Abstract
The mountain yellow-legged frog (Rana muscosa) was once a common inhabitant of the Sierra Nevada (California, USA), but has declined precipitously during the past century due in part to the introduction of nonnative fish into naturally fishless habitats. The objectives of the current study were to describe (1) the effect of fish removal from three lakes (located in two watersheds) on the small, remnant R. muscosa populations inhabiting those lakes, and (2) the initial development of metapopulation structure in each watershed as R. muscosa from expanding populations in fish-removal lakes dispersed to adjacent habitats. At all three fish-removal lakes, R. muscosa population densities increased significantly following the removal of predatory fish. The magnitude of these increases was significantly greater than that observed over the same time period in R. muscosa populations inhabiting control lakes that remained in their natural fishless condition. Following these population increases, R. muscosa dispersed to adjacent suitable (but unoccupied) sites, moving between 200 and 900 m along streams or across dry land. Together, these results suggest that large-scale removal of introduced fish could result in at least partial reversal of the decline of R. muscosa. Continued monitoring of R. muscosa at the fish-removal sites will be necessary to determine whether the positive effects of fish eradication are sustained over the long-term, especially in light of the increasingly important role played by an emerging infectious disease (chytridiomycosis, caused by Batrachochytrium dendrobatidis) in influencing R. muscosa populations.
Keywords: Alpine lakes, Amphibian decline, Batrachochytrium dendrobatidis, Fish eradication, Introduced species, Restoration, Sierra Nevada
1. Introduction
Amphibian species are experiencing severe population declines around the world. Thirty-two percent of the world’s 5743 recognized amphibian species are globally threatened and at least 43% of all amphibian species are experiencing declines (Stuart et al. 2004). The magnitude of many of these declines suggests the impending extinction of many amphibian species during coming decades. Many different factors have been suggested as contributing to these declines, including habitat loss (Dodd and Smith, 2003), increases in ultraviolet radiation (Blaustein et al., 2003), climate change (Pounds et al., 1999), over-exploitation (Jensen and Camp, 2003), introduction of nonnative species (Kats and Ferrer, 2003), chemical contaminants (Boone and Bridges, 2003), and emerging diseases (Daszak et al., 2003). In addition to the effects of these individual factors, synergisms between two or more factors may also be important as drivers of amphibian declines (Blaustein and Kiesecker, 2002; Kiesecker, 2002; Pounds et al., 2006).
Stressors such as climate change and disease offer daunting prospects for the conservation and restoration of amphibians because of our limited ability to reverse changes caused by these stressors over time scales relevant to current conservation efforts. However, some effects, such as those caused by nonnative species introductions, may be more readily reversed. The introduction of fish into fishless habitats worldwide provides a notable example. The mountains of western North America contain thousands of lakes that remained in their natural fishless condition until the late 1800s, when extensive programs were undertaken to expand recreational fisheries by introducing nonnative trout (Oncorhynchus sp., Salmo sp., Salvelinus sp.) into many of these sites (Pister, 2001; Schindler and Parker, 2002). These introductions resulted in dramatic changes to the distribution of many native aquatic species (Anderson, 1980; Stoddard, 1987; Bradford et al., 1998; Carlisle and Hawkins, 1998; Knapp et al., 2001), including amphibians (Tyler et al., 1998; Knapp and Matthews, 2000; Pilliod and Peterson, 2001; Knapp, 2005). The possibility that the negative effects of introduced fish on amphibians can be reversed was suggested by studies showing that amphibians often recovered following the termination of stocking and the subsequent disappearance of introduced fish populations (Funk and Dunlap, 1999; Knapp et al., 2001; Knapp et al., 2005). This apparent reversibility was recently confirmed for the mountain yellow-legged frog, Rana muscosa, through the use of a multi-lake fish removal experiment (Vredenburg, 2004).
Rana muscosa is endemic to the Sierra Nevada of California and Nevada and to the Transverse and Peninsular Ranges of southern California where it was once a common resident of lakes, ponds, and streams (Stebbins, 2003). During the past century, R. muscosa has disappeared from more than 90% of its historic localities (Vredenburg et al., 2006) due in part to the widespread introduction of nonnative fish, and its listing under the U.S. Endangered Species Act was recently found to be “warranted” (U.S. Fish and Wildlife Service, 2003). Given recent evidence suggesting that this decline might be at least partially reversed by removing nonnative fish (Knapp et al., 2001; Vredenburg, 2004; Knapp et al., 2005), several agencies have begun planning ambitious fish removal efforts. However, at least two key questions remain unanswered regarding the efficacy of fish eradication as a tool to restore R. muscosa populations. First, the only previous fish removal experiment (Vredenburg, 2004) was conducted in a single watershed in the southern Sierra Nevada. Therefore, the degree to which those results are generalizable to other parts of the native range of R. muscosa is unknown. This is particularly important given that potential stressors of amphibian populations in addition to introduced fish (e.g., pesticides) are unlikely to be evenly distributed across the native range of R. muscosa (Davidson, 2004). In addition, the R. muscosa populations reported in Vredenburg (2004) are actually highly genetically divergent from those in the central and northern Sierra Nevada (Vredenburg et al., 2006). Second, Vredenburg (2004) focused only on changes in R. muscosa population sizes in the fish-removal lakes themselves. As a result, considerable uncertainty currently exists regarding the extent to which frog populations that recover at a single site following fish removal will be able to recolonize adjacent water bodies to recreate historic metapopulation structures (Bradford et al., 1993).
The objectives of the current study were two-fold. First, we described the effect of fish removal from a series of lakes in two watersheds on the small, remnant R. muscosa populations inhabiting these sites. These watersheds are widely separated from the watershed used by Vredenburg (2004) and may therefore be subject to a different set of stressors that could influence experimental outcomes. In addition, the R. muscosa inhabiting our study watersheds are genetically distinct (Vredenburg et al. 2006) from those that were the focus of a previous fish removal experiment (Vredenburg 2004). Second, we described the initial establishment of R. muscosa metapopulation structures as frogs from the expanding R. muscosa populations at the fish-removal lakes dispersed to adjacent water bodies.
