death of IL3 withdrawn cells (data not shown), myrAkt better maintained surface Glut1 when cells were deprived of IL3. Together, these data indicate that in addition to Glut1 synthesis (Barthel et al., 1999; Plas et al., 2002), Akt is able to promote glucose uptake and Glut1 surface localization even in the absence of IL3.
Akt Activation of mTOR/RAPTOR Does Not Affect Glut1 Trafficking but Does Regulate Glut1 Transporter Activity
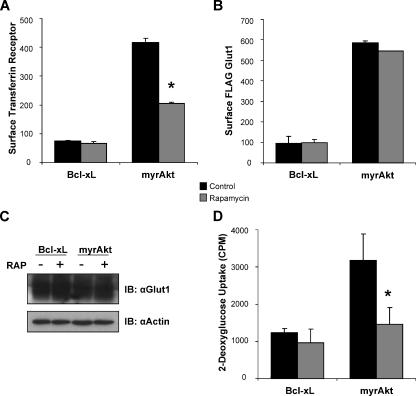
To determine the mechanism of Akt-mediated regulation of glucose uptake and Glut1, we next analyzed the role of specific Akt substrates on Glut1. Akt has previously been shown to affect the localization of several nutrient transporters in hematopoietic cells, including the TfR. Regulation of surface TfR levels by Akt was rapamycin sensitive and dependent on the mTOR/RAPTOR complex (Edinger and Thompson, 2002). We sought, therefore, to determine whether the Akt pathway for regulation of Glut1 localization in hematopoietic cells was regulated by mTOR/RAPTOR. Previously, myrAkt regulation of TfR had been most clearly demonstrated after 1-d IL3 withdrawal (Edinger and Thompson, 2002). To prevent cell death and allow analysis of surface FLAG-Glut1 at this time point for best comparison with regulation of TfR, the antiapoptotic protein Bcl-xL was expressed in control cells. The myrAkt itself was antiapoptotic and prevented cell death similar to Bcl-xL (data not shown). Bcl-xL expression had no effect on TfR or Glut1 trafficking in cytokine withdrawal (Rathmell et al., 2000; Edinger and Thompson, 2002). Surface levels of both TfR and Glut1 were reduced significantly in IL3-withdrawn Bcl-xL-expressing cells compared with cells that expressed myrAkt, which maintained high levels of both TfR and Glut1 in IL3 withdrawal (Figure 5, A and B). Levels of surface TfR and Glut1 in myrAkt expressing cells were comparable with levels observed in the presence of IL3 (data not shown). As reported, treatment of myrAkt cells with rapamycin led to a substantial decrease in surface TfR levels (Figure 5A) (Edinger and Thompson, 2002). Surface and total levels of FLAG-Glut1, however, were unaffected by rapamycin (Figure 5, B and C). These data show that Akt-mediated regulation of both surface and total Glut1 levels is mTOR/RAPTOR independent and is mediated by a pathway distinct from that of TfR.
Figure 5.
mTOR/RAPTOR does not regulate Glut1 cell surface localization but promotes Glut1 activity. (A and B) FL5.12 cells stably expressing FLAG-Glut1 were transfected with either Bcl-xL or myrAkt expression vectors and cultured in the presence of IL3 for 18 h. A vehicle control or 25 nM rapamycin was added at the time of growth factor withdrawal, and cells were cultured in the absence of IL3 for an additional 24 h. Mean surface levels of TfR (A) and FLAG-Glut1 (B) levels were analyzed with flow cytometry. (C and D) FL5.12 cells were transfected and cultured as described in A. Total endogenous Glut1 was measured via immunoblot with actin as a loading control (C), and glucose uptake was measured (D). Mean and SD of triplicate samples are shown. Asterisk (*) indicates p < 0.05 within the experiment. Representative results are shown for three or more repeated experiments.
Glucose uptake is controlled by both surface levels of glucose transporters and the activity of those transporters. Due to the central role of mTOR in regulation of nutrient uptake and use, we sought to determine whether mTOR/RAPTOR may influence Glut1 transporter activity rather than trafficking. Bcl-xL- and myrAkt-expressing FL5.12 cells were deprived of growth factor and treated with rapamycin, and glucose uptake was measured. Although surface FLAG-Glut1 and total Glut1 levels were unaffected, glucose uptake was significantly reduced (p < 0.05) by rapamycin (Figure 5, C and D). Together, these data indicate that although not directly able to regulate Glut1 surface localization or Glut1 protein levels, mTOR/RAPTOR seems to regulate glucose uptake by promoting Glut1 transporter activity.
