Abstract
The eukaryotic genome is divided into functional domains defined in part by local differences in chromatin structure and delimited in many cases by boundary elements. The HML and HMR loci in the yeast Saccharomyces cerevisiae are transcriptionally silent chromosome domains. Each locus is bracketed by two cis-acting sequences, designated E and I, that serve to establish and maintain repression of genes within each locus. We show that repression at HML is uniformly high between E and I but decreases sharply beyond I. The region of repression at HML generally correlates with the domain of histone hypoacetylation. Despite the sharp definition of the boundaries of HML, no sequence capable of blocking the spread of heterochromatin resides in the sequences flanking HML. We find, though, that inverting the orientation of I increases silencing outside of HML while weakening silencing within HML. These results indicate that the HML I silencer establishes a boundary between active and inactive chromatin at HML, but does so by organizing inactive chromatin in only one direction. This represents a different mechanism for delimiting the boundaries of a eukaryotic chromosome domain.
The eukaryotic genome is divided into domains of distinct regulatory potential. The same gene inserted into different sites in the Drosophila or mammalian genome can exhibit markedly different levels of expression (reviewed in ref. 1). This position effect on gene expression likely reflects local differences in chromatin structure as well as the particular distribution of enhancers and other regulatory elements throughout the genome. One question posed by the existence of different domains is how these local differences in expression potential are restricted to limited regions of the genome.
Studies of specific regions of the Drosophila and vertebrate genomes suggest that, at least for some domains, the regulatory potential of that domain is precisely delimited by boundary or insulator elements that serve to restrict the effects of transcriptional activity to the region lying between pairs of such elements. Two small segments, scs and scs′ (specialized chromatin structure), flank the 87A7 hsp70 heat shock locus of Drosophila and serve to limit the effects of heat shock activation to the locus (2, 3). These segments can function as nonspecific boundary elements: when bracketing a transgene they insulate it from position-effect variegation and when placed between an enhancer and a promoter either segment can block transcriptional activation of the promoter by the enhancer. The scs and scs′ elements contain binding sites for the zw5 protein (M. Gaszner and P. Schedl, personal communication) and the BEAF-32 protein (4), respectively. A cluster of binding sites for the suppresser of hairy wing [su(Hw)] protein constitute a second boundary element in Drosophila (5).
A DNase I hypersensitive site (5′HS4) from the chicken β-globin locus also exhibits boundary activity (6). This element resides at the transition between active chromatin of the β-globin locus, as marked by both DNase I sensitivity and high levels of histone acetylation, and the adjacent inactive chromatin domain. The 5′HS4 element can insulate transgenes from position-effect variegation and can function in Drosophila to block enhancer-mediated activation of an adjacent promoter. Thus, although the molecular mechanism of insulation is not yet resolved, these studies clearly demonstrate that at least some regulatory domains are restricted by the activity of specific boundary elements.
Examples of chromosome domains in the yeast Saccharomyces cerevisiae include the homothalic mating type loci, HML and HMR, as well as telomeres. Genes present at the expressed MAT locus on chromosome III determine the mating type of a haploid yeast cell. Identical mating type genes also exist at the HML or HMR loci on the same chromosome. However, at these loci, the mating type genes are not expressed, even though all the signals for expression are present (reviewed in ref. 7). Ectopic genes inserted at the HM loci are also subject to repression, indicating that this is a region-specific but gene-nonspecific event. This repression, known as silencing, results from the formation of a heterochromatin-like chromatin structure across the loci (reviewed in ref. 8). A heterochromatin-like structure also exists in sequences close to the telomeres in yeast, which causes transcriptional silencing of genes inserted near the telomeres.
Establishing and maintaining repression at the HM loci require a number of trans-acting factors and two cis-acting sites, named silencers, flanking each HM locus. Silencers are small (<250 bp) sequences composed of various combinations of binding sites for DNA binding proteins—Rap1, Abf1, and the origin recognition complex—whose binding are required for silencing (Fig. 1A). A variety of other proteins, including Sir1 through Sir4 and histones H3 and H4, are also required for transcriptional silencing (7). The extensive interactions among the trans-acting factors support the current model for silencing in which the silencer-binding proteins and Sir1 recruit a complex of Sir2, Sir3, and Sir4 to the silencer, which then extends along the neighboring nucleosomes, serving as an integral part of the silent chromatin (9).