2. Methods
2.1. Study area description
The two study areas, referred to here as Humphreys Basin and LeConte Basin, are located in the John Muir Wilderness (Sierra National Forest) and the adjacent northern Kings Canyon National Park, respectively. These basins are located 55 km (Humphreys Basin) and 40 km (LeConte Basin) north-northwest of Sixty Lake Basin where the fish eradication experiment described in Vredenburg (2004) was conducted. Our two study basins are located in the alpine zone and contain several oligotrophic lakes and ponds, all of which were naturally fishless (Knapp 1996). Lakes in the study basins were stocked with trout prior to 1950 and maintained self-sustaining trout populations between the time of initial stocking and when fish were removed during this study. The terrestrial environment is dominated by exposed granitic bedrock and scattered stands of whitebark pine (Pinus albicaulis) and lodgepole pine (Pinus contorta).
In Humphreys Basin, we described the distribution of R. muscosa and introduced trout in all water bodies in 1996 (Fig. 1A). Removal of the nonnative trout population from Marmot Lake (the only water body in the basin that contained R. muscosa) was initiated in September 1997 (Table 1). Following the successful removal of fish from Marmot Lake, we initiated trout population removal from Cony and No Good Lakes (Fig. 1A, Table 1). We chose to remove the trout populations from Cony and No Good Lakes despite the absence of any R. muscosa at these sites to increase the amount of fishless habitat in the vicinity of the R. muscosa population in Marmot Lake.
Fig. 1.

Map of the Humphreys Basin study area showing the distribution of Rana muscosa and nonnative fish populations (A) in 2000 when fish eradication from Marmot Lake was nearly complete and just beginning in Cony and No Good Lakes, and (B) in 2005, 3–5 years following fish eradication from the study lakes. Natural barriers to upstream fish movement are shown in (A) as thin black double lines.
Table 1.
Description of lakes from which nonnative fish populations were removed.
|
R. muscosa Presence
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lake Name | Basin Name | Lake Category | Maximum depth (m)1 | Surface area (ha)2 | Perimeter (m)2 | Elevation (m)3 | Fish Species4 | Pre-removal | Post-removal |
| Marmot | Humphreys | Primary | 7.8 | 3.1 | 833 | 3583 | GT × RT | Yes5 | Yes5 |
| Cony | Humphreys | Secondary | 3.2 | 1.5 | 531 | 3492 | BK | No | Yes6 |
| No Good | Humphreys | Secondary | 5.0 | 1.2 | 482 | 3516 | BK | No | Yes6 |
| Upper LeConte | LeConte | Primary | 5.7 | 1.5 | 579 | 3213 | GT × RT | Yes5 | Yes5 |
| Lower LeConte | LeConte | Primary | 1.5 | 0.5 | 293 | 3211 | GT × RT | Yes6 | Yes5 |
| Black Giant | LeConte | Secondary | 11.0 | 1.6 | 549 | 3153 | GT × RT | No | Yes6 |
Determined by sounding with a weighted line.
Obtained from a geographic information system hydrologic layer based on 7.5′ U.S. Geological Survey topographic maps.
Obtained from 7.5′ U.S. Geological Survey topographic maps.
BK: Brook trout (Salvelinus fontinalis); GT × RT: Golden trout (Oncorhynchus mykiss aguabonita) × rainbow trout (O. mykiss) hybrids.
Breeding population.
Non-breeding population.
We described the distribution of R. muscosa and introduced trout in all water bodies in LeConte Basin in 1997 (Fig. 2). Removal of the nonnative trout populations from Upper and Lower LeConte Lakes (Table 1; both lakes contained nonnative fish and small populations of R. muscosa) and the adjacent stream sections began in 2001 (Fig. 2). To increase the amount of fishless habitat in the vicinity of Upper and Lower LeConte Lakes, we also removed trout from adjacent habitats that did not contain R. muscosa. These included Black Giant Lake and the Middle Fork Kings River; Fig. 2, Table 1).
Fig. 2.

Map of the LeConte Basin study area in 2001 showing the distribution of Rana muscosa and nonnative fish populations just prior to the initiation of fish eradications in the three study lakes. Natural barriers to upstream fish movement are shown as thin black double lines. On the map, the Middle Fork Kings River flows from northwest to southeast. No map is provided of the fish and frog distribution at the end of the study because all fish had not yet been eradicated and fish distributions had therefore not changed markedly.
To distinguish the fish-removal lakes that were inhabited by R. muscosa before and during fish eradication (the primary focus of this study: Marmot, Upper LeConte, and Lower LeConte Lakes) from those that initially lacked R. muscosa (Cony, No Good, and Black Giant Lakes), we refer to the former as “primary” fish-removal lakes and to the latter as “secondary” fish-removal lakes.
