Abstract
Background
Hawthorn (Crataegus laevigata) leaves, flowers and berries are used by herbal practitioners in the UK to treat hypertension in conjunction with prescribed drugs. Small-scale human studies support this approach.
Aim
To investigate the effects of hawthorn for hypertension in patients with type 2 diabetes taking prescribed drugs.
Design of study
Randomised controlled trial.
Setting
General practices in Reading, UK.
Method
Patients with type 2 diabetes (n = 79) were randomised to daily 1200 mg hawthorn extract (n = 39) or placebo (n = 40) for 16 weeks. At baseline and outcome a wellbeing questionnaire was completed and blood pressure and fasting blood samples taken. A food frequency questionnaire estimated nutrient intake.
Results
Hypotensive drugs were used by 71% of the study population with a mean intake of 4.4 hypoglycaemic and/or hypotensive drugs. Fat intake was lower and sugar intake higher than recommendations, and low micronutrient intake was prevalent. There was a significant group difference in mean diastolic blood pressure reductions (P = 0.035): the hawthorn group showed greater reductions (baseline: 85.6 mmHg, 95% confidence interval [CI] = 83.3 to 87.8; outcome: 83.0 mmHg, 95% CI = 80.5 to 85.7) than the placebo group (baseline: 84.5 mmHg, 95% CI = 82 to 87; outcome: 85.0 mmHg, 95% CI = 82.2 to 87.8). There was no group difference in systolic blood pressure reduction from baseline (3.6 and 0.8 mmHg for hawthorn and placebo groups, respectively; P = 0.329). Although mean fat intake met current recommendations, mean sugar intake was higher and there were indications of potential multiple micronutrient deficiencies. No herb–drug interaction was found and minor health complaints were reduced from baseline in both groups.
Conclusions
This is the first randomised controlled trial to demonstrate a hypotensive effect of hawthorn in patients with diabetes taking medication.
Keywords: complementary therapies, Crataegus, herb–drug interactions, hypertension, phytotherapy, plant extracts
INTRODUCTION
Hawthorn berries, flowers and leaves (Crataegus laevigata [Poiret] DC) have been used traditionally throughout Europe to treat cardiovascular diseases including hypertension, myocardial dysfunction, angina and tachycardia.1 In France, it is also used for insomnia and anxiety.2 Twentieth century German research revealed the efficacy of hawthorn for the treatment of cardiac failure,3 and it is for this use that hawthorn is best known.
Similarities in therapeutic indications between hawthorn and cardioactive drugs have led to spurious conclusions about the toxicity of hawthorn resulting from its cardioactive glycosides akin to digoxins.4 However, hawthorn contains no cardioactive glycosides. The principle active components are flavonoids: non-toxic phytochemicals that are widespread in fruit and vegetables and that have health benefits.5 Hawthorn shows low toxicity in animal studies and minimal side effects in clinical trials.6 No drug–herb interaction has been reported in animal trials, and in a human study no interaction was observed between hawthorn and digoxin.7 Herbal practitioners use hawthorn for cardiovascular dysfunction, including mild manifestations in otherwise healthy people, without restriction on long-term use.1
A meta-analysis of clinical studies of hawthorn for cardiac failure provided preliminary evidence of its hypotensive effects.3 In a pilot study of mildly hypertensive, but otherwise healthy participants not taking prescribed drugs, there were promising hypotensive responses to 500 mg of hawthorn extract/day after 10 weeks.8 The hypotensive effect of hawthorn was studied in type 2 diabetic subjects because of the prevalence of hypertension and prescribed drug use among them.
Objective
To test if patients with type 2 diabetes consuming a typical western diet and taking prescribed medication with daily hawthorn extract show greater hypotensive effects over 16 weeks than a placebo group.
