Abstract
In higher eukaryotic cells pleiomorphic compartments composed of vacuoles, tubules and vesicles move from the endoplasmic reticulum (ER) and the plasma membrane to the cell center, operating in early biosynthetic trafficking and endocytosis, respectively. Besides transporting cargo to the Golgi apparatus and lysosomes, a major task of these compartments is to promote extensive membrane recycling. The endocytic membrane system is traditionally divided into early (sorting) endosomes, late endosomes and the endocytic recycling compartment (ERC). Recent studies on the intermediate compartment (IC) between the ER and the Golgi apparatus suggest that it also consists of peripheral (“early”) and centralized (“late”) structures, as well as a third component, designated here as the biosynthetic recycling compartment (BRC). We propose that the ERC and the BRC exist as long-lived “mirror compartments” at the cell center that also share the ability to expand and become mobilized during cell activation. These considerations emphasize the functional symmetry of endomembrane compartments, which provides a basis for the membrane rearrangements taking place during cell division, polarization, and differentiation.
INTRODUCTION
In the primordial eukaryotic cells the secretory and endocytic pathways most likely carried out clearly distinct tasks, export and import. Subsequent increase in cell size and the development of complex endomembranes involved the diversification of the secretory and endocytic compartments and boosting of their biosynthetic and sorting functions. Thus, looking at present-day metazoan cells, the functions of these pathways seem to overlap, both contributing to the sorting and transport of lipids and proteins to their correct functional locations. Accordingly, the molecular machineries of these transport routes, including coat proteins, regulatory GTPases, fusion and tethering factors, and molecular motors, as well as the sorting signals that they recognize, share many similarities (Bonifacino and Glick, 2004). Recent studies, providing increasing evidence for the complexity and flexibility of these pathways (Lippincott-Schwartz et al., 2000; Perret et al., 2005), have also revealed that they establish functional connections at various branch points (Lipschutz et al., 2003; Sannerud et al., 2003; Toikkanen et al., 2003; Ellis et al., 2006).
Regarding secretory and endocytic trafficking, a major difference between modern unicellular eukaryotes and metazoan cells is probably the requirement for extensive membrane recycling. Lower eukaryotes, like yeast, could be considered as biosynthetic machines. These small, rapidly dividing cells are equipped with an efficient secretory apparatus, but have low endocytic capacity, precluding the necessity to develop recycling organelles either between the endoplasmic reticulum (ER) and the Golgi apparatus, or between the plasma membrane (PM) and the vacuole (yeast counterpart of lysosomes). By contrast, metazoan cells have evolved in their early biosynthetic pathway a specialized membrane compartment, the so-called intermediate compartment (IC). The existence of the IC promotes not only molecular sorting and ER-to-Golgi transport but, in particular, membrane recycling, which is vital for the maintenance of the functional integrity of the ER. Similarly, the complex endocytic system of these cells includes the endocytic recycling compartment (ERC), whose main function is the retrieval of membrane and components back to the PM.
The emerging similarities of the IC and endosomes as sorting organelles have been addressed earlier (Saraste and Kuismanen, 1992; Lippincott-Schwartz, 1993; Aridor and Balch, 1996; Warren and Mellman, 1999). However, recent work on the IC has provided new insight on the spatial and functional organization of this compartment. Similarly, the endosomal recycling apparatus turns out to be functionally more complex than has been previously thought. For example, in addition to membrane recycling the ERC is involved in the biogenesis of specialized compartments and the mobilization of endomembranes in response to various stimuli (Maxfield and McGraw, 2004; van Ijzendoorn, 2006). On the basis of these developments, we compare here ER–Golgi trafficking and endocytosis, with particular emphasis on the emerging similarities between the ERC and a newly identified, tubular subcompartment of the IC.
MEMBRANE RECYCLING IN THE ENDOCYTIC PATHWAY
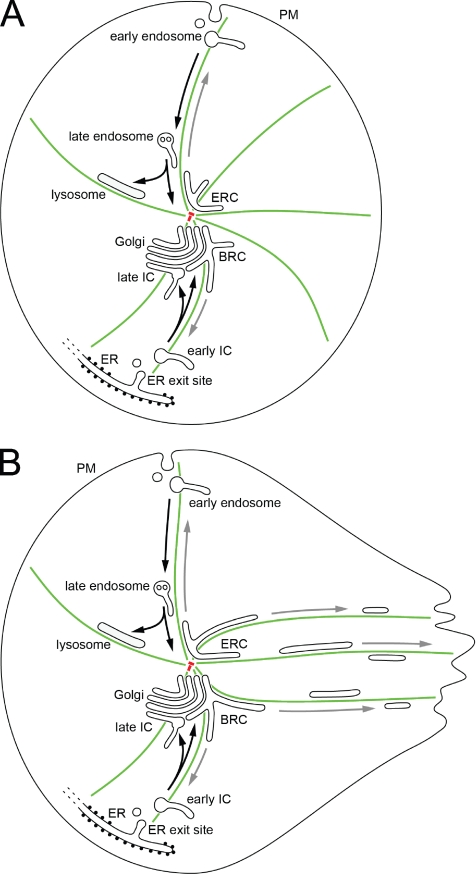
It is outside the scope of this review to give a detailed account of the complexity of the endocytic pathway (the reader is referred to a number of excellent reviews: Mellman, 1996; Conner and Schmid, 2003; Maxfield and McGraw, 2004; Perret et al., 2005; Roth, 2006), and we discuss only some of its features here. The endosomal system is classically divided into three main compartments: the early (sorting) endosomes and the late endosomes (or multivesicular bodies) that give rise to lysosomes, and the ERC (Figure 1). Because it has been estimated that the membrane surface engaged in endocytic trafficking exceeds the rate of membrane biosynthesis by 10-fold, it can be concluded that a major task of this system is in fact membrane recycling rather than delivery to lysosomes (Griffiths et al., 1989). Membrane retrieval most likely occurs from all three endosomal compartments.