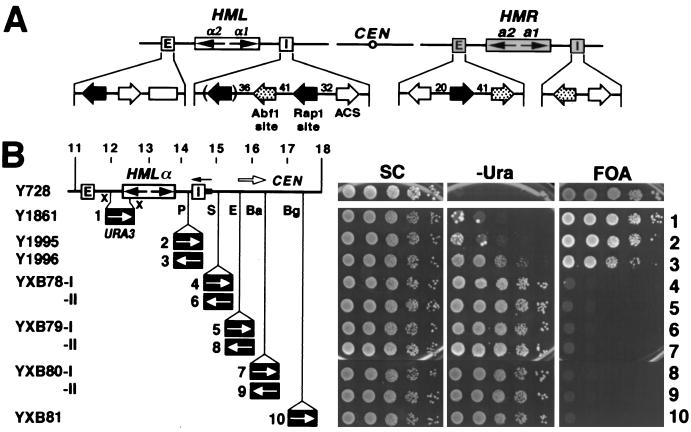
Figure 1.
Transcriptional silencing is uniformly high within HML but sharply decreases beyond the I silencer. (A) Schematic representation of the HML and HMR loci and their silencers on chromosome III. CEN, centromere. The open box in the HML E silencer represents the D-element (29). (B) The pattern of transcriptional silencing within and near HML. (Left) Strains used. The 11- to 18-kb fragment of chromosome III, including HML, is shown. The HML I silencer (I in open box) corresponds to position 14561–14701 (22). The filled bar to the right of HML I is 14702–14838. URA3 replaces 12015–12535 (XbaI–XbaI) in Y1861. Strains Y1995–YXB81 have URA3 inserted at the PvuII (P, 14441), SnaBI (S, 15016), EcoRV (E, 15410), BamHI (Ba, 16263), and BglII (Bg, 17344) sites, respectively, in both orientations. Copies of URA3 in these strains are designated 1–10, according to the positions of the URA3 promoter relative to HML I. X, XbaI site. (Right) Growth phenotypes. Cells of each strain were grown to late logarithmic phase, and serial dilutions (10-fold) were spotted on test plates and allowed to grow for 3 days. SC, synthetic complete medium; −Ura, SC medium lacking uracil; FOA, SC medium containing 5-fluoroorotic acid (5-FOA).
The fact that repressive chromatin emanates outward from a silencer raises the question of what prevents the repressive chromatin from extending indefinitely. Do boundary elements around the silent HM loci actively block the spread of heterochromatin elicited by silencers, or does silencing simply decay stochastically as a function of distance from the silencers? Recent studies revealed that certain sequences surrounding the HMR locus have the ability to block the spread of silencing from HMR (10). In two other independent studies, UASrpg, consisting of multiple binding sites of Rap1, and sequences containing multiple Reb1 and/or Tbf1 binding sites have both been identified as heterochromatin boundary elements in yeast (11, 12).
In this study, we defined the transcriptionally silent domain across the HML locus and tested for the presence of functional boundary elements surrounding the locus. We show that silencing is uniformly high within the region bracketed by the E and I silencers but sharply decreases beyond the I silencer. Unlike the situation for HMR, sequences surrounding the HML locus exhibit no detectable boundary activity. However, we found that inverting the I silencer extended silencing beyond I but diminished silencing within HML. These data, together with our previous finding that certain silencers promote ectopic silencing in only one orientation (13), suggest that HML silencers delimit a repression domain by unidirectional establishment of silencing.
Materials and Methods
Plasmids and Strains.
The plasmids described below were constructed by inserting a sequence of chromosome III into the polylinker of pUC19. Plasmid pUC26 contains the BamHI–HML–BamHI fragment corresponding to region 9666–16263 of chromosome III (base pair coordinates are from the complete sequence of chromosome III). The HpaI–HML I–HindIII (14561–14838) fragment in plasmid pUC26 was inverted to make plasmid pXB87. Plasmids pMB35, pAR61, pMB11, pMB10, and pDM33 contain the HindIII–HindIII (13679–14838), HindIII–BamHI (14838–16263), EcoRI–HindIII (14838–16713), HindIII–HindIII (17021–18961), and XbaI–XbaI (10838–12015) fragments, respectively. The HpaI–HML I–HindIII fragment (14561–14838) was inserted at HpaI site of pDM33 in both orientations to yield plasmids pGJ51 and pGJ52. The 1.1-kb BglII–URA3–BglII fragment of plasmid pFL44 (14) was used in constructing a series of plasmids for the insertion of URA3 at different sites within or near the HML locus on chromosome III. URA3 was inserted at the PvuII site of pMB35 in both orientations to make plasmids pMB36 and pMB37, at SnaBI of pAR61 to make plasmids pMB16a and -b, at the EcoRV site of pAR61 to generate plasmids pMB22a and -b, at the BamHI site of pMB11 to make plasmids pMB13a and -b, and at the BglII site of pMB10 to make plasmids pMB12a and -b. Plasmid pGJ8 contains the EcoRI–HindIII (11294–14838) fragment of chromosome III in which the XbaI–XbaI (12015–12535) fragment was replaced by URA3. Plasmid pMB21 consists of pRS404 (15) into which the SIR3 and SUP4-o genes have been inserted at the SacI + BamHI and BamHI sites, respectively. Plasmids pXB68, pXB69, pXB70, and pXB71 contain PCR fragments from chromosome III positions 9658–11158 (designated α, Fig. 4A); 14701–16201 (β); 289,776–291,276 (γ); and 293,628–295,128 (δ), respectively, inserted at the SpeI site of pYXB26 (11).