Inactivation of GSK3 Promotes Glut1 Cell Surface Localization but Does Not Alter Glut1 Internalization
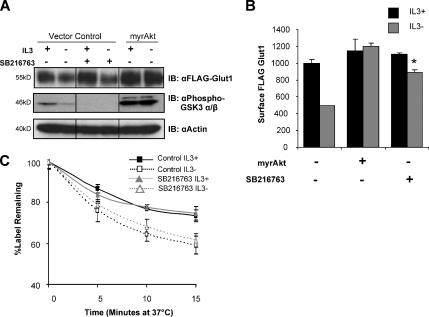
Another important Akt substrate that may affect Glut1 trafficking is GSK3. In this case, Akt phosphorylation of GSK3 leads to inhibition rather than activation of kinase activity (Jope and Johnson, 2004). Because GSK3 has recently been shown to inhibit recycling of internalized αvβ3 and α5β1 integrins back to the cell surface (Roberts et al., 2004), we sought to determine whether Akt-mediated inhibition of GSK3 may play a role to increase surface Glut1 levels. Stimulation of cells with IL3 led to inhibitory phosphorylation of GSK3 and expression of myrAkt was sufficient to sustain this inhibitory phosphorylation even in the absence of IL3 (Figure 6A). Treatment of cells with the GSK3 inhibitor compound SB216763 both prevented this phosphorylation and prevented GSK3 kinase activity. Inhibition of GSK3 did not affect total cellular expression of FLAG-Glut1 (Figure 6A). Similar to expression of myrAkt, treatment of cells with the GSK3 inhibitor during IL3-withdrawal did, however, largely maintain Glut1 cell surface levels (Figure 6B; p < 0.005). Similar results also were obtained with the GSK3 inhibitors SB415286 and AR-A0144-18 (data not shown).
Figure 6.
GSK3 activity promotes Glut1 surface localization, but it does not affect internalization. (A and B) FLAG-Glut1–expressing cells were transfected with control or myrAkt vectors. Cells were treated with a vehicle control or 10 μM SB216763 for 12 h before they were cultured in the presence or absence of IL3 for an additional 6 h. (A) Total levels of FLAG-Glut1 and phospho-GSK3 α/β were measured by immunoblot with actin as a loading control. Lanes are shown from the same blot and were uniformly contrasted and digitally rearranged (marked by black line) for ease of viewing. (B) Mean surface levels of FLAG-Glut1 were analyzed by flow cytometry. (C) Cells stably expressing FLAG-Glut1 were treated and cultured as described in A and B. Cells were stained with anti-FLAG antibody, washed, and cultured at 37°C for indicated times before staining with fluorescent secondary antibody. Mean and SD for triplicate samples are shown. Asterisk (*) indicates p < 0.005 within the experiment. Representative results are shown for three or more repeated experiments.
IL3 both attenuates Glut1 internalization and promotes Glut1 recycling (Figure 2, A and C). To determine which step of Glut1 trafficking may be regulated by GSK3, the effects of GSK3 inhibitors on Glut1 internalization were measured. FLAG-Glut1–expressing cells were treated with SB216763, and then they were cultured in the presence or absence of IL3, stained with anti-FLAG antibody, and washed free of unbound antibody. Remaining surface Glut1 staining was measured by addition of secondary antibody after various times in culture. Cell surface FLAG-Glut1 staining at each time point was normalized to the level of staining at the starting time point to determine percentage of labeled Glut1 protein that was retained on the cell surface over time (Figure 6C). Although cytokine-deprivation lead to increased GSK3 activity (Figure 6A) and more rapid Glut1 internalization of untreated cells (Figures 2A and 6C), treatment with the GSK3 inhibitor failed to maintain surface Glut1. Together, these data indicate that inhibition of GSK3 may play a prominent role in cytokine and Akt-mediated regulation of Glut1 surface localization but not via control of Glut1 internalization. Rather, GSK3 likely regulated Glut1 recycling similar to GSK3 regulation of integrin recycling (Roberts et al., 2004).