Figure 1.
Models for early biosynthetic and endocytic trafficking in nonpolarized (A) and polarized (B) cells. ER-to-Golgi and PM-to-lysosome transport are topologically similar, both involving the centripetal movements of pleiomorphic compartments toward the cell center (black arrows). This centralization takes place along microtubules (green) and provides a basis for the classification of IC elements and endosomes into peripheral/early and central/late structures that, in addition to their cellular location, also differ in age and composition. Consequently, tubular components of the IC and endosomes also accumulate at the cell center, in the region close to the centrosomes (red), generating the BRC and the ERC, long-lived compartments that are involved in the recycling of membrane back to the ER and PM, respectively (gray arrows). The polarization of cells due to differentiation or activation by external cues (B) involves the expansion and mobilization of these tubular compartments, and the centrifugal, microtubule-based movement of BRC- and ERC-derived tubules toward one pole of the cell, such as the leading edge of a migrating fibroblast.
Early endosomes that are formed by the coalescence of primary endocytic vesicles—and/or fusion of these vesicles with pre-existing compartments—consist of vacuolar and tubular subdomains (Mellman, 1996). The majority of the internalized receptors, such as the transferrin receptor, are recycled to the PM from this site (Hao and Maxfield, 2000; Sheff et al., 2002). As the surface area-to-volume ratio of the tubular domain is greater than that of the vacuolar domain, the tubules preferentially sort membrane components from soluble molecules. This geometry-based sorting has been well documented in the case of receptor-mediated endocytosis (see Roth, 2006 and references therein). It results in efficient partitioning of soluble ligands to a vacuolar pathway that leads via late endosomes to lysosomes, whereas membrane-bound receptors are sorted to the narrow tubules and recycled back to the cell surface. On top of this geometry-based device, additional mechanisms such as membrane-bound coat complexes contribute to the efficiency of the sorting process.
The ERC consists of an extensive network of narrow-diameter tubules, whereas both early and late endosomes are more vacuolar in appearance (Hopkins, 1983; Yamashiro et al., 1984). Depending on the cell type, it is predominantly found in the pericentriolar region or, in addition, dispersed into distinct foci throughout the cytoplasm (Ullrich et al., 1996; Casanova et al., 1999; Wilcke et al., 2000). The ERC has the potential to sort molecules to different destinations, such as the trans-Golgi network (Wilcke et al., 2000), but its best characterized function is the recycling of membrane back to the PM. Interestingly, cytoplasts are able to regenerate ERC-like structures (Sheff et al., 2002), implying that this compartment is highly dynamic and can be formed or expanded to fulfill the requirements for membrane recycling.
A “NEW” VIEW OF THE IC
Compared with the endocytic route, the boundaries between the compartments of the early biosynthetic pathway have remained less well defined. This is largely due to the enigmatic nature of the IC mediating communication between the ER and the Golgi apparatus (Saraste and Kuismanen, 1992; Appenzeller-Herzog and Hauri, 2006). The IC was originally described as large vacuoles (up to 1 μm in diameter) containing cargo proteins en route from the ER to the Golgi apparatus (Saraste and Kuismanen, 1984; Kuismanen and Saraste, 1989). Subsequent studies have verified these observations (Ying et al., 2000; Horstmann et al., 2002; Fan et al., 2003; Mironov et al., 2003), but also shown that cargo proteins at the ER–Golgi boundary are localized to vesicular-tubular clusters (VTCs; Balch et al., 1994, Martinez-Menarguez et al., 1999). However, the relationship between the pre-Golgi vacuoles and the VTCs has remained unclear. One possibility, suggested by studies employing live cell imaging, is that the VTCs represent (or contain) the nascent IC elements that reside temporarily close to ER exit sites (ERES), whereas the vacuoles correspond to the large, mobile structures that migrate from the stationary ERES to the central Golgi region (Presley et al., 1997; Scales et al., 1997; Mironov et al., 2003; Sannerud et al., 2006). Namely, the IC elements forming close to the widely distributed ERES are concentrated in the Golgi region by a microtubule-dependent process (Saraste and Svensson, 1991; Presley et al., 1997; Scales et al., 1997). The microtubule-based, long-distance movement of the large pre-Golgi structures across the viscous cytoplasm is associated with a shape change from vacuolar to elongated (Presley et al., 2002; Sannerud et al., 2006).
Supporting the above model, it has been reported that the IC consists of two kinetically distinct “early” and “late” components, which besides their overall cellular location and relation with the Golgi, also differ in composition (Marra et al., 2001). Additional evidence for a late IC compartment, located at the entry side of Golgi stacks, has been obtained by electron microscopic tomography (Ladinsky et al., 1999). Thus, in topological terms the IC can be compared with endosomes that based on their microtubule-dependent movements can be divided into peripheral (early) and more centrally located (late) structures (Mellman, 1996; Figure 1).
There are also apparent similarities in the sorting mechanisms that take place in the early IC and sorting endosomes. The peripheral IC elements acquire membrane-bound COPI (coat protein I) coats (Shima et al., 1999; Stephens et al., 2000; Presley et al., 2002), which may play multiple roles in these structures. They appear to contribute to the maturation of the IC elements into transport-competent structures (Bonifacino and Lippincott-Schwartz, 2003) and facilitate the recycling of selected proteins, such as the cargo receptor ERGIC-53/p58, back to the ER (Letourneur et al., 1994; Klumperman et al., 1998; Lee et al., 2004). More specifically, COPI coats may be involved in the formation of membrane subdomains within the IC, in analogy to the bilayered clathrin coats that associate with sorting endosomes (Sachse et al., 2002). The binding of these coats is probably important for the retention of ERGIC-53/p58 in the vacuolar portion of the IC, because their disassembly by brefeldin A leads to missorting of the protein to a tubular domain (Sannerud et al., 2006; see below). Similar missorting of ERGIC-53/p58 takes place when the lumen of the IC is neutralized (Palokangas et al., 1998). Interestingly, COPI coats have also been implicated in molecular sorting within the early endosomes (Guo et al., 1994; Whitney et al., 1995; Aniento et al., 1996), where their function seems to be regulated by endosomal acidification (Gu and Gruenberg, 2000).