METHOD
Participants and setting
Eighty patients with type 2 diabetes, hypertension, diastolic blood pressure 85–95 mmHg, and systolic pressure of 145–165 mmHg were recruited from general practice records in the Reading area in the south of England from 2001 to 2002. Pregnant women and patients with heart disease or major pathology were excluded. All patients gave written informed consent to participate. Medical history, lifestyle, prescribed medication and dietary supplements were recorded using a health questionnaire at baseline. Volunteers were asked to maintain their baseline dietary and lifestyle habits throughout the study. Changes in prescribed medication or dietary supplement use were recorded at clinic visits using a short questionnaire
Intervention
Participants were randomised to treatment or placebo groups using an established method.9 Identical pill boxes numbered 1 to 80 contained hawthorn or placebo tablets according to the code. They were assigned blindly in order of participants' enrolment into the trial. Participants were given placebo or hawthorn at 1200 mg extract/day which is equivalent to 6 g of dried flowering tops (Faros® 600 [LI 132, Lichtwer Pharma, Berlin] extract 3:1, standardised to 2.2% flavonoids). Participants were instructed to take one tablet before breakfast and another before the evening meal. Coding was undertaken by one of the authors, who had no direct contact with participants. Other team members were blinded to coding. Adherence was assessed by counting returned tablets at outcome, when participants were asked to guess their treatment.
Study design and analyses
The study was a double-blinded, parallel, placebo-controlled trial. Participants attended clinics at the Clinical Investigation Suite of the Hugh Sinclair Unit of Human Nutrition at The University of Reading on three occasions: at baseline and after 8 and 16 weeks of intervention. Data collected at week 8 were used for monitoring purposes (unless required for replacement of missing outcome data) and for calculation of nutrient intake using the validated DIETQ Food Frequency Questionnaire (Version 3, Tinuviel Software, Warrington, UK).
How this fits in
Previous clinical studies have confirmed the traditional use hawthorn as a cardioprotective agent. There has been no report of interactions with conventional cardiovascular medication. Hawthorn exerts hypotensive effects with no herb–drug interactions when taken with modern glycaemic, lipaemic and/or hypotensive drugs for type 2 diabetes.
Baseline and outcome measures
Participants were weighed at each visit wearing indoor clothing and no shoes. Height was also taken at baseline for calculation of body mass index (BMI). Weight was recorded at each clinic visit and three readings of systolic and diastolic blood pressure were performed at rest using an Omron 703CP automatic blood pressure monitor (Omron Terminals Ltd, Chessington, UK). First blood pressure readings were discarded and the mean of the last two were used for outcome measurement. Participants completed Bradley's Well-being Questionnaire10 on each visit.
At baseline and after 16 weeks of hawthorn treatment and placebo, blood samples were drawn after a 12-hour overnight fast into appropriate Becton Dickinson Vacutainer® blood collection tubes (containing lithium heparin, ethylenediaminetetraacetic acid or clot activator serum separation tube [SST™] gel) for routine electrolyte and liver and kidney function tests at the pathology division of the Royal Berkshire Hospital.
Plasma glucose concentrations were determined batchwise using a Monarch centrifugal analyser (Instrumentation Laboratories Ltd, Warrington, UK) which was equipped with an appropriate glucose hexokinase kit. Glycated haemoglobin (HbA1c) was measured by high-performance liquid chromatography (HA-8121, A Menarini Diagnostics Ltd, Wokingham, UK) and fructosamine was determined using the nitroblue tetrazolium method (Roche diagnostics, Basel, Switzerland) at the Royal Berkshire Hospital.
Statistical analysis
The number of participants required to give a significant difference (P = 0.05) between two treatment groups with 80% power (n = 80) was determined using data from a similar participant group (G Marakis, unpublished data, 2000) with mean resting diastolic blood pressure 84.7 (SD = 9.66) mmHg, assuming a treatment reduction of 7 mmHg and 25% dropouts. Intention-to-treat analysis was used, whereby missing final outcome data were replaced with those from the most recent data available (second clinic visit or baseline). Data analysis was carried out using SPSS version 13.0 (SPSS Inc, Chicago, US). Data are presented as with 95% confidence intervals (95% CI). Student's t-test for independent samples was used to compare group mean baseline values and response differences (outcome minus baseline) between the two groups. Planned Student's t-test for paired values was used to compare outcome versus baseline values within groups. Significant differences between mean data were determined using P<0.05 for primary and P<0.005 for secondary outcomes.