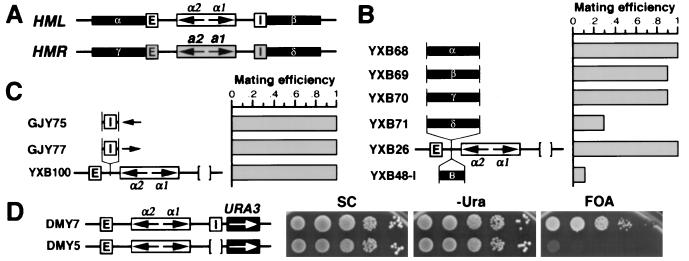
Figure 4.
Neither sequences flanking HML nor the HML I silencer actively block the spread of silencing. (A) Sequences (1.5 kb each, designated α–δ) flanking the HML and HMR loci. (B) Boundary element activity of sequences surrounding HM loci. YXB68-71 have the α–δ sequences inserted at HMLΔI of YXB26, respectively. YXB48-I has the 149-bp boundary element from the TEF2 promoter inserted at HMLΔI (11). Mating efficiency of each strain was determined as described (11). Mating efficiency of YXB26 was taken as one. (C and D) The HML I silencer is not a boundary element. (C) Strains GJY75 and GJY77 have the I silencer inserted at the HapI site of the HMLΔI locus in YXB100, but in opposite orientations. Mating efficiency of YXB100 was taken as one. (D) Growth phenotypes of strains DMY5 and DMY7 (16).
Strain Y851 is mata1 HMLα HMRα leu2–3,112 trp1Δ ade8 ura3–52, and strain Y1423 is identical to strain Y851 except for inactivation of SIR3 by insertion of LEU2. All other strains used in this study were derived from strain DMY1 (MATa ura3–52 leu2–3,112 ade2–1 lys1–1 his5–2 can1–100; ref. 16). Strain YXB76 was made by transforming strain DMY20 (DMY1, ΔE∷SUP4-o-HMLα-ΔI; ref. 16) to canavanine-resistance with the BamHI–E–HMLα–I(inverted)–BamHI fragment of plasmid pXB87. Strain YXB77 was derived from strain YXB76 by disrupting SIR3 with LEU2 as described (16). Strain Y1861s was constructed by transforming strain DMY2 (DMY1, sir3∷LEU2; ref. 16) to Ura+ with EcoRI plus HindIII-digested pGJ8 DNA. Strains Y1995s, Y1996s, YXB78-Is, YXB78-IIs, YXB79-Is, YXB79-IIs, YXB80-Is, YXB80-IIs, and YXB81s were similarly derived from DMY2 by transformation with appropriately digested pMB36, pMB37, pMB16a, pMB16b, pMB22a, pMB22b, pMB13a, pMB13b, and pMB12a DNAs, respectively. These strains were rendered SIR3+ by integrating pMB21 at TRP1 in the genome, resulting in the strains listed in Fig. 1B. All the strains listed in Fig. 5A were obtained by first transforming strain YXB77 to Ura+ with the plasmids listed above and then converting the resulting transformants to SIR3+ by integrating pMB21 at TRP1 in the genome. Strains YXB68 to YXB71 were made by transforming strain Y2047b (DMY1, ΔE∷SUP4-o-HMLα-ΔI LEU2-GAL10-FLP1 [cir0]; ref. 17) to canavanine-resistance with BamHI-digested plasmid pXB68 to pXB71, respectively. Strain DMY19s (DMY1, ΔE∷SUP4-o-HMLα-ΔI sir3∷LEU2) was transformed to canavanine-resistance by using XbaI-digested pGJ51 or pGJ52 to yield strains GJY75s and GJY77s, which were transformed with pMB21 to yield strains GJY75 and GJY77. Strain YXB100 is DMY1, E-HMLα-ΔI sir3∷LEU2 SIR3-SUP4-o. All strain constructions were confirmed by Southern blot analysis.