2.2. Amphibian surveys
At the beginning of this study, we used diurnal visual encounter surveys (Crump and Scott, 1994) of entire water body perimeters to describe the abundance of R. muscosa adults, subadults, and tadpoles at all lentic sites in the study basins. After using this information to select the fish-removal lakes, we conducted 1–6 visual encounter surveys at each of the fish-removal lakes starting the year in which fish eradication was initiated and continuing through 2005 (average = 3.1 surveys at each lake per year). R. muscosa subadults and adults (both hereafter referred to as “frogs”) as well as tadpoles have high detectabilities during diurnal shoreline surveys conducted during summer. Their high detectability is a consequence of several factors. First, in the Sierra Nevada, water clarity in alpine lentic habitats is exceedingly high (Diamond et al., 2005) and habitats are structurally simple (e.g., lacking aquatic vegetation and submerged logs). Second, frogs spend the majority of the ice-free season on shore immediately adjacent to water and tadpoles are found primarily in near-shore shallows (Camp, 1917; Zweifel, 1955). Third, frogs are highly aquatic and spend little or no time in terrestrial habitats (Matthews and Pope 1999). And fourth, tadpoles are present throughout the summer (and during all other seasons) due to the 2–3 year duration of this life stage in R. muscosa (Zweifel, 1955; Vredenburg et al., 2005). As a result of their high detectability, visual encounter surveys provide estimates of R. muscosa abundance that are highly correlated with actual abundances (Vredenburg and Knapp, unpublished data). To maximize the accuracy of R. muscosa count data, surveys were typically conducted on sunny days and between 1000 and 1500 h when air temperatures were near their daily maxima and frogs and tadpoles were likely to be basking close to shore (Bradford, 1984).
2.3. Fish surveys and fish population eradication
We described the presence or absence of trout in all water bodies in each basin using visual encounter surveys or gill nets (Knapp and Matthews, 2000). In shallow ponds (< 3 m deep) and in streams, trout presence or absence was determined using visual encounter surveys conducted while walking the entire pond or stream shoreline. In deeper water bodies, fish presence or absence was determined using both visual surveys and a single sinking monofilament gill net set for 8–12 hours.
Eradication of trout populations from the six study lakes was conducted using sinking monofilament gill nets (Knapp and Matthews, 1998). Nets were 36 m long and 1.8 m tall, each with six 6 m panels with bar mesh sizes of 10, 12.5, 18.5, 25, 33, and 38 mm (manufactured by Lundgrens Fiskredskapsfabrik AB, Stockholm, Sweden). Each net was deployed perpendicular to the lake shoreline, with the end of the net containing the smallest mesh size anchored to the shore and the end of the net containing the largest mesh size anchored in deep water. Three to 13 gill nets were deployed continuously in each lake during the fish eradication period, with the number of gill nets used being a function of lake surface area. During the ice-free months (June–October), captured fish were removed from nets every 12–24 hours when catch rates were high and every 2–5 days when catch rates were low. Each fall, just prior to when the lakes froze over, we moved all nets to deep water and allowed them to fish under the ice unattended for the entire winter. The following spring, these overwintered nets were cleaned of captured fish immediately after ice-out. Gill netting was continued at a lake until catch rates fell to zero for an entire summer.
Both study basins contained fish-bearing streams associated with the fish-removal lakes. In Humphreys Basin, these stream sections included the stream flowing from Marmot to Cony Lake, from Cony Lake to a natural fish barrier (steep cascade) 100 m downstream, and flowing into and out of No Good Lake (Fig. 1A). These stream sections are all ephemeral, and fish populations were eliminated when all stream sections dried during consecutive summers (see Results). In LeConte Basin, fish-bearing stream sections included a stream flowing from Upper to Lower LeConte Lake, from Lower LeConte Lake to its confluence with the Middle Fork Kings River, and the Middle Fork Kings River itself. All stream sections were perennial and required active fish removal. Fish were removed by electrofishing these stream sections every 2–10 days during each summer from 2001 to 2005. The number of R. muscosa observed in each stream section while electrofishing was also recorded. Fish were also removed from several large pools in the Middle Fork Kings River using short sections of gill nets. One to seven gill net sections were deployed continuously each summer from 2002 through 2005.
2.4. Statistical analysis
To allow comparisons among the study lakes, all R. muscosa count data were transformed into frog and tadpole densities (number/10 m of shoreline) by dividing total counts by the lake perimeter (Table 1). The statistical significance of changes in frog and tadpole density following fish eradication at each lake was determined by comparing densities from the first five surveys and the last five surveys using one-tailed Wilcoxon rank-sum tests. In Marmot Lake, the first five surveys included one conducted in 1996, three in 1997, and the first survey conducted in 1998. The last five surveys included three surveys conducted in 2004 and two surveys conducted in 2005. In Upper and Lower LeConte Lakes, the first five surveys included one survey conducted in 1997, one in 2001, and the first three surveys conducted in 2002. The last five surveys were all conducted in 2005. Significant increases in R. muscosa populations in all fish-removal lakes would provide reasonably strong evidence that the negative effects of fish on this species can be reversed. However, it is possible that the R. muscosa populations in the fish-removal lakes increased not solely due to fish removal but also due to favorable environmental conditions that caused R. muscosa population increases across the Kings Canyon National Park-John Muir Wilderness region. Therefore, we conducted a second analysis in which we compared the magnitude of changes in tadpole and frog density between 1997 and 2005 ([2005 density]-[1997 density]) in the three primary fish-removal lakes versus in 22 control lakes that remained in their natural fishless condition and contained R. muscosa populations in both 1997 and 2005.
Control lakes were located in Kings Canyon National Park, the only portion of the native range of R. muscosa that still contains robust populations (Knapp, unpublished data). Lakes were selected using habitat criteria that maximized their similarity to the primary fish-removal lakes (maximum water depth ≥ 1.5 m, surface area ≥ 0.3 ha, elevation = 3000–3600 m; Table 1). In addition, we only selected control lakes whose R. muscosa populations showed no evidence of chytridiomycosis, the disease caused by the amphibian chytrid fungus (Batrachochytrium dendrobatidis). This additional criterion was important as B. dendrobatidis has not yet been detected in R. muscosa at any of the primary fish-removal lakes but is currently spreading across Kings Canyon National Park and in R. muscosa typically causes severe population declines or extinctions of (Rachowicz et al., 2006). Surveys for R. muscosa and fish were conducted at all control lakes in both 1997 and 2005 using the methods described in Sections 2.2 and 2.3. We determined the presence/absence of B. dendrobatidis in R. muscosa populations at the fish removal and control lakes during at least one survey between 2002 and 2005 (average = 2.5 surveys). Infection status was assessed using tadpole mouthpart inspections during 2002, 2003, and 2004, and molecular assays in 2004 and 2005 (Knapp and Morgan, 2006). The 22 control lakes that met our criteria came from nine different basins scattered across the eastern edge of Kings Canyon National Park. Primary fish-removal and control lakes likely experienced similar climatic conditions because all were located in the same alpine ecosystem (average elevation = 3336 m and 3358 m, respectively; average distance between fish-removal and control lakes = 25 km). Fish-removal and control lakes also had similar maximum depths (average = 5.7 m and 8.7 m, respectively) and surface areas (average = 1.7 ha and 2.9 ha, respectively).