Forward membrane flow at the ER–Golgi boundary must be counteracted by extensive membrane recycling to maintain the biosynthetic functions of the ER (Martinez-Menarguez et al., 1999; Sannerud et al., 2003). In addition, newly synthesized cargo en route to the Golgi and post-Golgi locations must be efficiently segregated from resident ER components, which are retrieved back to ER (Pelham, 1989; Martinez-Menarguez et al., 1999). A third analogy between ER–Golgi trafficking and endocytosis is illustrated by recent studies revealing that the IC also includes a tubular component with striking resemblance to endosomal tubules. This domain of the IC could be the functional counterpart of the ERC. Namely, the pre-Golgi tubules, which are enriched in the GTPase Rab1, are largely devoid of secretory cargo, suggesting that they operate in retrograde transport at the ER–Golgi boundary (Palokangas et al., 1998). Live cell imaging employing fluorescent Rab1 showed that these narrow-diameter tubules emerge from the vacuolar domains of the IC and move in retrograde direction toward the ERES, providing additional evidence for their function in membrane recycling (Sannerud et al., 2006; Figure 1). The separation of the IC into vacuolar and tubular subdomains can also be seen at the cellular level, as shown by our studies of polarized PC12 cells (Sannerud et al., 2006). Because of the properties of the tubules and their resemblance to the ERC, we propose the term biosynthetic recycling compartment (BRC) to describe this domain of the IC.
ERC AND BRC: PLASTICITY AND FUNCTIONAL DIVERSITY
Eukaryotic cells have the remarkable capacity to mobilize or reorganize their endomembrane compartments during various cellular responses. This organelle plasticity is an essential part of many activation processes, and is required, e.g., during cell motility, polarization, and differentiation (Singer and Kupfer, 1986; Nabi, 1999; Lecuit and Pilot, 2003). It is noteworthy that these membrane rearrangements primarily involve the proliferation of tubular domains of organelles. Because activation often involves the delivery of membranes to specific regions of the cell, it requires a source that can be rapidly mobilized and expanded. Dynamic tubular compartments, like ERC and BRC, appear to qualify as such membrane reservoirs.
Mobilization of ERC and BRC
The expansion and microtubule-based mobilization of the ERC has been shown to occur in a number of situations involving cell activation (van Ijzendoorn, 2006). For example, movement of membranes from the pericentriolar ERC to the leading lamella takes place in motile fibroblasts (Hopkins et al., 1994). Phagocytosis, a process that requires the recruitment of endomembranes to the nascent phagosome, provides another example of a cellular activation process that involves the mobilization of ERC-derived tubules (Jutras and Desjardins, 2005).
In addition, there are several examples of cell types, in which ERC-derived membranes can be used for the buildup of specialized internal compartments. For instance, Birbeck granules, rod-shaped structures specific to epidermal Langerhans cells, which may be involved in antigen presentation, appear to be derived from subdomains of the ERC (McDermott et al., 2002). The vesicular compartment of adipocytes and cardiomyocytes, containing the glucose transporter GLUT4, also seems to be generated from the ERC (Zeigerer et al., 2002; Uhlig et al., 2005). A further example is the subapical recycling compartment found in epithelial cells. This compartment that plays a key role in the biogenesis and maintenance of the apical and basolateral domains of the polarized cells is located at the crossroads of several intracellular transport pathways (Hoekstra et al., 2004; Ellis et al., 2006).
It now seems evident that the IC can also expand and become mobilized during cell activation or differentiation. Interestingly, the Rab1-positive pre-Golgi tubules move to the neurites of the polarized PC12 cells (Sannerud et al., 2006), indicating that the BRC is capable of expansion and functional specialization. An important observation is that the tubules that are devoid of anterograde markers contain HMG-CoA-reductase, a key enzyme of cholesterol biosynthesis, as well as other ER proteins (BiP and calreticulin), suggesting that they correspond to smooth ER (SER; Sannerud et al., 2006; unpublished results). These dynamic tubules segregating from the vacuolar parts of the IC can thus be functionally uncoupled from ER–Golgi trafficking to create a Golgi-bypass pathway that connects the IC with cell periphery (Sannerud et al., 2006). Already in the 1970s neuronal SER was visualized by heavy metal impregnation as a continuous tubular network extending from the cell body to the axon terminals and establishing close contacts with the axonal PM. Autoradiographic studies further suggested that the SER establishes a direct pathway for fast axonal transport of membrane-bound components, such as phospholipids and glycoproteins, between RER in the cell body and PM of the axon (see Rambourg and Droz, 1980 and references therein).
The BRC is also mobilized in migrating cells, radiating tubules to the pseudopodia or the leading lamella (Sannerud et al., 2006; Dale, Marie, and Saraste, unpublished results). Phagocytosis provides another example of a situation that may involve the mobilization of the BRC. First, engulfment of large particles by macrophages depends on the function of Sec22b/ERS-24 (Becker et al., 2005), a fusion protein (SNARE) that is specifically enriched in the IC (Zhang et al., 1999). Another ER/IC-localized SNARE, syntaxin 18, has also been implicated in this process (Hatsuzawa et al., 2006). Second, IC membranes participate in the formation of phagocytic vacuoles containing internalized intracellular pathogens, such as Legionella and Chlamydia (Kagan and Roy, 2002; Rzomp et al., 2003). Rapid recruitment of Sec22b and Rab1 to Legionella-containing vacuoles (Derre and Isberg, 2004; Kagan et al., 2004) suggests the involvement of BRC-derived tubules. Similarly, mobilization of SER membranes has been implicated in phagocytosis (Stendahl et al., 1994; Jutras and Desjardins, 2005).