Akt Promotes Glut1 Surface Localization via Attenuating Glut1 Internalization in Absence of Growth Factor
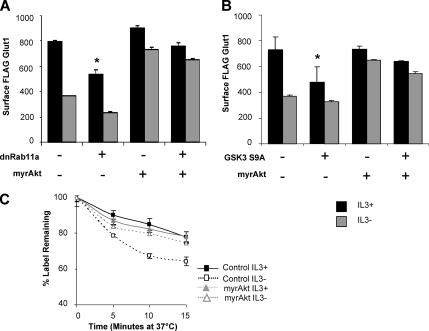
In addition to promoting recycling of internalized Glut1 through inhibition of GSK3, Akt may also regulate Glut1 endocytosis. We sought to determine, therefore, whether Akt required efficient Rab11a-mediated recycling and inhibition of GSK3 to maintain surface Glut1. Cells stably expressing FLAG-Glut1 were transiently transfected with control, myrAkt, dnRab11a, or both myrAkt and dnRab11a. Surface FLAG-Glut1 levels were measured by flow cytometry after a 6-h growth factor withdrawal (Figure 7A). The myrAkt maintained surface expression of FLAG-Glut1 in the absence of IL3 even when endocytic recycling of Glut1 was blocked with dnRab11a. Furthermore, myrAkt was capable of largely maintaining surface Glut1 even in the presence of activated GSK3 (Figure 7B). Transfection of constitutively active form of GSK3 (GSK3 S9A) reduced surface levels of FLAG-Glut1 in control cells even in the presence of IL3 (p < 0.05). In cells expressing myrAkt, however, GSK3 S9A caused only a modest decrease in surface Glut1 that was maintained upon cytokine withdrawal. This suggested that myrAkt regulated Glut1 through pathways in addition to and upstream or dominant to those regulated by GSK3, such as would occur if Akt also prevented Glut1 internalization. To directly test whether activated Akt could prevent Glut1 endocytosis upon growth factor withdrawal, we used a pulse-chase assay to directly measure internalization of Glut1. Cells stably expressing FLAG-Glut1 were transfected with either control or myrAkt and cultured in the presence or absence of IL3 for 6 h followed by the pulse-chase assay (Figure 7C). Indeed, myrAkt was sufficient to attenuate Glut1 internalization in the absence of IL3. Together, these data indicate that Akt promotes maximal levels of surface Glut1 by both attenuating endocytosis and promoting recycling of Glut1.
Figure 7.
Akt prevents internalization of Glut1 upon growth factor withdrawal independently of recycling. (A) FLAG-Glut1 cells were transiently transfected with control, dominant-negative Rab11a (Rab11aS25N, dnRab11a), and/or myrAkt vectors. Cells were cultured in IL3 or were withdrawn from IL3 for 6 h, and mean surface FLAG-Glut1 fluorescence levels were measured. (B) FLAG-Glut1 cells were transfected with control, constitutively active GSK3 (GSK3 S9A) and/or myrAkt constructs for 18 h and then cultured in the presence or absence of IL3 for an additional 6 h. Surface FLAG-Glut1 was measured by flow cytometry. (C) Cells stably expressing FLAG-Glut1 were transfected with control or myrAkt vectors and cultured in the presence of absence of IL3 for 6 h. Cells were stained with anti-FLAG antibody, washed, and cultured at 37°C for indicated times before staining with fluorescent secondary antibody. Mean and SD for triplicate samples are shown. Asterisk (*) indicates p < 0.05 within the experiment. Representative results are shown of three or more repeated experiments.
DISCUSSION
It has been previously shown that Glut1 synthesis and glucose uptake are dependent on cytokine growth factors in hematopoietic cells (Rathmell et al., 2001; Vander Heiden et al., 2001; Frauwirth and Thompson, 2004). In addition, glucose uptake and metabolism are often greatly increased in cancer cells, with Glut1 overexpression occurring frequently (Warburg, 1956; Macheda et al., 2005). Direct evidence for regulation of glucose uptake and Glut1 trafficking by noninsulin growth factors or oncogenes, however, has been lacking. We show here using direct protein trafficking assays, including cell surface and pulse-chase staining assays, that the hematopoietic growth factor IL3 and the oncogene Akt can regulate trafficking of Glut1 to the cell surface, independently of protein synthesis. IL3 regulated Glut1 through PI3K and its downstream effector, Akt via activation of mTOR, inactivation of GSK3, and other pathways to control Glut1 activity, recycling, and internalization.