The magnitude of changes in tadpole and frog densities in fish-removal lakes versus control lakes was compared using one-tailed Wilcoxon rank-sum tests. If changes in R. muscosa densities at the fish-removal lakes were due primarily to fish eradication and not to larger-scale environmental effects, density changes in the fish-removal lakes should be significantly larger than those in the control lakes. In all of our analyses, we used nonparametric tests instead of parametric tests because the data were typically not normally distributed and could not be transformed to approximate normality.
3. Results
3.1. Fish eradication
Following initiation of intensive gill netting, fish populations in the six fish-removal lakes were quickly reduced to a fraction of their original densities (Fig. 3). In the three fish-removal lakes in Humphreys Basin, complete eradication of all fish was accomplished by early in the third or fourth year of gill netting (Fig. 3A, B, C). Continued gill netting during subsequent years confirmed the complete eradication of each of these fish populations. A total of 345, 642, and 485 fish were removed from Marmot, Cony, and No Good Lakes, respectively. Fish inhabiting the stream sections between Marmot and Cony Lakes, immediately below Cony Lake, and above and below No Good Lake (Fig. 1A) were eliminated when these sections dried completely in 2000 and 2001. Fish remain in the stream below Cony Lake (Fig. 1B), but their reinvasion of Cony Lake is prevented by an impassible cascade on the outlet stream 100 m below the lake.
Fig. 3.

Catch rates of nonnative trout (number of fish captured/net-hour) through time in the Humphreys Basin (A, B, C) and LeConte Basin (D, E, F) fish-removal lakes. Primary fish-removal lakes are (A) Marmot, (D) Upper LeConte, and (E) Lower LeConte, and secondary fish-removal lakes are (B) Cony, (C) No Good, and (F) Black Giant. Open symbols indicate when eradication was complete. In LeConte Basin, complete eradication had not yet been achieved in any of the three fish-removal lakes by the end of the 2005 field season.
Fish removal from the three fish-containing lakes in LeConte Basin followed a similar pattern to that in Humphreys Basin (Fig. 3D, E, F), with rapid depletion of the fish populations in the first few weeks of gill netting. By the end of 2005, a total of 556, 490, and 297 fish had been removed by gill netting from Upper LeConte, Lower LeConte, and Black Giant Lakes, respectively. A total of 143 hours were spent electrofishing the stream sections adjacent to Upper to Lower LeConte Lakes and in the Middle Fork Kings River (Fig. 2) during the study period, and resulted in large reductions in fish density (LeConte stream; 2001: 23.4 fish captured/hour, 2005: 3.0 fish captured per hour; Middle Fork Kings River; 2001: 20.8 fish captured/hour, 2005: 2.8 fish captured per hour). By the end of the 2005 field season, we had removed 226 fish from the stream sections adjacent to Upper and Lower LeConte Lakes and 1913 fish from the Middle Fork Kings River. However, unlike in Humphreys Basin where fish eradication was complete by the fourth summer, in LeConte Basin small numbers of fish were still being caught during the fifth year of fish eradication in all three fish-removal lakes and in all stream sections. This slower rate of fish eradication was due to the difficulty of eradicating fish from stream sections. Despite the fact that fish were still present in LeConte Basin by the end of the study, trout densities and therefore potential predation by trout on R. muscosa had clearly been dramatically reduced.
3.2. Change in R. muscosa population size at the primary fish-removal lakes
In Humphreys Basin, the density of R. muscosa tadpoles and frogs in Marmot Lake was very low both before and during the period of fish eradication (Fig. 4A). In 2000, the year in which the last fish were captured, the tadpole population began to increase and this increase continued through 2003. In 2004, tadpole density decreased markedly and remained at this lower level in 2005 (Fig. 4A). Frog density began to increase in 2001 and this increase continued through 2005 (Fig. 4A). The rate of growth appeared to be fastest in 2003, slowing somewhat in 2004, and slowing further in 2005. Comparisons of R. muscosa densities in the first five surveys (1996, 1997, 1998) and the last five surveys (2004 (2005) indicated that during this period average tadpole density increased by 39-fold (from 0.3 to 11.6; P = 0.004) and average frog density increased by 40-fold (from 0.6 to 24.3; P = 0.004).
Fig. 4.

Density (± 1 S.E.) of Rana muscosa in (A) Marmot Lake, (B) Upper LeConte Lake, and (C) Lower LeConte Lake before, during, and after the eradication of nonnative fish. Arrows indicate the date on which fish removal was initiated.