Role of Rab1 and Rab11
The molecular mechanisms underlying the expansion and mobilization of the ERC and BRC remain largely unknown. However, the two GTPases of the Rab family, Rab1 and Rab11, appear as key players in these events. Based on the general model suggesting that Rab GTPases bring lipids and proteins together to determine the properties of organelle membranes (Zerial and McBride, 2001; Munro, 2004), the BRC and ERC could be defined by Rab1 and Rab11, respectively. Accordingly, in addition to its established role in endocytic recycling, Rab11 appears to be directly involved in the biogenesis and maintenance of specialized ERC-derived compartments. For example, it seems to play a direct role in the biogenesis of Birbeck granules in Langerhans cells (Uzan-Gafsou, Bausinger, Proamer, Monier, Lipsker, Cazenave, Goud, de la Salle, Salamero, and Hanau, unpublished results). Rab11 is also required for the formation of bile canaliculi in liver cells (Wakabayashi et al., 2005), GLUT-4 containing vesicles in adipocytes (Zeigerer et al., 2002), and the subapical recycling compartment in epithelial cells (Casanova et al., 1999). The role of Rab1 in the BRC requires further investigation. Evidence exists that Rab1 is required for ER-to-Golgi transport (Tisdale et al., 1992; Nuoffer et al., 1994) but, as discussed above, it could as well function in membrane recycling. Moreover, in analogy to Rab11, Rab1 can be involved in the formation a specialized tubular compartment derived from the BRC, as illustrated by the studies of differentiating PC12 cells (Sannerud et al., 2006).
By coordinating input from several compartments, Rab proteins could be ideal candidates for modulating the plasticity and expansion of pre-existing membrane entities. For example, Rab11 participates in the rapid mobilization of ERC membranes to the nascent phagosome (Cox et al., 2000; Murray et al., 2005) and has been recently shown to be involved in neurite outgrowth by regulating membrane trafficking through its interaction with protrudin (Shirane and Nakayama, 2006). As it is well established that Rab proteins can interact directly or indirectly with microtubule- or actin-based motors (Goud, 2002; Grosshans et al., 2006), expansion of the ERC and BRC is likely to be mediated by motors that interact with Rab1 and Rab11. Myosin Vb has been characterized as one of the effectors of Rab11 (Lapierre et al., 2001), whereas the motors that associate with Rab1 remain to be identified. Finally, there is evidence that Rab proteins associate with the tubular domains of both endocytic and secretory compartments (Klumperman et al., 1993; Palokangas et al., 1998; Weigert et al., 2004; Hattula et al., 2006; Sannerud et al., 2006), besides binding to the vacuolar domains. Other Ras-like GTPases are able to directly induce the formation of tubules, such as Sar1 at ERES (Aridor et al., 2001), and Arl1, a member of the Arf family, in the Golgi (Lu et al., 2001). Whether Rab proteins also have this capacity requires further investigation.
ERC AND BRC: MIRROR COMPARTMENTS?
We have seen that endosomes and the IC are pleiomorphic compartments whose major function is membrane sorting for anterograde and, especially, retrograde (recycling) purposes. They may use common mechanisms, including geometry-based sorting, coat proteins and Rab GTPases to generate distinct sorting domains. In analogy to endosomes that can be divided into three compartments: early (sorting) and late endosomes and the ERC, we propose that the IC is similarly composed of three domains, peripheral/early, centralized/late, and the BRC. It is then possible to outline a simplified map that highlights the functional symmetry of the IC and the endosomal system (Figure 1). In this topological context the BRC and ERC occupy equivalent positions and could be considered as mirror compartments. In nonpolarized (stationary) cells, these tubular compartments, like the Golgi (Rios and Bornens, 2003), are typically localized to the cell center, most likely because of the microtubule-based centralization of the pre-Golgi elements and endosomes (Figure 1A). Generally, the centralization of endomembrane compartments most likely favors the establishment of gradients and the integration of signaling events between the periphery of the cell and its center. In the case of BRC and ERC, it may also be required for the regulated mobilization of these compartments upon cell activation, for example during the delivery of membranes to the leading lamella of motile cells (Figure 1B). Moreover, it may be necessary for the concerted reorganization of these compartments during cell division, polarization, and differentiation. This topological symmetry also suggests that the biosynthetic and endocytic pathways developed synergistically during the evolution of complex endomembranes. The apparent cooperation of pre-Golgi membranes and endosomes during phagocytosis is an indication such early interactions.
The similar positioning of the BRC and ERC at the cell center and their comparable dynamics during cell activation and polarization (Figure 1) set the stage for their functional connection. The establishment of close contacts between them could promote nonvesicular exchange of ions and small molecules, such as lipids (Levine, 2004). Furthermore, the emerging role of the ERC as a common sorting site along the endocytic and secretory pathways provides a precedent for their possible communication via membrane traffic. Namely, recent studies of polarized epithelial cells have shown that the ERC can operate as a way station in biosynthetic protein transport from the Golgi to the PM (Ang et al., 2004; Lock and Stow, 2005; Murray et al., 2005). There is also increasing evidence that the sorting of selected proteins destined to the different PM domains is initiated at pre-Golgi locations (Alfalah et al., 2005; Tveit et al., 2005; Ellis et al., 2006). Further, experiments with both yeast and mammalian cells have revealed that the sorting of proteins can take place at or close to their exit from the ER (Bagnat et al., 2000; Watanabe and Riezman, 2004). If such pre-Golgi sorting events coincided with the segregation of specific cargo to IC tubules, they could lead to the use of an alternative pathway distinct from the traditional route via trans-Golgi, involving direct communication between the BRC and ERC.