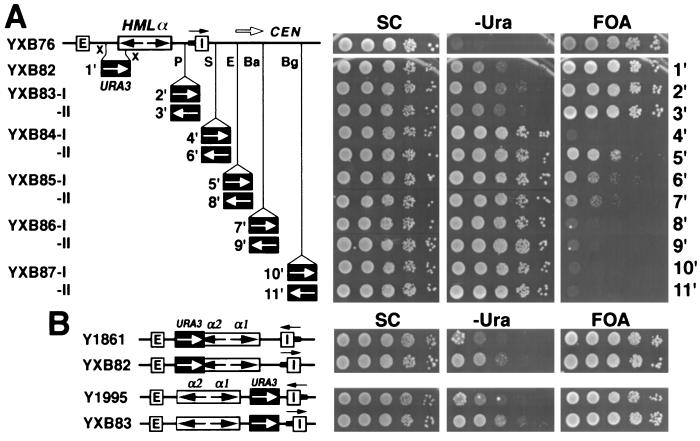
Figure 5.
The HML I silencer functions as a heterochromatin domain boundary by directional silencing. (A) Growth phenotypes of strains with URA3 positioned within or centromere-proximal to HML when the I silencer is inverted. The strains used are the same as those in Fig. 1B, except that the HML I silencer plus a small region to its right (14561–14838) is inverted in each. Copies of URA3 in these strains are designated 1′–11′, according to the positions of the URA3 promoter relative to HML I. (B) Effect of flipping HML I on silencing within HML. Cell growth on different media was tested as described in the legend to Fig. 1B.
Chromatin Immunoprecipitation.
Chromatin immunoprecipitation assays were performed as described previously (18).
Results
Transcriptional Silencing Is Uniformly High Within HML but Sharply Decreases Beyond the I Silencer.
The URA3 gene provides a convenient reporter for studying transcriptional silencing. URA3 expression can be assessed by cell viability assays on medium containing 5-fluoroorotic acid (5-FOA) or on medium lacking uracil. The URA3 gene product converts the drug 5-FOA to a toxic metabolite, so that cells with basal level expression of URA3 are sensitive to 5-FOA, whereas ura3 cells or cells in which URA3 expression is repressed below basal level can grow on 5-FOA medium (19). Depletion of uracil from the medium activates the Ppr1 transactivator, which in turn binds to the upstream activating sequence (UAS) of URA3 and increases expression of the gene. Therefore, while cell growth on 5-FOA medium indicates repression of the basal level of transcription of URA3, growth on uracil minus (−Ura) medium reflects the ability of the cell to activate expression of URA3. Telomeric silencing can repress basal expression but not the activated expression of URA3 mediated by Ppr1 (20). In contrast, HM silencing represses both basal and activated URA3 expression (see below).
To define the domain of transcriptional repression associated with the silent HML locus, we first examined the extent of repression of URA3 inserted at various locations between the silencers (Fig. 1B). Viability assays on 5-FOA medium indicated that basal expression of URA3 was repressed in these strains: the 5-FOA-resistant fraction of cells from each strain was essentially equivalent to that of a ura3 strain (Y728). Viability assay on −Ura medium also showed significant repression of activated expression of URA3 within HML (Y1861 and Y1995), except for URA3 in Y1996, whose promoter is located only ≈110 bp from I. In summary, within the HML locus, silencing of both basal and activated transcription of URA3 is, in general, uniformly high. The slightly diminished silencing of activated transcription of URA3 in Y1996 likely results from the unique chromatin context near I attributable to the occupancy by silencer-binding proteins. This is supported by evidence presented below.
We next examined transcriptional repression in the region immediately adjacent to HML. We inserted the URA3 gene at various sites centromere-proximal to HML I and then assessed its basal and activated levels of expression (Fig. 1B). In these experiments and those above, we integrated all the URA3 reporter genes into a sir3− strain and only subsequently converted the strains to SIR+ (see Materials and Methods). This precluded inadvertently selecting activated, or silencing resistant, reporter genes in the course of strain construction. Data presented in Fig. 1B indicate that neither basal nor activated expression of URA3 in strains YXB78-81 is repressed. Note that URA3 in strain YXB78-I resides very close (300 bp centromere-proximal) to I and yet is not silenced. The loss of silencing potential immediately outside the I site suggests that I or sequences in the vicinity of I constitute a boundary for the silent chromatin domain across HML.
The Domain of Histone Hypoacetylation Correlates with the Domain of Transcriptional Repression at HML.