Densities of R. muscosa tadpoles and frogs in Upper and Lower LeConte Lakes were also very low before fish eradication began, but increased rapidly following the initiation of fish removal (Fig. 4B, C). Maximum tadpole densities in both lakes peaked in 2004, and in 2005 decreased either markedly (Upper LeConte) or slightly (Lower LeConte). Initial increases in frog densities lagged those of tadpoles by one year (Upper LeConte) or two years (Lower LeConte). In Upper LeConte Lake, the frog population increased rapidly in 2003 and 2004, but population growth halted in 2005 (Fig. 4B). In Lower LeConte Lake, by 2005 the frog population was only in its second year of rapid growth, but also showed some indication of a slowing rate of increase (Fig. 4C). Comparisons of R. muscosa densities in the first five surveys (1997, 2001, 2002) and the last five surveys (2005) indicated that during this period average tadpole density increased by 7-fold in Upper LeConte Lake (from 12.2 to 91.0; P = 0.008) and by nearly 13-fold in Lower LeConte Lake (from 0.3 to 4.4; P = 0.008). During this same period, average frog density increased by 12-fold in Upper LeConte Lake (from 2.4 to 28.9; P = 0.006) and by 18-fold in Lower LeConte Lake (from 1.3 to 24.0; P = 0.004).
The increase in frog and tadpole densities in Marmot, Upper LeConte, and Lower LeConte Lakes between 1997 and 2005 was significantly greater than in the fishless control lakes (Fig. 5). The average change in tadpole density in the primary fish-removal lakes and control lakes was +35.2 and +2.3, respectively (P = 0.025). The average change in frog density in the fish removal-lakes and control lakes was +24.9 and +0.4, respectively (P = 0.0004). This provides strong support for the hypothesis that the changes in R. muscosa populations in the primary fish-removal lakes was the result of fish eradication and not regionally favorable conditions for R. muscosa population growth.
Fig. 5.
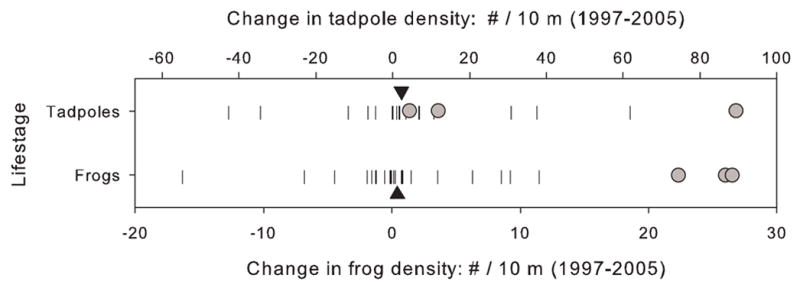
Change in density of Rana muscosa tadpoles and frogs in the 22 fishless control lakes (vertical lines) and in the three primary fish-removal lakes (gray circles) between 1997 and 2005. Black arrowheads indicate the average change in density of tadpoles and frogs in the control lakes.
3.3. Indications of R. muscosa metapopulation development
Coincident with the increase in R. muscosa in Marmot Lake, R. muscosa began dispersing to neighboring fishless lakes (Fig. 1). In 1996 and 2001, no R. muscosa were detected at Cony Lake (Fig. 1A). In late-June 2002 (approximately one week after ice-out and when the last fish was captured), one R. muscosa subadult was observed in Cony Lake near the mouth of the stream that drains from Marmot Lake (Fig. 1). A survey conducted in late August 2002 indicated the presence of at least 10 frogs, and the number of frogs in Cony Lake in subsequent years has continued to increase (maximum frog counts; 35 in 2003, 46 in 2004, 67 in 2005). No tadpoles were observed through 2005, indicating that all of the frogs in Cony Lake were the result of dispersal from Marmot Lake and not within-lake recruitment. No R. muscosa were detected in No Good Lake (Fig. 1A) during surveys conducted in 1996, 1999, 2000, 2001, or 2002. However, three frogs were detected at this site in 2003, one in 2004, and three in 2005 (Fig. 1B). Again, no evidence of reproduction (i.e., tadpoles) was found in No Good Lake through 2005. In Frog Lake, a total of five surveys were conducted in 2001, 2002, and early-2003 and no R. muscosa were detected (Fig. 1A). However, in late August 2003 three frogs were observed (Fig. 1B). Surveys conducted in 2004 (2 surveys) and 2005 (2 surveys) indicated maximum frog counts of six in both years. No evidence of reproduction was observed through 2005. Although the smaller ponds in the study basin are too shallow to support permanent R. muscosa populations and so have not been routinely surveyed, surveys conducted in 2004 and casual observations made in 2005 indicated that many of these sites are now occupied seasonally by small numbers of R. muscosa (Fig. 1B).
Similar results were observed in LeConte Basin where, following the initiation of fish removal, R. muscosa dispersed from the upper part of the basin into previously unoccupied portions of the Middle Fork Kings River and LeConte Meadow, and into Black Giant Lake (Fig. 2). No R. muscosa were detected in the fish-removal portion of the Middle Fork Kings River (upstream of the fish barrier below Black Giant Lake) in 2001. During surveys conducted in 2002, 2003, 2004, and 2005, the maximum number of frogs detected was 11, 8, 25, and 41, respectively. Similarly, surveys of Black Giant Lake conducted from 2001 through 2004 indicated a lack of R. muscosa. In 2005, R. muscosa were detected in Black Giant Lake, with 3–5 frogs counted in each of four surveys. No tadpoles were observed in these newly populated areas until 2005 (observed in the Middle Fork Kings River), indicating that all or most of the frogs in these areas were the result of dispersal from upstream sites and not within-site reproduction.
4. Discussion
4.1. R. muscosa population expansion
The rapid increases in R. muscosa population sizes in all three of the primary fish-removal lakes provides experimental support for the suggestion made previously (based on observational studies) that R. muscosa populations might recover following the disappearance of nonnative trout populations (Knapp et al., 2001; Knapp et al., 2005). These results are also consistent with those of a recent experiment in a different part of the Sierra Nevada (Vredenburg, 2004) that indicated rapid recovery of R. muscosa populations following fish removal. Collectively, results from these observational and experimental studies all indicate that nonnative trout are a major factor limiting R. muscosa populations but that these populations can quickly rebound following fish removal.