RESULTS
Recruitment
Eighty-nine participants were assessed for eligibility (Figure 1), of whom 80 met the study criteria. There were three withdrawals from the placebo group for adverse events (facial hypersensitivity, scalp infection, and skin rash). Among those finishing per protocol, there was 95.6% (n = 34) adherence to intervention among the hawthorn group and 96.7% (n = 31: 1 datum missing) adherence in the placebo group.
Figure 1.
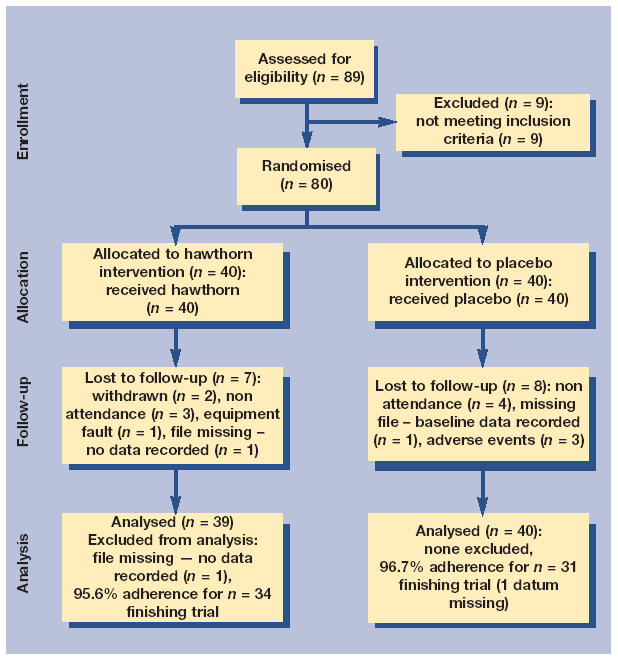
CONSORT (consolidated standards of reporting trials) diagram of study participants.
Participants
Personal and lifestyle characteristics for the two treatment groups were similar at baseline (Table 1). Participants were mostly male and non-smokers; mean age was about 60 years. Most were overweight at baseline, which was unchanged at outcome: 82% in the hawthorn group and 90% in the placebo group had BMI over the target maximum for health (BMI ≥25) according the many authorities.11
Table 1.
Personal and lifestyle characteristics of participants at baseline, including prescribed drug usage, according to treatment group.a
| Hawthorn (n = 39) (95% CI) | Placebo (n = 40) (95% CI) | |
|---|---|---|
| Sex: males/females (n) | 27/12 | 28/12 |
| Age (years) | 62.6 (60.07 to 65.13) | 61.3 (58.16 to 64.44) |
| Body mass index (kg/m2)b | 28.8 (27.55 to 30.05) | 30.2 (28.79 to 31.61) |
| Exercise: ‘moderate’ to ‘great’/‘hardly any’ to ‘light’ (%) | 40/60 | 45/55 |
| Stress: ‘moderate’ to ‘great’/‘not stressful’ (%) | 57.5/42.5 | 45/55 |
| Smoking: yes/no (%) | 2.5/97.5 | 2.5/97.5 |
| Waist circumference: males (cm)c | 104.0 (100.10 to 107.90) | 105.0 (101.5 to 108.05) |
| Waist circumference: females (cm)d | 98.6 (93.36 to 103.84) | 102.5 (96.06 to 108.94) |
| Waist to hip ratio: males (n)e | 0.97 (0.95 to 0.99) | 0.97 (0.95 to 0.99) |
| Waist to hip ratio: females (n)f | 0.89 (0.85 to 0.93) | 0.89 (0.86 to 0.92) |
| Participants on hypoglycaemic medication (n) | ||
| Low-dose insulin | 4 | 3 |
| Metformin | 19 | 20 |
| Gliclazide | 12 | 16 |
| Others | 6 | 7 |
| Participants on hypotensive medication (n) | ||
| ACE inhibitors | 19 | 15 |
| Calcium channel blockers | 8 | 10 |
| β-blockers | 6 | 7 |
| Diuretics | 10 | 4 |
| Others | 2 | 4 |
ACE = angiotensin converting enzyme.
Mean values (95% CI) as appropriate. Recommended values:
<25;
<94 cm;
<80 cm;
0.95;
0.85.