In insulin-responsive tissues, Glut4 trafficking has been widely described to regulate acute glucose uptake, and although some aspects of Glut1 regulation described here may be shared with Glut4, others are distinct. Glut4 has been known to undergo rapid translocation from intracellular storage vesicles to the cell surface in response to insulin in insulin-responsive tissues (Welsh et al., 2005). Glut1 also responds to insulin by accumulation on the cell surface in these cell types, albeit to a lower extent and at a much slower rate than Glut4 (Piper et al., 1991). Akt regulates surface trafficking of both Glut4 and Glut1, with Akt2-mediated phosphorylation of AS160 (Zeigerer et al., 2004; Larance et al., 2005; Gonzalez and McGraw, 2006) critical to promote Glut4 translocation. Mechanisms by which insulin-stimulated Akt activity may regulate Glut1 are less certain, but they may include mechanisms described here. Our results demonstrate that Glut1 cell surface trafficking is not unique to insulin; it also occurs in response to growth factor and oncogenic stimulation in noninsulin-responsive tissues. A key difference between IL3-mediated trafficking of Glut1 to the cell surface and insulin-induced Glut4 trafficking in insulin-responsive tissues is that Glut4 is present in storage vesicles poised to respond to insulin and traffic to the cell surface. Glut1, in contrast, must be synthesized for delivery to the cell surface or returned to the cell surface through a recycling pool. Thus, Glut4 translocation provides for rapid glucose uptake for acute use, whereas Glut1 trafficking is slower and allows glucose uptake in chronic conditions, such as required for cell growth. Because both Glut1 and Glut4 are insulin responsive, defining shared and distinct mechanisms in regulation of Glut1 and Glut4 trafficking will aid in understanding insulin-regulated glucose homeostasis.
In addition to promoting Glut1 expression (Barthel et al., 1999; Plas et al., 2001), we provide direct evidence with cell surface staining that Akt maintains Glut1 protein on the surface even upon growth factor withdrawal. Akt has also been shown to promote surface accumulation of nutrient transporters such as amino acid, LDL, and transferrin receptors (Edinger and Thompson, 2002). In these cases, the inhibitor of the mTOR/RAPTOR complex, rapamycin, blocked Akt-mediated regulation of nutrient transporter localization. In contrast, our results with IL3 stimulation and Akt are similar to what has been reported in 3T3-L1 adipocytes (Tremblay et al., 2005), where insulin-stimulated Glut1 translocation to cell surface was reported to be insensitive to rapamycin treatment (Figure 5B). These data indicate that Akt regulates Glut1 surface localization differently than other nutrient transporters. It remains unclear how this selective trafficking of Glut1 is regulated.
Despite the inability of mTOR/RAPTOR inhibition to alter Glut1 protein levels or localization, rapamycin lead to a sharp reduction in glucose uptake. These data suggest that mTOR/RAPTOR may affect Glut1 transporter activity. Glut1 activity can be regulated by intracellular ATP binding, which increases glucose transport activity by modulating substrate binding affinity (Levine et al., 1998; Lachaal et al., 2001; Liu et al., 2001). Glycosylation of Glut1 has also been suggested to increase Glut1 glucose transport activity (Asano et al., 1991). Modulation of Glut1 transport activity by signaling components, however, is not completely understood. In principle, mTOR/RAPTOR may regulate Glut1 activity through either of these pathways by modulation of downstream metabolism to promote ATP generation or by altering protein glycosylation patterns. Alternatively, mTOR/RAPTOR can affect a variety of other cellular processes that may impact Glut1 activity. Although our data do not distinguish between direct or indirect mechanisms of mTOR/ RAPTOR regulation of Glut1 activity, Tremblay et al. (2005) showed that acute inhibition of mTOR/RAPTOR led to increased insulin-stimulated glucose uptake rather than the decreased glucose uptake observed here using longer treatments of rapamycin. Together, these findings suggest that either mTOR/RAPTOR has differential effects on Glut4 and Glut1 or that rapamycin's inhibition of glucose uptake occurs only after chronic mTOR/RAPTOR inhibition. Because mTOR/ RAPTOR inhibitors are becoming increasingly used in a variety of clinical settings, it will be particularly important to further study this novel function of mTOR/RAPTOR on glucose uptake and glucose transporter activity.