In addition to delivering membranes, the ERC and BRC could transport mRNA between central and peripheral parts of the cell. Namely, recent studies have shown that mRNA transport and membrane traffic are coupled events, implicating the Rab11-positive ERC both in the localization of viral RNA and the transport of mRNA in Drosophila (Cohen, 2005). Moreover, work with yeast Saccharomyces cerevisiae has revealed a functional connection between mRNA transport and ER–Golgi trafficking (Trautwein et al., 2004), as well as a link between mRNA localization, cortical ER, and local protein synthesis (Jüschke et al., 2004). Interestingly, selected yeast proteins appear to utilize a novel, Sec18-independent pathway to gain access from the cortical ER to the PM (Jüschke et al., 2005). Based on the properties of the BRC and ERC discussed above, it is tempting to speculate that these dynamic tubular compartments could be involved in the corresponding events in mammalian cells.
In conclusion, studies carried out during the last decade or so have begun to reveal the extraordinary dynamics of membrane compartments and the communication pathways that connect them. One outcome has been the recognition that these dynamic events are not incidental, but organized according to distinct topological rules. To understand cellular physiology, the challenge for the future is to integrate our knowledge of molecular events with a combined picture of cell dynamics and global organization.
ACKNOWLEDGMENTS
We are grateful to Michel Bornens, Esa Kuismanen, Jean Salamero, Anni Vedeler, and Joanne Young for critical reading of the manuscript. We also thank the people in our laboratories for fruitful discussions and Michaël Marie for additional help with the models in Figure 1. Finally, special thanks to the two anonymous reviewers whose extensive and constructive comments helped us to improve the manuscript. This work was partly supported by the E. de Rotschild and Y. Mayent Fellowship to J.S.
Abbreviations used:
- BRC
biosynthetic recycling compartment
- COP
coat protein
- ERES
ER exit site
- ERC
endocytic recycling compartment
- IC
intermediate compartment.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-10-0933) on January 31, 2007.
REFERENCES
- Alfalah M., Wetzel G., Fischer I., Busche R., Sterchi E. E., Zimmer K. P., Sallmann H. P., Naim H. Y. A novel type of detergent-resistant membranes may contribute to an early protein sorting event in epithelial cells. J. Biol. Chem. 2005;280:42636–42643. doi: 10.1074/jbc.M505924200. [DOI] [PubMed] [Google Scholar]
- Ang A. L., Taguchi T., Francis S., Fölsch H., Murrels L. J., Pypaert M., Warren G., Mellman I. Recycling endosomes can serve as intermediates during trans-port from the Golgi to the plasma membrane in MDCK cells. J. Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniento F., Gu F., Parton R. G., Gruenberg J. An endosomal β-COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J. Cell Biol. 1996;133:29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenzeller-Herzog C., Hauri H.-P. The ER-Golgi intermediate compartment: in search of its identity and function. J. Cell Sci. 2006;119:2173–2183. doi: 10.1242/jcs.03019. [DOI] [PubMed] [Google Scholar]
- Aridor M., Balch W. E. Principles of selective transport: coat complexes hold the key. Trends Cell Biol. 1996;6:315–320. doi: 10.1016/0962-8924(96)10027-1. [DOI] [PubMed] [Google Scholar]
- Aridor M., Fish K. N., Bannykh S., Weissman J., Roberts T. H., Lippincott-Schwartz J., Balch W. E. The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J. Cell Biol. 2001;152:213–229. doi: 10.1083/jcb.152.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnat M., Keränen S., Shevchenko A., Shevchenko A., Simons K. Lipid raft function in biosynthetic delivery of proteins to the cell surface in yeast. J. Biol. Chem. 2000;97:3254–3259. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., McCaffery J. M., Plutner H., Farquhar M. G. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Becker T., Volchuk A., Rothman J. E. Differential use of endoplasmic reticulum membrane for phagocytosis in J774 macrophages. Proc. Natl. Acad. Sci. USA. 2005;102:4022–4026. doi: 10.1073/pnas.0409219102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., Glick B. S. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat. Rev. Mol. Cell Biol. 2003;4:409–414. doi: 10.1038/nrm1099. [DOI] [PubMed] [Google Scholar]
- Casanova J. E., Wang X., Kumar R., Bhartur S. G., Navarre J., Woodrum J. E., Altschuler Y., Ray G. S., Goldenring J. R. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol. Biol. Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. S. The role of membranes and membrane trafficking in RNA localization. Biol. Cell. 2005;97:5–18. doi: 10.1042/BC20040056. [DOI] [PubMed] [Google Scholar]
- Conner S. D., Schmid S. L. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Cox D., Lee D. J., Dale B. M., Calafat J., Greenberg S. A Rab11-containing rapidly recycling compartment in macrophages that promotes phagocytosis. Proc. Natl. Acad. Sci. USA. 2000;97:680–685. doi: 10.1073/pnas.97.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derre I., Isberg R. R. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect. Immun. 2004;72:3048–3053. doi: 10.1128/IAI.72.5.3048-3053.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M. A., Potter B. A., Cresawn K. O., Weisz O. Polarized biosynthetic traffic in renal epithelial cells: sorting, sorting everywhere. Am. J. Physiol. Renal Physiol. 2006;291:707–713. doi: 10.1152/ajprenal.00161.2006. [DOI] [PubMed] [Google Scholar]
- Fan J. Y., Roth J., Zuber C. Ultrastructural analysis of transitional endoplasmic reticulum and pre-Golgi intermediates: a highway for cars and trucks. Histochem. Cell Biol. 2003;120:455–463. doi: 10.1007/s00418-003-0597-1. [DOI] [PubMed] [Google Scholar]