Histones H3 and H4 in nucleosomes constituting silent chromatin exhibit a reduced level of acetylation compared with that in nucleosomes constituting active chromatin (8, 18, 21). Accordingly, we investigated whether the domain of gene silencing across HML locus correlated with the pattern of chromatin hypoacetylation. To determine the extent of Sir-dependent histone H4 hypoacetylation at and around HML, we immunoprecipitated chromatin isolated from isogenic SIR+ (Y851) and sir− (Y1423) strains with Abs against acetylated histone H4 (18). The presence of various sequences in or near HML in the immunoprecipitated chromatin was detected by slot blot hybridization of the immunoprecipitated DNA with appropriate genomic probes (Fig. 2B, a–h). The fraction of a particular sequence present in the immunoprecipitate was determined by densitometry analysis of the probed blots and normalized relative to the amount of MAT sequence present in the same immunoprecipitate, a value defined as the relative fraction (Fig. 2A). Consistent with previous observations, we found that DNA from within HML in a SIR+ strain was underrepresented in the acetylated fraction, indicating that hypoacetylated nucleosomes package the silent HML locus. Nucleosome hypoacetylation at HML was Sir-dependent, as evidenced by the fact that the equivalent amounts of HML and MAT were recovered in acetylated chromatin immunoprecipitated from a sir3− strain (Fig. 2A).
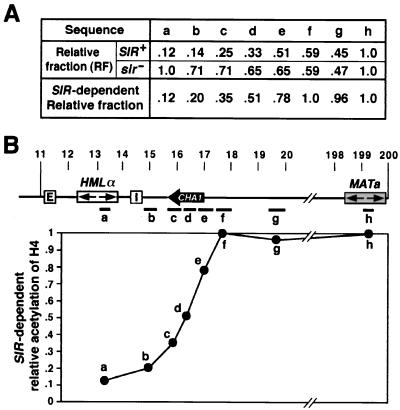
Figure 2.
The domain of histone H4 hypoacetylation at HML. (A) Relative fraction of different sequences within and adjacent to HML immunoprecipitated with anti-acetylated histone H4 Ab. See text for calculations. (B) SIR-dependent relative acetylation of histone H4 as a function of position on chromosome III. (Upper) Schematic representation of the 11- to 200-kb sequence of chromosome III analyzed in the chromatin immunoprecipitation assay. The HMLα and MATa loci and the CHA1 gene are indicated. Various DNA sequences (coordinates in parentheses) used to make probes for slot blot analysis are: a (13172–13418), b (14839–15360), c (15730–16264), d (16264–16714), e (16714–17562), f (17562–18049), g (19574–20029), and h, the MATa-specific probe (16). (Lower) SIR-dependent relative acetylation of histone H4 described in A, plotted as the function of the positions of the a–h probes.
To determine the domain of hypoacetylation around HML, we probed the immunoprecipitates obtained with anti-acetylated histone H4 with DNA probes located at increasing distances outward from HML toward the centromere. As noted in Fig. 2A, the amount of DNA in the immunoprecipitate, relative to the amount of MAT DNA in the immunoprecipitate, increased with increasing distance from HML for chromatin isolated from a SIR+ strain. In contrast, the relative amount of DNA in the immunoprecipitate decreased slightly with increasing distance from HML for chromatin isolated from a sir− strain. Accordingly, to focus on the Sir-dependent acetylation effects, we normalized the relative fraction of a sequence in the immunoprecipitate from the SIR+ strain to that of the same sequence in the immunoprecipitate in the sir− strain to yield a Sir-dependent relative fraction value for each sequence (Fig. 2A). As is evident from the data, the relative acetylation level of H4 is low within HML (Fig. 2B, a) and increases in the centromere-proximal region of I (Fig. 2B, b–g). The lack of a sharply defined transition in histone acetylation may be because of the limitations of the chromatin immunoprecipitation assay, in which immunoprecipitation was performed on chromatin fragments of random, variable sizes. Nonetheless, the pattern of acetylation generally corresponds with the pattern of transcriptional repression of the URA3 gene inserted around HML.
The HML I Silencer Defines the Heterochromatin Boundary by Initiating Silencing in Only One Direction.