In addition to Glut1 activity, glucose uptake is regulated through the net efflux and influx of Glut1 proteins from the cell surface and Akt regulated this balance through GSK3 dependent and independent means. Akt can directly phosphorylate GSK3α/β on serines 21/9 to inhibit GSK3 kinase activity (Jope and Johnson, 2004) and maintain surface Glut1 levels even in the absence of IL3. This maintenance of surface Glut1 by GSK3 inhibition was likely due to enhanced efflux of Glut1 relative to control cells, as Glut1 influx was not altered by GSK3. These data are consistent with results from Roberts et al. (Roberts et al., 2004), who recently demonstrated that inhibition of GSK3 lead to enhanced recycling of integrins. Akt did not, however, require GSK3 inhibition or Rab11a-dependent recycling to maintain surface Glut1 levels as Akt also attenuated Glut1 internalization, thus obviating a requirement for maximal recycling activity to maintain surface Glut1. The mechanism by which Akt regulates Glut1 endocytosis is not clear, but does not appear to involve mTOR/RAPTOR or GSK3.
Here, we show that Glut1 trafficking is a regulated event that is critical in growth factor-regulated glucose homeostasis, and we define three distinct mechanisms by which Akt may promote glucose uptake. Activation of the proapoptotic molecule Bax and programmed cell death occurs if sufficient levels of glucose are not maintained in immune cells or during development (Moley and Mueckler, 2000; Rathmell et al., 2003). Conversely, increased glucose uptake occurs in immune cell activation and in poor prognosis cancers and is required for increased cellular proliferation (Gatenby and Gillies, 2004; Macheda et al., 2005). We demonstrate that growth factors and oncogenic Akt regulate Glut1 activity and trafficking. It will be important in future studies to further define these pathways to determine how growth factors and oncogenes promote sufficient glucose uptake to match intracellular demands to allow cell growth and to prevent cell death.
ACKNOWLEDGMENTS
We thank Dr. Christopher Newgard, Yuxing Zhao, Jonathan Coloff, and Erin Rees for helpful comments. We also like thank Dr. Michael Ehlers (Duke University) for generously providing dnRab11a and dnRme1 plasmids and Dr. Xiao-Fan Wang (Duke University) for the GSK3 S9A plasmid. We thank the Department of Pharmacology and Cancer Biology shared microscopy facility and the Duke University Flow Cytometry Shared Resource. J.C.R. was funded by a Howard Temin KO1 Career Development Award, the Sidney Kimmel Foundation for Cancer Research Scholar Award, and The V Foundation for Cancer Research Scholar Award. This work was also supported by grant R01-A1063345 from the National Institute of Allergy and Infectious Diseases.
Abbreviations used:
- 2-DOG
2-deoxy-d-glucose
- CHX
cycloheximide
- GFP
green fluorescent protein
- GSK
glycogen synthase kinase 3
- HRP
horseradish peroxidase
- IL
interleukin
- LDL
low-density lipoprotein
- myrAkt
myristoylated Akt
- PBS
phosphate-buffered saline
- PI3K
phosphatidylinositol 3-kinase
- TfR
transferrin receptor.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/10.1091/mbc.E06-07-0593) on February 14, 2007.