With regard to waist circumference, 56% and 63% of participants taking hawthorn and placebo respectively, were categorised as being at ‘substantial risk’ of coronary heart disease and diabetes (≥102 cm for men and 88 cm for women12) and a further 29% and 32%, respectively, were at ‘increased risk’ (≥94 cm for men and 80 cm for women12). Most participants were over target (0.95 for males and 0.85 for females) for waist to hip ratio (64% hawthorn, 65% placebo).13
Prescription drug treatment was similar between groups (Table 1). Low-dose insulin was equally distributed between groups (hawthorn n = 4, placebo n = 3). Mean hypoglycaemic drug use was 1.9 and 2.1 drugs per participant in the hawthorn and placebo groups, with use of 0, 1, 2, or 3 drugs by 15, 13, 9, and 2 participants in the hawthorn and 9, 21, 8, and 2 participants in the placebo group, respectively. Mean hypotensive drug use was 2.5 and 2.2 drugs per participant in the hawthorn and placebo groups, with use of 0, 1, 2, 3, or 4 drugs by 11, 13, 9, 4, and 2 participants in the hawthorn and 12, 16, 9, 2, and 1 participants in the placebo group, respectively. In the entire study group, 71% (n = 28 in both groups) used one or more hypotensive drugs. Statins, aspirin, clofibrates and digoxins were prescribed for 9, 8, 1, and 0 participants in the hawthorn and 6, 6, 3, and 1 in the placebo group respectively. During the study there were 11 changes in drug regime (six in the hawthorn and five in the placebo group): seven dosage increases, three new drug introductions, and one drug cessation.
Table 2 shows mean energy intake and percentage contributions to energy intake from protein, fat, carbohydrate, and alcohol as calculated by the DIETQ Food Frequency Questionnaire. Mean total fat intake was below the recommended level of 35% of total energy intake for patients with diabetes,14 except for seven participants on hawthorn and eight on placebo. Mean intake of energy from polyunsaturated fatty acids was well within the guidelines of <10% of total energy,14 except for one participant in the placebo group. However, mean intake of saturated fat on hawthorn and 18 on placebo were over target. Group mean values for energy contribution from monounsaturated fatty acids were close to the lower limit of the recommended 10–20% of total energy:14 12 participants on hawthorn and 11 on placebo had lower intakes. For sugar, group mean intakes were above recommendations: 30 participants on hawthorn and 29 on placebo had sugar intake greater than 10% of total energy.
Table 2.
Mean daily nutrient intake implicated in maintenance of normal blood pressure.
| Hawthorn (95% CI) | Placebo (95% CI) | |||
|---|---|---|---|---|
| Nutrient | Males (n = 21) | Females (n = 10) | Males (n = 22) | Females (n = 9) |
| Energy (kcal) | 1847 (1676 to 2018) | 1769 (1654 to 1884) | 1898 (1710 to 2086) | 1555 (1339 to 1771) |
| Protein energy (% total energy) | 20.4 (19.40 to 21.40) | 21.4 (19.43 to 23.37) | 21.0 (19.86 to 22.14) | 22.8 (21.41 to 24.16) |
| Fat energy (% total energy)a | 30.2 (27.98 to 32.42) | 30.7 (28.54 to 32.86) | 31.5 (29.11 to 33.89) | 28.1 (24.40 to 31.80) |
| Carbohydrate energy (% total energy) | 48.2 (46.39 to 50.01) | 47.8 (44.86 to 50.74) | 46.9 (44.59 to 49.21) | 48.8 (45.61 to 51.99) |
| Alcohol energy (% total energy) | 1.3 (0.86 to 1.74) | 0.2 (0 to 0.43) | 0.7 (0.37 to 1.03) | 0.4 (0 to 0.83) |
| Saturated fat (% total energy)b | 11.33 (9.95 to 12.71) | 11.3 (9.92 to 12.68) | 12.01 (10.48 to13.54) | 10.69 (8.13 to 13.25) |