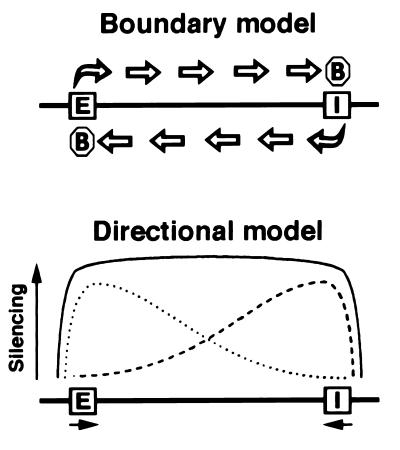
The above experiments on the pattern of gene silencing and the profile of histone H4 acetylation around the HML I silencer indicate that the I silencer lies at or near the breakpoint between fully repressed and fully activated domains of gene expression. We can envision two distinct models to account for the colocalization of the breakpoint and the I silencer (Fig. 3). The “boundary model” posits elements flanking the HML locus that actively block the spread of silencing initiated at the silencers, as is the case for at least the rightward (telomere-proximal) side of HMR (10). In this model, the boundary element would lie close to the HML I silencer, either as a distinct element or overlapping the I site. The “directional model” proposes that no boundary elements surround HML. Rather, the E and I silencers would initiate silenced chromatin that would emanate outward in only one direction, but that would decay stochastically with increasing distance from the silencer. We assume that the activities of the two silencers would be additive so that repression of sequences lying between them would be uniformly high. Both models yield a domain of repression consistent with that observed at HML.
Figure 3.
Proposed mechanisms for delimiting transcriptionally silenced domains. The E and I silencers are indicated. The octagons represent putative boundary elements. See text for details.
To distinguish between these two models, we tested whether sequences flanking HML were capable of protecting a promoter from the repressive effects of heterochromatin initiated at a silencer. This “blocking” assay has been used to identify and characterize other heterochromatin boundary elements in yeast (10–12). Fragments 1.5 kb in length corresponding to the flanking regions of HML and HMR were inserted between the E silencer and the α genes in the HMLΔI locus of strain YXB26 (Fig. 4A). The effect of each sequence on silencing of the HML α genes imposed by the E silencer could be determined by quantitative mating, because the mating efficiency of this MATa strain is inversely proportional to the level of expression of the α genes resident at HML. As is evident from the data in Fig. 4B, no sequences with silencer blocking activity lies within the 1.5-kb fragments flanking the HML locus (compare YXB68 and YXB69 to YXB26). In contrast, the 1.5-kb sequence to the right of HMR I (telomere proximal) exhibits silencer blocking activity (Fig. 4, YXB71), consistent with an earlier identification of boundary activity within a 1-kb sequence encompassed by this fragment (10). Similarly, a 149-bp fragment from the TEF2 promoter that we identified previously as possessing boundary activity (11) was also able to block repression of the HML α genes by silencing initiated at the E silencer (Fig. 4, YXB48-I). However, the 1.5-kb sequence from the left of HMR E (centromere proximal) did not exhibit blocking activity, although it overlaps ≈500 bp with the 1-kb leftward boundary of the HMR repression domain (10) (Fig. 4, YXB70). Finally, we also tested the HML I silencer, as defined by Feldman et al. (22), for silencer-blocking activity. As shown in Fig. 4C, insertion of I in either orientation between the E silencer and the α genes did not alleviate silencing of the α genes. Therefore, neither sequences surrounding the HML locus nor the HML I silencer can restrict the spread of transcriptional repression initiated at a silencer. These observations are inconsistent with the “boundary model” described above.
As a second test of the “boundary model,” we examined the effect of eliminating the I silencer on the extent of repression of a reporter gene lying immediately beyond I. If the I silencer comprises a boundary element capable of blocking the spread of heterochromatin, then deleting the I silencer should allow heterochromatin initiated at the E silencer to spread beyond the I site. This would result in increased repression of a gene lying immediately outside the I site. Accordingly, we examined the expression state of a URA3 gene inserted 100 bp centromere-proximal to I in two isogenic strains, in one of which the I site is intact and in one of which the I site has been deleted. As seen in Fig. 4D, the level of repression of the URA3 gene actually decreases on deletion of I, as evidenced by the fact that the DMY5 cells were not able to grow on FOA medium. This result adds further weight to the conclusion that the I site does not have silencer-blocking activity.