REFERENCES
- Asano T., et al. The role of N-glycosylation of GLUT1 for glucose transport activity. J. Biol. Chem. 1991;266:24632–24636. [PubMed] [Google Scholar]
- Barnes K., McIntosh E., Whetton A. D., Daley G. Q., Bentley J., Baldwin S. A. Chronic myeloid leukaemia: an investigation into the role of Bcr-Abl-induced abnormalities in glucose transport regulation. Oncogene. 2005;24:3257–3267. doi: 10.1038/sj.onc.1208461. [DOI] [PubMed] [Google Scholar]
- Barthel A., Okino S. T., Liao J., Nakatani K., Li J., Whitlock J. P., Jr, Roth R. A. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J. Biol. Chem. 1999;274:20281–20286. doi: 10.1074/jbc.274.29.20281. [DOI] [PubMed] [Google Scholar]
- Bentley J., Itchayanan D., Barnes K., McIntosh E., Tang X., Downes C. P., Holman G. D., Whetton A. D., Owen-Lynch P. J., Baldwin S. A. Interleukin-3-mediated cell survival signals include phosphatidylinositol 3-kinase-dependent translocation of the glucose transporter GLUT1 to the cell surface. J. Biol. Chem. 2003;278:39337–39348. doi: 10.1074/jbc.M305689200. [DOI] [PubMed] [Google Scholar]
- Cham C. M., Gajewski T. F. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J. Immunol. 2005;174:4670–4677. doi: 10.4049/jimmunol.174.8.4670. [DOI] [PubMed] [Google Scholar]
- Ciofani M., Zuniga-Pflucker J. C. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat. Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- Cong L. N., Chen H., Li Y., Zhou L., McGibbon M. A., Taylor S. I., Quon M. J. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol. Endocrinol. 1997;11:1881–1890. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- Dang C. V. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol. Cell Biol. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh M., Vasilakos J., Deckwerth T. L., Lampe P. A., Shivers B. D., Johnson E. M., Jr Genetic and metabolic status of NGF-deprived sympathetic neurons saved by an inhibitor of ICE family proteases. J. Cell Biol. 1996;135:1341–1354. doi: 10.1083/jcb.135.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger A. L., Thompson C. B. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol. Biol. Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran P. G., Fletcher L. M., Oatey P. B., Mohammed N., Dolly J. O., Tavare J. M. Protein kinase B stimulates the translocation of GLUT4 but not GLUT1 or transferrin receptors in 3T3–L1 adipocytes by a pathway involving SNAP-23, synaptobrevin-2, and/or cellubrevin. J. Biol. Chem. 1999;274:28087–28095. doi: 10.1074/jbc.274.40.28087. [DOI] [PubMed] [Google Scholar]
- Fox C. J., Hammerman P. S., Cinalli R. M., Master S. R., Chodosh L. A., Thompson C. B. The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev. 2003;17:1841–1854. doi: 10.1101/gad.1105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauwirth K. A., Thompson C. B. Regulation of T lymphocyte metabolism. J. Immunol. 2004;172:4661–4665. doi: 10.4049/jimmunol.172.8.4661. [DOI] [PubMed] [Google Scholar]
- Gatenby R. A., Gillies R. J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- Gonzalez E., McGraw T. E. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol. Biol. Cell. 2006;17:4484–4493. doi: 10.1091/mbc.E06-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlob K., Majewski N., Kennedy S., Kandel E., Robey R. B., Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig C. W., Saunders T., Brosius F. C., 3rd, Moley K., Heilig K., Baggs R., Guo L., Conner D. Glucose transporter-1-deficient mice exhibit impaired development and deformities that are similar to diabetic embryopathy. Proc. Natl. Acad. Sci. USA. 2003;100:15613–15618. doi: 10.1073/pnas.2536196100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope R. S., Johnson G. V. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Kan O., Baldwin S. A., Whetton A. D. Apoptosis is regulated by the rate of glucose transport in an interleukin 3 dependent cell line. J. Exp. Med. 1994;180:917–923. doi: 10.1084/jem.180.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. S., Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp. Cell Res. 1999;253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- Kansara M., Berridge M. V. Oncogenes modulate cell sensitivity to apoptosis induced by glucose deprivation. Anticancer Res. 2004;24:2503–2510. [PubMed] [Google Scholar]
- Krauss S., Brand M. D., Buttgereit F. Signaling takes a breath–new quantitative perspectives on bioenergetics and signal transduction. Immunity. 2001;15:497–502. doi: 10.1016/s1074-7613(01)00205-9. [DOI] [PubMed] [Google Scholar]