| PUFA (% total energy) | 5.41 (4.81 to 6.01) | 4.65 (4.11 to 5.19) | 5.42 (4.84 to 6.00) | 4.54 (4.24 to 4.84) |
| MUFA (% total energy)c | 10.8 (10.06 to 11.54) | 10.4 (9.41 to 11.39) | 11.3 (11.38 to 12.22) | 9.91 (8.73 to 11.09) |
| Sugar (% total energy)d | 17.0 (8.6 to 25.4) | 21.4 (18.97 to 23.83) | 16.9 (15.21 to 18.59) | 18.9 (15.85 to 21.95) |
| Fibre: Englyst method (g) | 19.2 (16.98 to 21.42) | 21.1 (18.86 to 23.34) | 20.4 (17.94 to 22.86) | 20.4 (16.78 to 24.02) |
| Sodium (mg) | 2413 (2099 to 2727) | 2484 (2137 to 2831) | 2449 (2194 to 2704) | 2327 (1875 to 2779) |
| Potassium (mg) | 3473 (3138 to 3808) | 3511 (3278 to 3744) | 3603 (3312 to 3894) | 3047 (2589 to 3505) |
| Calcium (mg) | 1026 (905 to 1147) | 1081 (939 to 1223) | 1046 (945 to 1147) | 783 (562 to 1004) |
| Magnesium (mg) | 337 (302.70 to 371.3) | 351 (319.10 to 382.90) | 359 (328.50 to 389.50) | 308 (261.50 to 354.50) |
| Selenium (mcg) | 65 (53.9 to 76.1) | 69 (51.20 to 86.80) | 68 (56.20 to 79.80) | 67 (46.00 to 88.00) |
| Vitamin A (mcg) | 933 (726 to 1140) | 1250 (775 to 1725) | 1506 (648 to 2364) | 930 (523 to 1337) |
| Vitamin C (mg) | 81 (67.70 to 94.30) | 120 (96.60 to 143.40) | 90 (76.30 to 103.70) | 84 (61.20 to 106.80) |
| Vitamin E (mg) | 3.71 (3.26 to 4.16) | 4.91 (4.33 to 5.49) | 4.52 (3.81 to 5.23) | 3.63 (3.17 to 4.09) |
| Vitamin D (mcg) | 4.1 (3.05 to 5.15) | 5.0 (3.19 to 6.81) | 4.5 (2.89 to 6.11) | 5.2 (3.02 to 7.38) |
| Folic acid (mcg) | 280 (243.70 to 316.30) | 298 (247.80 to 348.20) | 279 (251.3 to 306.70) | 264 (234.60 to 293.40) |
PUFA = polyunsaturated fatty acids. MUFA = monounsaturated fatty acids. Recommended intake:
<35%;
<10%;
10–20%;
<10%.
The micronutrients tested for were chosen because of their involvement in countering raised blood pressure.15–21 There was no significant difference in intake of nutrients for men between the two groups and only vitamin E levels were significantly different between women in the two groups. Mean intakes according to group were generally above the reference nutrient levels.22 However, mean intake was greater than the reference nutrient intake for vitamin D for both groups. Mean intake of potassium was greater among men on hawthorn and women on placebo. Mean selenium intake was greater among men in both groups and copper intake was greater among women in the placebo group. Mean intake of vitamin E in men on hawthorn was lower than the recommended safe intake.22
Participants who did not reach daily target intakes included 71% of the study population with vitamin D intakes less than the reference nutrient intake, 48% for potassium, 46% for selenium, 35% for copper, 29% for iodine, 27% for vitamin A and vitamin E, 16% for magnesium, and 8% for folate. Participant intakes of iron, zinc, iodine, manganese, vitamins B1, B2, B3, and B6 fell below their targets for between 1 and 10% of the study population. All participants reached target intakes for B12, biotin and pantothenic acid. Only three participants in the study group met all dietary targets: 24 were below nutrient targets for 1–3 nutrients, 22 for 4–6, eight for 7–9, and three were below target for 10–12 micronutrients.
Dietary supplement use between the two treatment groups at baseline was similar. For the study population, 40.5% were taking dietary supplements (n = 15 hawthorn, n = 17 placebo). The most popular dietary supplements were omega-3 (29.0% study population), multi-nutrients (12.7%), and vitamin C (10.0%).