To test the “directional model,” we inverted the I silencer and examined its effect on the repression of URA3 gene inserted at different sites within and centromere proximal to HML (Fig. 5A). The strains shown in Fig. 5A are identical to those in Fig. 1B, except that the HML I silencer is inverted in each of them. We found that inverting the orientation of I resulted in significant changes in the repression pattern of URA3 expression in the region to the right of the I silencer. First, repression of basal transcription of URA3 was increased such that a fraction of the cells were able to grow on 5-FOA medium (compare YXB84-II to YXB87-II in Fig. 5A with their counterparts in Fig. 1B). Second, as the distance between the URA3 promoter and the I silencer increased, both the number and the size of the colonies on 5-FOA medium decreased, indicating that silencing diminishes with increasing distance. We noted a discontinuity in the repression pattern, in that strain YXB84-I failed to grow on FOA, indicating that the URA3 gene inserted immediately adjacent to I was completely derepressed, whereas those inserted farther away were repressed. This is consistent with previous observations, which indicate that chromatin in the region immediately adjacent to a silencing organizing center is not repressive (23, 24). We also observed changes in silencing of activated transcription of URA3 within HML (Fig. 5B). Strain Y1861 harboring URA3 within HML cannot grow on −Ura medium, indicating full repression of activated URA3 expression. However, strain YXB82 derived from Y1861 by inverting the I silencer shows limited growth on −Ura medium, indicating that inversion of I diminished repression of activated expression of URA3. Similar results were obtained for strains Y1995 and YXB83 (Fig. 5B). In summary, we conclude that with I in its natural orientation, silencing is strong within HML but weak or nonexistent in the region centromere proximal to I. Inversion of I weakens silencing within HML but strengthens silencing in the centromere proximal region next to I. This is in full accordance with the “directional model.”
Discussion
The results presented in this report describe a different means of establishing a distinct chromatin domain within a eukaryotic genome. Previous studies examining the region of transition between active and inactive chromatin domains have identified elements that possess either enhancer-blocking activity or heterochromatin-blocking activity (reviewed in ref. 1). In this study, we were able to map quite precisely the transition between active and inactive chromatin on one side of the silent HML locus in yeast. In this case, though, we found that no sequence with the ability to block the spread of heterochromatin resides at or near this transition point. Rather, we found that the boundary appears to derive from the fact that the silencer promotes formation of heterochromatin in only one direction. Accordingly, heterochromatin forms to one side of the silencer but not to the other, effectively creating a boundary.
To account for the pattern of repression we observe at HML, we assume that the E and I silencers promote formation of heterochromatin that propagates inward toward the mating-type genes. We further assume that the repressive effect imposed by each silencer diminishes with increasing distance from the silencer, similar to the pattern of telomeric silencing (25–27). Finally, we assume that the repressive effects of the two silencers are additive. In this sense, our model is consistent with the concept proposed by Boscheron et al. (28), which states that the silencers at HML cooperate. However, our results reinforce the notion that such a cooperation does not represent a physical interaction between the two silencers, which would delimit silencing to the loop of DNA formed between them. This possibility is most clearly eliminated by the results presented in Fig. 4C, in which we showed that moving I to a site between E and the α mating genes does not eliminate repression of the α genes. Finally, we previously showed that I is capable of promoting repression of genes at MAT only when inserted next to MAT and pointing toward the MAT genes (that is, with I oriented so that the MAT genes are to the same side of I as the α genes are at HML). Insertion of I at this same site next to MAT but in the opposite orientation failed to elicit repression of the MAT genes (13). Thus, our previous demonstration of the orientation dependence of I-mediated repression is consistent with our current hypothesis that silencing spreads outward from I at HML only in the direction of the α genes.
While the HML I and HMR E silencers both contain binding sites for origin recognition complex, Rap1, and Abf1 with relatively similar arrangements (Fig. 1A), they differ in strength and orientation dependence. In vitro binding assays have shown that the Rap1 site in HML I has significantly lower affinity for Rap1 than does the site in HMR E (28). Footprinting studies revealed a second weak, nonessential, Rap1-binding site in HML I (Fig. 1A, Rap1 site in parentheses; ref. 28). The affinity of origin recognition complex or Abf1 to its binding site in the two silencers has not been directly compared. The difference in the potency of silencing by HML I and HMR E silencers could reflect any combination of a number of possible factors, including the small differences in the spacing of the three protein-binding sites, the affinities of individual sites to their respective factors, or the sequence context of the sites. However, the difference in the directionality of silencing between the two silencers is more difficult to interpret. Further experiments need to address whether a single component of a silencer or the concerted action of all components is responsible for the observed directionality of the silencer and how that local structure is translated into a unidirectional initiation of heterochromatin.
Acknowledgments
We thank Dr. C. D. Allis for the gift of anti-H4 Ab and Karun Shekeri for technical assistance. This work was supported by National Institutes of Health Grant GM48540 (to J.R.B.) and by Postdoctoral Fellowship PF4298 from the American Cancer Society (to X.B.).