The pattern of R. muscosa population increase in the three primary fish-removal lakes was remarkably similar. In all three populations, tadpole density increased rapidly for several years and then declined. Frog densities also increased rapidly following fish removal, but the rate of increase slowed noticeably by the end of the study period. In addition, average frog densities in the final year of the study (2005) were similar between the three lakes (24.0–28.9/10 m of shoreline) and much more so than were tadpole densities (4.4–91.0/10 m of shoreline). Although future monitoring of these populations will be necessary to describe their longer-term density trajectories and the mechanisms underlying these trajectories, the consistent decreases in tadpole density following initial increases and comparable frog densities across lakes by the end of the study period suggest the action of similar factors limiting or regulating these populations following the removal of nonnative fish.
In both Humphreys and LeConte Basins, recovery of R. muscosa populations at the primary fish-removal lakes and the eradication of fish from adjacent streams resulted in large numbers of emigrating frogs that subsequently recolonized nearby unoccupied habitats, including secondary fish-removal lakes. In Humphreys Basin, the numbers of R. muscosa counted at previously unoccupied lakes in the vicinity of the Marmot Lake source population suggest that most frogs dispersing from Marmot Lake moved down the outlet stream to Cony Lake. This dispersal required a minimum distance moved of 880 m (Fig. 1), a distance consistent with previous observations that R. muscosa are capable of movements exceeding 1 km (Pope and Matthews, 2001). The R. muscosa that recolonized Frog and No Good Lakes could have done so by following streams or by traveling over land (Fig. 1). The fact that no frogs were ever seen in the fish-containing stream below Cony Lake or in the stream between No Good and Frog Lakes suggests that R. muscosa may more likely have dispersed over land (Marmot Lake to Frog Lake, and from Marmot Lake to Cony Lake and then over-land to No Good Lake). If so, the required over-land movements of at least 400 m and 200 m, respectively, would represent remarkable dispersal events for this highly aquatic species (Matthews and Pope, 1999; Pope and Matthews, 2001). Interestingly, R. muscosa were first detected at both Frog and No Good Lakes immediately following an unusually rainy 3-week period in July 2003. This may suggest that R. muscosa dispersal propensity was increased by the wet conditions or that wet conditions increased survival during dispersal. In LeConte Basin, the recolonization by R. muscosa of previously unoccupied habitat was likely the result of frogs dispersing down streams, but we were unable to calculate minimum dispersal distances because of the multiple potential source populations (Fig. 2).
Although no evidence of R. muscosa reproduction has yet been observed at the four lakes that were recently colonized by R. muscosa (Cony, No Good, Frog, Black Giant), this is likely the result of insufficient time and not an indication of the eventual failure by R. muscosa to establish self-sustaining populations at these four sites. The first frogs detected at these sites were all small, suggesting that most dispersing individuals were young subadults. Given that the transition from recently metamorphosed subadult to sexually mature adult may take four years in R. muscosa (Vredenburg et al., 2005), the first evidence of reproduction by R. muscosa was not expected until at least 2006 in Cony Lake, 2007 in No Good and Frog Lakes, and 2009 in Black Giant Lake. We plan future population surveys to document the eventual fate of what appear to be developing R. muscosa metapopulations in both Humphreys and LeConte Basins.
4.2. Implications for conservation
The results of our experiment clearly demonstrate that intensive gill netting is an effective means of eradicating nonnative trout populations from mountain lakes (Knapp and Matthews, 1998; Parker et al., 2001). Removal of these nonnative predators allowed for rapid increases in resident populations of R. muscosa and subsequent dispersal of R. muscosa from these source populations to adjacent suitable habitats. Our results are very similar to those reported by Vredenburg (2004) in a different portion of the range of R. muscosa, and together suggest that expanding the scope of fish eradication efforts across this range could reverse the decline of R. muscosa. However, we posit that the conservation of R. muscosa is considerably more complicated than the outcome of our experiments would suggest. Although the distribution of R. muscosa is clearly controlled in part by introduced fish, other factors may also have controlling influences. Of particular concern is the ongoing spread of chytridiomycosis through R. muscosa populations in the Sierra Nevada (Rachowicz et al., 2006). Outbreaks of this emerging infectious disease typically result in the extinction of R. muscosa populations, including those inhabiting lakes that remain in their natural fishless condition (Rachowicz et al., 2006). The spread of this disease suggests that although fish eradications done in areas not yet infected by B. dendrobatidis may result in rapid increases of R. muscosa over the short-term (Vredenburg, 2004; this study), these populations may subsequently decline following B. dendrobatidis outbreaks. Exactly this scenario is currently playing out in the fish-removal lakes described in Vredenburg (2004).
Despite this grim prognosis, there are several reasons why fish eradication is likely to remain a critically important tool for restoring R. muscosa populations. First, patterns of B. dendrobatidis spread remain enigmatic (Knapp, unpublished data) and it is not clear that B. dendrobatidis will eventually spread to all R. muscosa populations. Second, although the arrival of B. dendrobatidis may reduce the benefits to R. muscosa populations conveyed by fish eradications, our understanding of B. dendrobatidis and its effects on R. muscosa is still far from complete, and fish eradications may in fact confer long-term benefits. For example, the presence of nonnative fish has relegated many R. muscosa populations to marginal habitats (e.g., shallow ponds, lakes at the highest elevations; Knapp et al., 2003) that only support small frog populations or have increased the degree of R. muscosa population isolation (Bradford et al., 1993). As a result of small population sizes and isolation, these populations may have a lower likelihood of surviving stochastic events such as disease outbreaks. If some R. muscosa populations do persist following a disease outbreak, this creates the potential for host-pathogen evolution that may over time favor more resistant frogs and/or a less virulent strain of B. dendrobatidis.