Outcome measures
No significant difference was found between groups on mean baseline values for blood pressure measurements or indices of glycaemic control (Table 3). The primary outcome measure of diastolic blood pressure showed a significant reduction of 2.6 mmHg in the hawthorn group compared with the placebo group (P = 0.035) (Figure 2). No significant difference was found between treatment responses for systolic blood pressure: a paired t-test of baseline versus outcome showed a non-significant (P = 0.096) decline of 3.6 mmHg in the hawthorn group. There was no significant outcome difference between groups in the indices of glycaemic control, that is, fasting glucose, glycated haemoglobin and fructosamine.
Table 3.
Mean values for blood pressure and indices of glycaemic control at baseline and outcome.
| Hawthorn (n = 39) | Placebo (n = 40) | ||||||
|---|---|---|---|---|---|---|---|
| Outcome | Baseline (95% CI) | 16 weeks (95% CI) | (P-value)b | Baseline (95% CI) | 16 weeks (95% CI) | (P-value)b | Treatment response difference (P-value)c |
| Diastolic BP, mmHg | 85.6 (83.33 to 87.87) | 83.00 (80.53 to 85.47) | 0.016 | 84.50 (82.00 to 87.00) | 85.0 (82.16 to 87.84) | 0.645 | 0.035 |
| Systolic BP, mmHg | 152.3 | 148.7 | 0.096 | 147.40 | 146.6 | 0.771 | 0.329 |
| (147.20 to 157.4) | (143.13 to 154.27) | (143.36 to 151.44) | (141.74 to 151.46) | ||||
| Fasting glucose | 8.91 (7.91 to 9.91a) | 8.31 (7.37 to 9.25a) | 0.176 | 8.12 (7.65 to 8.59a) | 7.96 (7.53 to 8.39a) | 0.800 | 0.170 |
| HbA1c | 7.6 (7.09 to 8.11a) | 7.50 (7.13 to 7.87a) | 0.634 | 8.12 (7.65 to 8.59a) | 7.96 (7.53 to 8.39a) | 0.194 | 0.694 |
| Fructosamine | 313.9 (297.70 to 330.10) | 314.70 (300.60 to 328.80) | 0.950 | 335.40 (319.70 to 351.10a) | 339.50 (319.50 to 359.50a) | 0.468 | 0.796 |
BP = blood pressure. HbA1c = glycated haemoglobin.
One datum missing.
Paired t-test of outcome versus baseline.
Independent samples t-test of response to treatment (baseline minus outcome).
Figure 2.
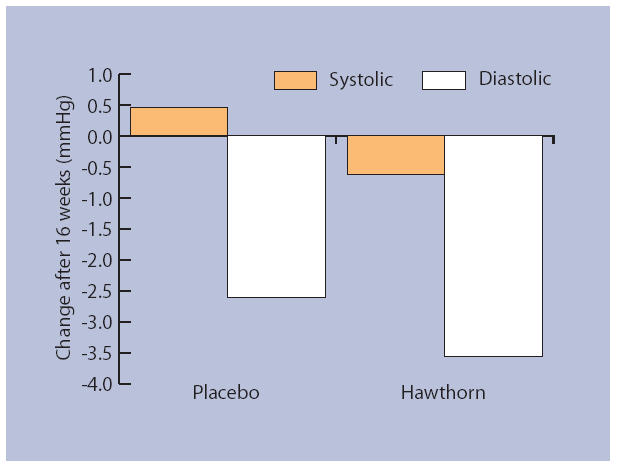
Change in blood pressure after 16 weeks of daily supplementation with hawthorn extract compared with placebo. Diastolic blood pressure was significantly reduced in the hawthorn group compared with the placebo group (P = 0.035).
All mean values for liver and kidney function tests were normal for both groups at baseline and outcome, except γ-glutamyl transpeptidase which was 70 and 75 U/L at baseline and outcome respectively for the placebo group (normal range 12–58 U/l). Eight participants on hawthorn and nine on placebo had slightly raised levels at baseline; two participants in the placebo group had levels >250 U/l.
No significant difference was noted in total wellbeing score using Bradley's Well-being Questionnaire, or subscores of depression, anxiety, energy or positive wellbeing. Mean total scores at baseline were 50.5 (95% CI = 48.62 to 52.38) and 49.4 (95% CI = 47.68 to 51.12) for hawthorn and placebo groups respectively; at outcome these scores were 50.4 (95% CI = 48.51 to 52.29) and 50.6 (95% CI = 48.86 to 52.34) respectively. Minor health complaints at 16 weeks of treatment were reduced in both groups compared with baseline (Table 4).