Abbreviation
- 5-FOA
5-fluoorotic acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Udvardy A. EMBO J. 1999;18:1–8. doi: 10.1093/emboj/18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kellum R, Schedl P. Cell. 1991;64:941–950. doi: 10.1016/0092-8674(91)90318-s. [DOI] [PubMed] [Google Scholar]
- 3.Kellum R, Schedl P. Mol Cell Biol. 1992;12:2424–2431. doi: 10.1128/mcb.12.5.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao K, Hart C M, Laemmli U K. Cell. 1995;81:879–889. doi: 10.1016/0092-8674(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 5.Geyer P K, Corces V G. Genes Dev. 1992;6:1865–1873. doi: 10.1101/gad.6.10.1865. [DOI] [PubMed] [Google Scholar]
- 6.Chung J H, Whiteley M, Felsenfeld G. Cell. 1993;74:505–514. doi: 10.1016/0092-8674(93)80052-g. [DOI] [PubMed] [Google Scholar]
- 7.Laurenson P, Rine J. Microbiol Rev. 1992;56:543–560. doi: 10.1128/mr.56.4.543-560.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braunstein M, Holmes S G, Broach J R. In: Nuclear Organization, Chromatin Structure and Gene Expression. van Driel R, Otte A P, editors. Oxford: Oxford Univ. Press; 1997. pp. 250–275. [Google Scholar]
- 9.Lustig A J. Curr Opin Genet Dev. 1998;8:233–239. doi: 10.1016/s0959-437x(98)80146-9. [DOI] [PubMed] [Google Scholar]
- 10.Donze D, Adams C R, Rine J, Kamakaka R T. Genes Dev. 1999;13:698–708. doi: 10.1101/gad.13.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bi X, Broach J R. Genes Dev. 1999;13:1089–1101. doi: 10.1101/gad.13.9.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fourel G, Revardel E, Koering C E, Gilson E. EMBO J. 1999;18:2522–2537. doi: 10.1093/emboj/18.9.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shei G J, Broach J R. Mol Cell Biol. 1995;15:3496–3506. doi: 10.1128/mcb.15.7.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chevallier M R, Bloch J C, Lacroute F. Gene. 1980;11:11–19. doi: 10.1016/0378-1119(80)90082-7. [DOI] [PubMed] [Google Scholar]
- 15.Sikorski R, Hieter P. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahoney D J, Broach J R. Mol Cell Biol. 1989;9:4621–4630. doi: 10.1128/mcb.9.11.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes S, Broach J R. Genes Dev. 1996;10:1021–1032. doi: 10.1101/gad.10.8.1021. [DOI] [PubMed] [Google Scholar]
- 18.Braunstein M, Rose A B, Holmes S G, Allis C D, Broach J R. Genes Dev. 1993;7:592–604. doi: 10.1101/gad.7.4.592. [DOI] [PubMed] [Google Scholar]
- 19.Boeke J D, Trueheart J, Natsoulis G, Fink G R. Methods Enzymol. 1987;154:164–175. doi: 10.1016/0076-6879(87)54076-9. [DOI] [PubMed] [Google Scholar]
- 20.Gottschling D E, Aparicio O M, Billington B L, Zakian V A. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- 21.Braunstein M, Allis C D, Turner B M, Broach J R. Mol Cell Biol. 1996;16:4349–4356. doi: 10.1128/mcb.16.8.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman J B, Hicks J B, Broach J R. J Mol Biol. 1984;178:815–834. doi: 10.1016/0022-2836(84)90313-9. [DOI] [PubMed] [Google Scholar]
- 23.Wright J H, Gottschling D E, Zakian V A. Genes Dev. 1992;6:197–210. doi: 10.1101/gad.6.2.197. [DOI] [PubMed] [Google Scholar]
- 24.Loo S, Rine J. Science. 1994;264:1768–1771. doi: 10.1126/science.8209257. [DOI] [PubMed] [Google Scholar]
- 25.Renauld H, Aparicio O M, Zierath P D, Billington B L, Chhablani S K, Gottschling D E. Genes Dev. 1993;7:1133–1145. doi: 10.1101/gad.7.7a.1133. [DOI] [PubMed] [Google Scholar]
- 26.Hecht A, Laroche T, Strahl-Bolsinger S, Grunstein M. Nature (London) 1996;383:92–96. doi: 10.1038/383092a0. [DOI] [PubMed] [Google Scholar]
- 27.Pryde F E, Louis E J. EMBO J. 1999;18:2538–2550. doi: 10.1093/emboj/18.9.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boscheron C, Maillet L, Marcand S, Trai-Pflugfelder M, Gasser S, Gilson E. EMBO J. 1996;15:2184–2195. [PMC free article] [PubMed] [Google Scholar]
- 29.Mahoney D J, Marquardt R, Shei G J, Rose A B, Broach J R. Genes Dev. 1991;5:605–615. doi: 10.1101/gad.5.4.605. [DOI] [PubMed] [Google Scholar]