- Goud B. How Rab proteins link motors to membranes. Nat. Cell Biol. 2002;4:77–78. doi: 10.1038/ncb0402-e77. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Back R., Marsh M. A quantitative analysis of the endocytic pathway in baby hamster kidney cells. J. Cell Biol. 1989;109:2703–2720. doi: 10.1083/jcb.109.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans B. L., Ortiz D., Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc. Natl. Acad. Sci. USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F., Gruenberg J. ARF1 regulates pH-dependent COP functions in the early endocytic pathway. J. Biol. Chem. 2000;275:8154–8160. doi: 10.1074/jbc.275.11.8154. [DOI] [PubMed] [Google Scholar]
- Guo Q., Vasile E., Krieger M. Disruptions in Golgi structure and membrane traffic in a conditional lethal mammalian cell mutant are corrected by epsilon-COP. J. Cell Biol. 1994;125:1213–1224. doi: 10.1083/jcb.125.6.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao M., Maxfield F. R. Characterization of rapid membrane internalization and recycling. J. Biol. Chem. 2000;275:15279–15286. doi: 10.1074/jbc.275.20.15279. [DOI] [PubMed] [Google Scholar]
- Hatsuzawa K., Tamura T., Hashimoto H., Hashimoto H., Yokoya S., Miura M., Nagaya H., Wada I. Involvement of syntaxin 18, an endoplasmic reticulum (ER)-localized SNARE protein, in ER-mediated phagocytosis. Mol. Biol. Cell. 2006;17:3964–3977. doi: 10.1091/mbc.E05-12-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattula K., Furuhjelm J., Tikkanen J., Tanhuanpää K., Laakkonen P., Peränen J. Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J. Cell Sci. 2006;119:4866–4877. doi: 10.1242/jcs.03275. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., Tyteca D., van Ijzendoorn S.C.D. The sub-apical compartment: a traffic center in membrane polarity development. J. Cell Sci. 2004;117:2183–2192. doi: 10.1242/jcs.01217. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R. Intracellular routing of transferrin and transferrin receptors in epidermoid carcinoma A431 cells. Cell. 1983;35:321–330. doi: 10.1016/0092-8674(83)90235-0. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Gibson A., Shipman M., Strickland D. K., Trowbridge I. S. In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then routed to the plasma membrane of the leading lamella. J. Cell Biol. 1994;125:1265–1274. doi: 10.1083/jcb.125.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann H., Ng C. P., Tang B. L., Hong W. Ultrastructural characterization of endoplasmic reticulum-Golgi transport containers (EGTC) J. Cell Sci. 2002;115:4263–4273. doi: 10.1242/jcs.00115. [DOI] [PubMed] [Google Scholar]
- Jutras I., Desjardins M. Phagocytosis: at the crossroads of innate and adaptive immunity. Annu. Rev. Cell Dev. Biol. 2005;21:511–527. doi: 10.1146/annurev.cellbio.20.010403.102755. [DOI] [PubMed] [Google Scholar]
- Jüschke C., Ferring D., Jansen R. P., Seedorf M. Novel transport pathway for a yeast plasma membrane protein encoded by a localized mRNA. Curr. Biol. 2004;14:406–411. doi: 10.1016/j.cub.2004.02.034. [DOI] [PubMed] [Google Scholar]
- Jüschke C., Wächter A., Schwappach B., Seedorf M. SEC18/NSF-independent, protein-sorting pathway from the yeast cortical ER to the plasma membrane. J. Cell Biol. 2005;169:613–622. doi: 10.1083/jcb.200503033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J. C., Roy C. R. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat. Cell Biol. 2002;4:945–954. doi: 10.1038/ncb883. [DOI] [PubMed] [Google Scholar]
- Kagan J. C., Stein M. P., Pypaert M., Roy C. R. Legionella subvert the functions of Rab1 and Sec22b to create a replicative organelle. J. Exp. Med. 2004;199:1201–1211. doi: 10.1084/jem.20031706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J., Hille A., Veenendaal T., Oorschot V., Stoorvogel W., von Figura K., Geuze H. J. Differences in the endosomal distributions of the two mannose 6-phosphate receptors. J. Cell Biol. 1993;121:997–1010. doi: 10.1083/jcb.121.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J., Schweizer A., Clausen H., Tang B. L., Hong W., Oorschot V., Hauri H. P. The recycling pathway of protein ERGIC-53 and dynamics of the ER-Golgi intermediate compartment. J. Cell Sci. 1998;111:3411–3425. doi: 10.1242/jcs.111.22.3411. [DOI] [PubMed] [Google Scholar]
- Kuismanen E., Saraste J. Low temperature-induced transport blocks as tools to manipulate membrane traffic. Methods Cell Biol. 1989;32:257–274. doi: 10.1016/s0091-679x(08)61174-7. [DOI] [PubMed] [Google Scholar]
- Ladinsky M. S., Mastronarde D. N., McIntosh J. R., Howell K. E., Staehelin L. A. Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J. Cell Biol. 1999;144:1135–1149. doi: 10.1083/jcb.144.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre L. A., Kumar R., Hales C. M., Navarre J., Bhartur S. G., Burnette J. O., Provance D. W., Jr., Mercer J. A., Bahler M., Goldenring J. R. Myosin Vb is associated with plasma membrane recycling systems. Mol. Biol. Cell. 2001;12:1843–1857. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit T., Pilot F. Developmental control of cell morphogenesis: a focus on membrane growth. Nat. Cell Biol. 2003;5:103–108. doi: 10.1038/ncb0203-103. [DOI] [PubMed] [Google Scholar]