Table 4.
Number of participants at baseline and outcome with minor health complaints.a
| Hawthorn | Placebo | |||
|---|---|---|---|---|
| Baseline | 16 weeks | Baseline | 16 weeks | |
| Nausea | 1 | 2 | 2 | 1 |
| Bloating | 5 | 2 | 3 | 3 |
| Flatulence | 11 | 7 | 9 | 6 |
| Diarrhoea | 2 | 2 | 2 | 1 |
| Rash | 5 | 4 | 2 | 0 |
| Fatigue | 11 | 10 | 11 | 7 |
| Cold hands | 6 | 5 | 3 | 4 |
| Cold feet | 8 | 8 | 2 | 3 |
| Total | 136 | 119 | 97 | 77 |
Minor health complaints ‘all the time’ or ‘sometimes’ in the past week.
Participants who finished the study were asked to guess whether they had been assigned to the treatment or placebo group. Eleven participants on hawthorn made correct guesses while none made an incorrect guess. Two participants in the placebo group guessed correctly and two participants guess incorrectly. The remainder did not know which group they had been assigned to.
DISCUSSION
Summary of main findings
This is the first study to show a reduction in diastolic blood pressure using hawthorn intervention in participants with type 2 diabetes taking prescribed medication. Although hawthorn has been traditionally used in France as a mild sedative,2 no effect on anxiety, mood, or wellbeing was found in the current study.
Strengths and limitations of the study
Although statistically significant, the difference between diastolic blood pressure of participants taking hawthorn and those taking placebo was modest. Furthermore, the study was not designed to yield information on the effects of hawthorn on longer-term clinical outcomes, such as ischaemic heart disease and stroke.
The acceptance of herbal treatments in conventional medicine has been limited by the possibility of herb–drug interactions. This study investigated the efficacy of hawthorn taken as an adjunct to modern drugs. Although medication varied between participants, drug treatment was similar between the groups that were similar in age, BMI, smoking habits, and diet.
This study provides further evidence for the safe use of hawthorn, a herb with no restrictions on its long-term use.1 There was no significant change in liver or kidney function for both groups between baseline and outcome. Numbers of participants lost at follow up were similar for treatments and side effects were minor and fewer at the end of the trial compared with baseline. Minor health complaints cannot be attributed to the study intervention as they were present at baseline. Further investigation is needed to establish any links between these complaints and multiple medication and/or low nutrient intake.
A nutrient intake perspective was included in this trial to provide more information on participant group profiles. Although mean total fat intake of participants met current recommendations, mean sugar intake was high and potential micronutrient deficits were revealed, some of which could have contributed to hypertension.23–25 Nutritional adequacy is especially important for patients with diabetes to prevent complications, especially in relation to glycaemic fluctuation.26,27
More participants in the hawthorn than the placebo group correctly guessed the treatment condition they were assigned to. However, this represented only 11% of the study population, so it is unlikely to have greatly affected outcome.
Comparison with existing literature
Placebo-controlled studies of hawthorn extracts have focused on cardiac function in heart failure. One study showed a hypotensive effect compared with placebo as a secondary outcome.28 This finding prompted a pilot study in which hawthorn was given to mildly hypertensive, non-diabetic participants (500 mg extract/day for 10 weeks).8 This pilot study showed a promising reduction in diastolic blood pressure compared with placebo (P = 0.081). In the present study, a significant drop in diastolic pressure for participants with diabetes resulted from increasing the hawthorn dosage to 1200 mg extract/day for 16 weeks. This dosage is at the high end of reported dosage range, but the herb is well tolerated with no contraindications or reports of adverse effects of overdose.6
Implications for future research and clinical practice
The finding that hawthorn has a hypotensive effect in patients with type 2 diabetes taking prescribed medication should stimulate further studies. Future research could examine the hypotensive effects of hawthorn for other hypertensive patient groups, including non-diabetic, newly-diagnosed patients. Micronutrient deficits among patients with type 2 diabetes are of concern, especially as adequate intake of several nutrients is necessary to avoid hypertension. Further investigation into the influences of nutrient deficits on glycaemic, lipaemic and haemodynamic control is warranted.