In conclusion, nonnative trout have been widely introduced into mountain lakes worldwide (Pister, 2001; Schindler and Parker, 2002) and these introductions have resulted in negative impacts to numerous amphibian species (Braña et al., 1996; Tyler et al., 1998; Knapp and Matthews, 2000; Pilliod and Peterson, 2001). As such, the eradication of fish populations is likely to be a key element of any attempt to conserve and restore amphibian populations inhabiting these montane lentic environments, regardless of whether other anthropogenic factors also exert a controlling influence on these populations.
Acknowledgments
Financial support was provided by the National Science Foundation (DEB-9629473 and DEB-0075509 to R. Knapp and O. Sarnelle), National Institutes of Health/National Science Foundation Ecology of Infectious Disease Program (R01ES12067 to C. Briggs, R. Knapp, C. Moritz, and J. Taylor), and Sequoia and Kings Canyon National Parks. Research was permitted by the California Department of Fish and Game (Scientific Collecting Permits 801016–02 and 801088–03), the U.S. Forest Service, and Sequoia and Kings Canyon National Parks. Animal use was approved by University of California, Santa Barbara IACUC (Protocol 4–98–478 and 5– 01–478). We thank the following people for their invaluable assistance with this research: K. Armstrong, C. Archer, J. Asarian, M. Bibeau, E. Cole, L. Conrad, B. Czibesz, P. Epanchin, D. Ford, S. Giery, A. Hackmann, J. Kohen, A. Martinez, J. McClory, E. Meyer, J. Moore, P. Kirchner, A. Kramer, R. Peek, N. Perrill, C. Ray, S. Roll, E. Spies, L. Tauzer, T. Tunstall, M. Van Scoyoc, and H. Werner.
Footnotes
Publisher's Disclaimer: This is a PDF le of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its nal citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RS. Relationships between trout and invertebrate species as predators and the structure of the crustacean and rotiferan plankton in mountain lakes. In: Kerfoot WC, editor. Evolution and Ecology of Zooplankton Communities. University Press of New England; Hanover: 1980. pp. 635–641. [Google Scholar]
- Blaustein AR, Kiesecker JM. Complexity in conservation: lessons from the global decline of amphibian populations. Ecology Letters. 2002;5:597–608. [Google Scholar]
- Blaustein AR, Romansic JM, Kiesecker JM, Hatch AC. Ultraviolet radiation, toxic chemicals and amphibian population declines. Diversity and Distributions. 2003;9:123–140. [Google Scholar]
- Boone MD, Bridges CM. Effects of pesticides on amphibian populations. In: Semlitsch RD, editor. Amphibian Conservation. Smithsonian; Washington, D.C.: 2003. pp. 152–167. [Google Scholar]
- Bradford DF. Temperature modulation in a high-elevation amphibian, Rana muscosa. Copeia. 1984;1984:966–976. [Google Scholar]
- Bradford DF, Cooper SD, Jenkins TM, Jr, Kratz K, Sarnelle O, Brown AD. Influences of natural acidity and introduced fish on faunal assemblages in California alpine lakes. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:2478–2491. [Google Scholar]
- Bradford DF, Tabatabai F, Graber DM. Isolation of remaining populations of the native frog, Rana muscosa, by introduced fishes in Sequoia and Kings Canyon National Parks, California. Conservation Biology. 1993;7:882–888. [Google Scholar]
- Braña F, Frechilla L, Orizaola G. Effect of introduced fish on amphibian assemblages in mountain lakes of northern Spain. Herpetological Journal. 1996;6:145–148. [Google Scholar]
- Camp CL. Notes on the systematic status of the toads and frogs of California. University of California Publications in Zoology. 1917;17:115–125. [Google Scholar]
- Carlisle DM, Hawkins CP. Relationships between invertebrate assemblage structure, 2 trout species, and habitat structure in Utah mountain lakes. Journal of the North American Benthological Society. 1998;17:286–300. [Google Scholar]
- Crump ML, Scott NJ., Jr . Visual encounter surveys. In: Heyer WR, Donnelly MA, McDiarmid RW, Hayek L-AC, Foster MS, editors. Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians. Smithsonian; Washington, DC: 1994. pp. 84–91. [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. Infectious disease and amphibian population declines. Diversity and Distributions. 2003;9:141–150. [Google Scholar]
- Davidson C. Declining downwind: amphibian population declines in California and historic pesticide use. Ecological Applications. 2004;14:1892–1902. [Google Scholar]
- Diamond SA, Trenham PC, Adams MJ, Hossack BR, Knapp RA, Stark SL, Bradford D, Corn PS, Czarnowski K, Brooks PD, Fagre D, Breen B, Detenbeck NE, Tonnessen K. Estimated ultraviolet radiation doses in wetlands in six national parks. Ecosystems. 2005;8:462–477. [Google Scholar]
- Dodd CK, Smith LL. Habitat destruction and alteration: historical trends and future prospects for amphibians. In: Semlitsch RD, editor. Amphibian Conservation. Smithsonian; Washington, D.C.: 2003. pp. 94–112. [Google Scholar]
- Funk WC, Dunlap WW. Colonization of high-elevation lakes by long-toed salamanders (Ambystoma macrodactylum) after the extinction of introduced trout populations. Canadian Journal of Zoology. 1999;77:1759–1767. [Google Scholar]
- Jensen JB, Camp CD. Human exploitation of amphibians: direct and indirect impacts. In: Semlitsch RD, editor. Amphibian Conservation. Smithsonian; Washington, D.C.: 2003. pp. 199–213. [Google Scholar]
- Kats LB, Ferrer RP. Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Diversity and Distributions. 2003;9:99–110. [Google Scholar]