- Lachaal M., Spangler R. A., Jung C. Y. Adenosine and adenosine triphosphate modulate the substrate binding affinity of glucose transporter GLUT1 in vitro. Biochim. Biophys. Acta. 2001;1511:123–133. doi: 10.1016/s0005-2736(01)00272-3. [DOI] [PubMed] [Google Scholar]
- Larance M., et al. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J. Biol. Chem. 2005;280:37803–37813. doi: 10.1074/jbc.M503897200. [DOI] [PubMed] [Google Scholar]
- Levine K. B., Cloherty E. K., Fidyk N. J., Carruthers A. Structural and physiologic determinants of human erythrocyte sugar transport regulation by adenosine triphosphate. Biochemistry. 1998;37:12221–12232. doi: 10.1021/bi980585y. [DOI] [PubMed] [Google Scholar]
- Lin S. X., Grant B., Hirsh D., Maxfield F. R. Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat. Cell Biol. 2001;3:567–572. doi: 10.1038/35078543. [DOI] [PubMed] [Google Scholar]
- Liu Q., Vera J. C., Peng H., Golde D. W. The predicted ATP-binding domains in the hexose transporter GLUT1 critically affect transporter activity. Biochemistry. 2001;40:7874–7881. doi: 10.1021/bi002850x. [DOI] [PubMed] [Google Scholar]
- Macheda M. L., Rogers S., Best J. D. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J. Cell Physiol. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R., McGraw T. E. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Moley K. H., Mueckler M. M. Glucose transport and apoptosis. Apoptosis. 2000;5:99–105. doi: 10.1023/a:1009697908332. [DOI] [PubMed] [Google Scholar]
- Piper R. C., Hess L. J., James D. E. Differential sorting of two glucose transporters expressed in insulin-sensitive cells. Am. J. Physiol. 1991;260:C570–C580. doi: 10.1152/ajpcell.1991.260.3.C570. [DOI] [PubMed] [Google Scholar]
- Plas D. R., Rathmell J. C., Thompson C. B. Homeostatic control of lymphocyte survival: potential origins and implications. Nat. Immunol. 2002;3:515–521. doi: 10.1038/ni0602-515. [DOI] [PubMed] [Google Scholar]
- Plas D. R., Talapatra S., Edinger A. L., Rathmell J. C., Thompson C. B. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J. Biol. Chem. 2001;276:12041–12048. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- Rathmell J. C., Farkash E. A., Gao W., Thompson C. B. IL-7 enhances the survival and maintains the size of naive T cells. J. Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- Rathmell J. C., Fox C. J., Plas D. R., Hammerman P. S., Cinalli R. M., Thompson C. B. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol. Cell Biol. 2003;23:7315–7328. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell J. C., Vander Heiden M. G., Harris M. H., Frauwirth K. A., Thompson C. B. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol. Cell. 2000;6:683–692. doi: 10.1016/s1097-2765(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Roberts M. S., Woods A. J., Dale T. C., Van Der Sluijs P., Norman J. C. Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of alpha v beta 3 and alpha 5 beta 1 integrins. Mol. Cell Biol. 2004;24:1505–1515. doi: 10.1128/MCB.24.4.1505-1515.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. E., Thompson C. B. Putting the rap on Akt. J. Clin. Oncol. 2004;22:4217–4226. doi: 10.1200/JCO.2004.01.103. [DOI] [PubMed] [Google Scholar]
- Tremblay F., Gagnon A., Veilleux A., Sorisky A., Marette A. Activation of the mammalian target of rapamycin pathway acutely inhibits insulin signaling to Akt and glucose transport in 3T3–L1 and human adipocytes. Endocrinology. 2005;146:1328–1337. doi: 10.1210/en.2004-0777. [DOI] [PubMed] [Google Scholar]
- Ullrich O., Reinsch S., Urbe S., Zerial M., Parton R. G. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden M. G., Chandel N. S., Williamson E. K., Schumacker P. T., Thompson C. B. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- Vander Heiden M. G., Plas D. R., Rathmell J. C., Fox C. J., Harris M. H., Thompson C. B. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol. Cell Biol. 2001;21:5899–5912. doi: 10.1128/MCB.21.17.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Watson R. T., Kanzaki M., Pessin J. E. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr. Rev. 2004;25:177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- Welsh G. I., Hers I., Berwick D. C., Dell G., Wherlock M., Birkin R., Leney S., Tavare J. M. Role of protein kinase B in insulin-regulated glucose uptake. Biochem. Soc. Trans. 2005;33:346–349. doi: 10.1042/BST0330346. [DOI] [PubMed] [Google Scholar]
- Zeigerer A., McBrayer M. K., McGraw T. E. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol. Biol. Cell. 2004;15:4406–4415. doi: 10.1091/mbc.E04-04-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]