- Lee M. C., Miller E. A., Goldberg J., Orci L., Schekman R. Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- Letourneur F., Gaynor E. C., Hennecke S., Demolliere C., Duden R., Emr S. D., Riezman H., Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Levine T. Short-range intracellular trafficking of small molecules across endoplasmic reticulum junctions. Trends Cell Biol. 2004;14:483–490. doi: 10.1016/j.tcb.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Lipschutz J. H., Lingappa V. R., Mostov K. E. The exocyst affects protein synthesis by acting on the translocation machinery of the endoplasmic reticulum. J. Biol. Chem. 2003;278:20954–20960. doi: 10.1074/jbc.M213210200. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J. Bidirectional membrane traffic between the endoplasmic reticulum and Golgi apparatus. Trends Cell Biol. 1993;3:81–88. doi: 10.1016/0962-8924(93)90078-f. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Roberts T. H., Hirschberg K. Secretory protein trafficking and organelle dynamics in living cells. Annu. Rev. Cell Dev. Biol. 2000;16:557–589. doi: 10.1146/annurev.cellbio.16.1.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J. G., Stow J. L. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol. Biol. Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Horstmann H., Ng C., Hong W. Regulation of Golgi structure and function by ARF-like protein 1 (Arl1) J. Cell Sci. 2001;114:4543–4555. doi: 10.1242/jcs.114.24.4543. [DOI] [PubMed] [Google Scholar]
- Marra P., Maffucci T., Daniele T., Tullio G. D., Ikehara Y., Chan E. K., Luini A., Beznoussenko G., Mironov A., De Matteis M. A. The GM130 and GRASP65 Golgi proteins cycle through and define a subdomain of the intermediate compartment. Nat. Cell Biol. 2001;3:1101–1113. doi: 10.1038/ncb1201-1101. [DOI] [PubMed] [Google Scholar]
- Martinez-Menarguez J. A., Geuze H. J., Slot J. W., Klumperman J. Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COPI-coated vesicles. Cell. 1999;98:81–90. doi: 10.1016/S0092-8674(00)80608-X. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R., McGraw T. E. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- McDermott R., et al. Birbeck granules are subdomains of endosomal recycling compartment in human epidermal Langerhans cells, which form where Langerin accumulates. Mol. Biol. Cell. 2002;13:317–335. doi: 10.1091/mbc.01-06-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Mironov A. A., et al. ER-to-Golgi carriers arise through direct en bloc protrusion and multistage maturation of specialized ER exit domains. Dev. Cell. 2003;5:583–594. doi: 10.1016/s1534-5807(03)00294-6. [DOI] [PubMed] [Google Scholar]
- Munro S. Organelle identity and the organization of membrane traffic. Nat. Cell Biol. 2004;6:469–472. doi: 10.1038/ncb0604-469. [DOI] [PubMed] [Google Scholar]
- Murray R. Z., Kay J. G., Sangermani D. G., Stow J. L. A role for the phagosome in cytokine secretion. Science. 2005;310:1492–1495. doi: 10.1126/science.1120225. [DOI] [PubMed] [Google Scholar]
- Nabi I. R. The polarization of the motile cell. J. Cell Sci. 1999;112:1803–1811. doi: 10.1242/jcs.112.12.1803. [DOI] [PubMed] [Google Scholar]
- Nuoffer C., Davidson H. W., Matteson J., Meinkoth J., Balch W. E. A GDP-bound of Rab1 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J. Cell Biol. 1994;125:225–237. doi: 10.1083/jcb.125.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palokangas H., Ying M., Väänänen K., Saraste J. Retrograde transport from the pre-Golgi intermediate compartment and the Golgi complex is affected by the vacuolar H+-ATPase inhibitor bafilomycin A1. Mol. Biol. Cell. 1998;9:3561–3578. doi: 10.1091/mbc.9.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H.R.B. Control of protein exit from the endoplasmic reticulum. Ann. Rev. Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Perret E., Lakkaraju A., Deborde S., Schreiner R., Rodriguez-Boulan E. Evolving endosomes: how many varieties and why? Curr. Opin. Cell Biol. 2005;17:1–12. doi: 10.1016/j.ceb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Presley J. F., Cole N. B., Schroer T. A., Hirschberg K., Zaal K. J., Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Presley J. F., Ward T. H., Pfeffer A. C., Siggia E. D., Phair R. D., Lippincott-Schwartz J. Dissection of COPI and Arf1 dynamics in vivo and role in Golgi membrane transport. Nature. 2002;417:187–193. doi: 10.1038/417187a. [DOI] [PubMed] [Google Scholar]
- Rambourg A., Droz B. Smooth endoplasmic reticulum and axonal transport. J. Neurochem. 1980;35:16–25. doi: 10.1111/j.1471-4159.1980.tb12484.x. [DOI] [PubMed] [Google Scholar]
- Rios R. M., Bornens M. The Golgi apparatus at the cell centre. Curr. Opin. Cell Biol. 2003;15:60–66. doi: 10.1016/s0955-0674(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Roth M. G. Clathrin endocytosis before fluorescent proteins. Nat. Rev. Mol. Cell Biol. 2006;7:63–68. doi: 10.1038/nrm1783. [DOI] [PubMed] [Google Scholar]
- Rzomp K. A., Scholtes L. D., Briggs B. J., Whittaker G. R., Scidmore M. A. Rab GTPases are recruited to Chlamydial inclusions in both a species-dependent and species-independent manner. Infect. Immun. 2003;71:5855–5870. doi: 10.1128/IAI.71.10.5855-5870.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse M., Urbe S., Oorschot V., Strous G. J., Klumperman J. Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol. Biol. Cell. 2002;13:1313–1328. doi: 10.1091/mbc.01-10-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannerud R., Saraste J., Goud B. Retrograde traffic in the biosynthetic-secretory route: pathways and machinery. Curr. Opin. Cell Biol. 2003;15:438–445. doi: 10.1016/s0955-0674(03)00077-2. [DOI] [PubMed] [Google Scholar]
- Sannerud R., Marie M., Nizak C., Dale H. A., Pernet-Gallay K., Perez F., Goud B., Saraste J. Rab1 defines a novel pathway connecting the pre-Golgi intermediate compartment with the cell periphery. Mol. Biol. Cell. 2006;17:1514–1526. doi: 10.1091/mbc.E05-08-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., Kuismanen E. Pre- and post-Golgi vacuoles operate in the transport of Semliki Forest virus membrane glycoproteins to the cell surface. Cell. 1984;38:535–549. doi: 10.1016/0092-8674(84)90508-7. [DOI] [PubMed] [Google Scholar]