- Kiesecker JM. Synergism between trematode infection and pesticide exposure: A link to amphibian limb deformities in nature? Proceedings of the National Academy of Sciences, USA. 2002:152098899. doi: 10.1073/pnas.152098899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp RA. Pages 363–407 in Sierra Nevada Ecosystem Project: Final Report to Congress. III. University of California, Davis; Centers for Water and Wildland Resources: 1996. Non-native trout in natural lakes of the Sierra Nevada: an analysis of their distribution and impacts on native aquatic biota. (Available online at http://ceres.ca.gov/snep/pubs/v3.html.) [Google Scholar]
- Knapp RA. Effects of nonnative fish and habitat characteristics on lentic herpetofauna in Yosemite National Park, USA. Biological Conservation. 2005;121:265–279. [Google Scholar]
- Knapp RA, Hawkins CP, Ladau J, McClory JG. Fauna of Yosemite National Park lakes has low resistance but high resilience to fish introductions. Ecological Applications. 2005;15:835–847. [Google Scholar]
- Knapp RA, Matthews KR. Eradication of non-native fish by gill-netting from a small mountain lake in California. Restoration Ecology. 1998;6:207–213. [Google Scholar]
- Knapp RA, Matthews KR. Non-native fish introductions and the decline of the mountain yellow-legged frog from within protected areas. Conservation Biology. 2000;14:428–438. [Google Scholar]
- Knapp RA, Matthews KR, Preisler HK, Jellison R. Developing probabilistic models to predict amphibian site occupancy in a patchy landscape. Ecological Applications. 2003;13:1069–1082. [Google Scholar]
- Knapp RA, Matthews KR, Sarnelle O. Resistance and resilience of alpine lake fauna to fish introductions. Ecological Monographs. 2001;71:401–421. [Google Scholar]
- Knapp RA, Morgan JAT. Tadpole mouthpart depigmentation as an accurate indicator of chytridiomycosis, an emerging disease of amphibians. Copeia. 2006;2006:188–197. [Google Scholar]
- Matthews KR, Pope KL. A telemetric study of the movement patterns and habitat use of Rana muscosa, the mountain yellow-legged frog, in a high-elevation basin in Kings Canyon National Park, California. Journal of Herpetology. 1999;33:615–624. [Google Scholar]
- Parker BR, Schindler DW, Donald DB, Anderson RS. The effects of stocking and removal of a nonnative salmonid on the plankton of an alpine lake. Ecosystems. 2001;4:334–345. [Google Scholar]
- Pilliod DS, Peterson CR. Local and landscape effects of introduced trout on amphibians in historically fishless watersheds. Ecosystems. 2001;4:322–333. [Google Scholar]
- Pister EP. Wilderness fish stocking: history and perspective. Ecosystems. 2001;4:279–286. [Google Scholar]
- Pope KL, Matthews KR. Movement ecology and seasonal distribution of mountain yellow-legged frogs, Rana muscosa, in a high-elevation Sierra Nevada basin. Copeia. 2001;101:787–793. [Google Scholar]
- Pounds JA, Bustamante MR, Coloma LA, Consuegra JA, Fogden MPL, Foster PN, Marca EL, Masters KL, Merino-Viteri A, Puschendorf R, Ron SR, Sánchez-Azofeifa GA, Still CJ, Young BE. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- Pounds JA, Fogden MPL, Campbell JH. Biological response to climate change on a tropical mountain. Nature. 1999;398:611–615. [Google Scholar]
- Rachowicz LJ, Knapp RA, Morgan JAT, Stice MJ, Vredenburg VT, Parker JM, Briggs CJ. Emerging infectious disease as a proximate cause of amphibian mass mortality. Ecology. 2006;87:1671–1683. doi: 10.1890/0012-9658(2006)87[1671:eidaap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Rachowicz LJ, Knapp RA, Morgan JAT, Stice MJ, Vredenburg VT, Parker JM, Briggs CJ. Emerging infectious disease as a proximate cause of amphibian mass mortality. Ecology. 2006;87:1671–1683. doi: 10.1890/0012-9658(2006)87[1671:eidaap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Schindler DW, Parker BR. Biological pollutants: alien fishes in mountain lakes. Water, Air, and Soil Pollution: Focus. 2002;2:379–397. [Google Scholar]
- Stebbins RC. A Field Guide to Western Reptiles and Amphibians. 3. Houghton Mifflin; Boston: 2003. [Google Scholar]
- Stoddard JL. Microcrustacean communities of high-elevation lakes in the Sierra Nevada, California. Journal of Plankton Research. 1987;9:631–650. [Google Scholar]
- Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- Tyler T, Liss WJ, Ganio LM, Larson GL, Hoffman R, Deimling E, Lomnicky G. Interaction between introduced trout and larval salamanders (Ambystoma macrodactylum) in high-elevation lakes. Conservation Biology. 1998;12:94–105. [Google Scholar]
- U.S. Fish and Wildlife Service. 12-month finding for a petition to list the Sierra Nevada distinct population segment of the mountain yellow-legged frog (Rana muscosa) Federal Register. 2003;68:2283–2303. [Google Scholar]
- Vredenburg VT. Reversing introduced species effects: Experimental removal of introduced fish leads to rapid recovery of a declining frog. Proceedings of the National Academy of Sciences, USA. 2004;101:7646–7650. doi: 10.1073/pnas.0402321101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vredenburg VT, Fellers G, Davidson C. The mountain yellow-legged frog (Rana muscosa) In: Lannoo MJ, editor. Status and Conservation of U.S. Amphibians. University of California; Berkeley: 2005. pp. 563–566. [Google Scholar]
- Vredenburg VT, Bingham R, Knapp RA, Morgan JAT, Moritz C, Wake D. Concordant molecular and phenotypic data delineate new taxonomy and conservation priorities for the endangered mountain yellow-legged frog (Ranidae: Rana muscosa) Journal of Zoology. 2006 in press. [Google Scholar]
- Zweifel RG. Ecology, distribution, and systematics of frogs of the Rana boylei group. University of California Publications in Zoology. 1955;54:207–292. [Google Scholar]