- Saraste J., Kuismanen E. Pathways of protein sorting and membrane traffic between the rough endoplasmic reticulum and the Golgi complex. Semin. Cell Biol. 1992;3:343–355. doi: 10.1016/1043-4682(92)90020-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., Svensson K. Distribution of the intermediate elements operating in ER to Golgi transport. J. Cell Sci. 1991;100:415–430. doi: 10.1242/jcs.100.3.415. [DOI] [PubMed] [Google Scholar]
- Scales S. J., Pepperkok R., Kreis T. E. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Sheff D., Pelletier L., O'Connell C. B., Warren G., Mellman I. Transferrin receptor recycling in the absence of perinuclear recycling endosomes. J. Cell Biol. 2002;156:797–804. doi: 10.1083/jcb.20111048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima D. T., Scales S. J., Kreis T. E., Pepperkok R. Segregation of COPI-rich and anterograde-cargo-rich domains in endoplasmic-reticulum-to-Golgi transport complexes. Curr. Biol. 1999;9:821–824. doi: 10.1016/s0960-9822(99)80365-0. [DOI] [PubMed] [Google Scholar]
- Shirane M., Nakayama K. I. Protrudin induces neurite formation by directional membrane trafficking. Science. 2006;314:818–821. doi: 10.1126/science.1134027. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Kupfer A. The directed migration of eukaryotic cells. Annu. Rev. Cell Biol. 1986;2:337–365. doi: 10.1146/annurev.cb.02.110186.002005. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Krause K. H., Krischer J., Jerstrom P., Theler J. M., Clark R. A., Carpentier J. L., Lew D. P. Redistribution of intracellular Ca2+ stores during phagocytosis in human neutrophils. Science. 1994;265:1439–1441. doi: 10.1126/science.8073285. [DOI] [PubMed] [Google Scholar]
- Stephens D. J., Lin-Marq N., Pagano A., Pepperkok R., Paccaud J. COPI-coated ER-to-Golgi transport complexes segregate from COPII in close proximity to ER exit sites. J. Cell Sci. 2000;113:2177–2185. doi: 10.1242/jcs.113.12.2177. [DOI] [PubMed] [Google Scholar]
- Tisdale E. J., Bourne J. R., Khosravi-Far R., Der C. J., Balch W. E. GTP-binding mutants of Rab1 and Rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex J. Cell Biol. 1992;119:749–761. doi: 10.1083/jcb.119.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toikkanen J. H., Miller K. J., Söderlund H., Jäntti J., Keränen S. The beta subunit of the Sec61p endoplasmic reticulum translocon interacts with the exocyst complex in Saccharomyces cerevisiae. J. Biol. Chem. 2003;278:20946–20953. doi: 10.1074/jbc.M213111200. [DOI] [PubMed] [Google Scholar]
- Trautwein M., Dengjel J., Schirle M., Spang A. Arf1p provides an unexpected link between COPI vesicles and mRNA in Saccharomyces cerevisiae. Mol. Biol. Cell. 2004;15:5021–5037. doi: 10.1091/mbc.E04-05-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveit H., Dick G., Skibeli V., Prydz K. A proteoglycan undergoes different modifications en route to the apical and basolateral surfaces of Madin-Darby canine kidney cells. J. Biol. Chem. 2005;280:29596–29603. doi: 10.1074/jbc.M503691200. [DOI] [PubMed] [Google Scholar]
- Uhlig M., Passlack W., Eckel J. Functional role of Rab11 in GLUT4 trafficking in cardiomyocytes. Mol. Cell. Endocrinol. 2005;235:1–9. doi: 10.1016/j.mce.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Ullrich O., Reinsch S., Urbe S., Zerial M., Parton R. G. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ijzendoorn S. C. Recycling endosomes. J. Cell Sci. 2006;119:1679–1681. doi: 10.1242/jcs.02948. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y., Dutt P., Lippincott-Schwartz J., Arias I. M. Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells. Proc. Natl. Acad. Sci. USA. 2005;102:15087–15092. doi: 10.1073/pnas.0503702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G., Mellman I. Bulk flow redux? Cell. 1999;98:125–127. doi: 10.1016/s0092-8674(00)81006-5. [DOI] [PubMed] [Google Scholar]
- Watanabe R., Riezman H. Differential ER exit in yeast and mammalian cells. Curr. Opin. Cell Biol. 2004;16:350–355. doi: 10.1016/j.ceb.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Weigert R., Yeung A. C., Li J., Donaldson J. G. Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol. Biol. Cell. 2004;15:3758–3770. doi: 10.1091/mbc.E04-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney J. A., Gomez M., Sheff D., Kreis T. E., Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Wilcke M., Johannes L., Galli T., Mayau V., Goud B., Salamero J. Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-Golgi network. J. Cell Biol. 2000;151:1207–1220. doi: 10.1083/jcb.151.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro D. J., Tycko B., Fluss S. R., Maxfield F. R. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- Ying M., Flatmark T., Saraste J. The p58-positive pre-Golgi intermediates consist of distinct subpopulations of particles that show differential binding of COPI and COPII coats and contain vacuolar H+-ATPase. J. Cell Sci. 2000;113:3623–3638. doi: 10.1242/jcs.113.20.3623. [DOI] [PubMed] [Google Scholar]
- Zeigerer A., Lampson M. A., Karylowski O., Sabatini D. D., Adesnik M., Ren M., McGraw T. E. GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps. Mol. Biol. Cell. 2002;13:2421–2435. doi: 10.1091/mbc.E02-02-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M., McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhang T., Wong S. H., Tang B. L., Xu Y., Hong W. Morphological and functional association of Sec22b/ERS-24 with the pre-Golgi intermediate compartment. Mol. Biol. Cell. 1999;10:435–453. doi: 10.1091/mbc.10.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]