This study showed no herb–drug interactions arising from hawthorn administration. Taken concomitantly with prescribed medications, the herb demonstrated a hypotensive effect for patients with type 2 diabetes.
Acknowledgments
We wish to thank the study participants and Ann Byrne and Chandra Patterson for their technical assistance.
Funding body
Lichtwer Pharma UK funded GM's Fellowship and donated the supplements
Ethics committee
The University of Reading Ethics and Research and the West Berkshire Local Ethics Committees
Competing interests
The authors have stated that there are none
REFERENCES
- 1.Mills S, Bone K. Principles and practice of phytotherapy: modern herbal medicine. Edinburgh: Churchill Livingstone; 2000. [Google Scholar]
- 2.British Herbal Medicine Association. A guide to traditional herbal medicines: a sourcebook of accepted traditional uses of medicinal plants within Europe. Bournemouth: BHMA; 2003. [Google Scholar]
- 3.Pittler MH, Schmidt K, Ernst E. Hawthorn extract for treating chronic heart failure: meta-analysis of randomized trials. Am J Med. 2003;114(8):665–674. doi: 10.1016/s0002-9343(03)00131-1. [DOI] [PubMed] [Google Scholar]
- 4.British Heart Foundation. Herbs and the heart. Factfile 09/2003. London: BHF; 2003. [Google Scholar]
- 5.Yao LH, Jiang YM, Shi J, et al. Flavonoids in food and their health benefits. Plant Foods Hum Nutr. 2004;59(3):113–122. doi: 10.1007/s11130-004-0049-7. [DOI] [PubMed] [Google Scholar]
- 6.Mills S, Bone K. The essential guide to herbal safety. Edinburgh: Churchill Livingstone; 2005. [Google Scholar]
- 7.Tankanow R, Tamer HR, Streetman DS, et al. Interaction study between digoxin and a preparation of hawthorn (Crataegus oxyacantha) J Clin Pharmacol. 2003;43(6):637–642. [PubMed] [Google Scholar]
- 8.Walker AF, Marakis G, Morris AP, Robinson PA. Promising hypotensive effect of hawthorn extract: a randomized double-blind pilot study of mild, essential hypertension. Phytother Res. 2002;16(1):48–54. doi: 10.1002/ptr.947. [DOI] [PubMed] [Google Scholar]
- 9.Pocock SJ. Clinical trials: a practical approach. Chichester: John Wiley & Sons; 1983. [Google Scholar]
- 10.Bradley C, Gamsu DS. Guidelines for encouraging psychological well-being: report of a Working Group of the World Health Organization Regional Office for Europe and International Diabetes Federation European Region St Vincent Declaration Action Programme for Diabetes. Diabet Med. 1994;11(5):510–516. doi: 10.1111/j.1464-5491.1994.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 11.Department of Health. Report on health and social subjects No 46. Nutritional aspects of cardiovascular disease. London: HMSO; 1994. [PubMed] [Google Scholar]
- 12.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Bethesda MD: National Institutes of Health; National Heart, Lung, and Blood Institute; 1998. [Google Scholar]
- 13.Molarius A, Seidell JC. Selection of anthropometric indicators for classification of abdominal fatness — a critical review. Int J Obes Relat Metab Disord. 1998;22(8):719–727. doi: 10.1038/sj.ijo.0800660. [DOI] [PubMed] [Google Scholar]
- 14.Connor H, Annan F, Bunn E, et al. The implementation of nutritional advice for people with diabetes. Diabet Med. 2003;20(10):786–807. doi: 10.1046/j.1464-5491.2003.01104.x. [DOI] [PubMed] [Google Scholar]
- 15.Cappuccio FP, Elliott P, Allender PS, et al. Epidemiologic association between dietary calcium intake and blood pressure: a meta-analysis of published data. Am J Epidemiol. 1995;142(9):935–945. doi: 10.1093/oxfordjournals.aje.a117741. [DOI] [PubMed] [Google Scholar]
- 16.Duffy SJ, Gokce N, Holbrook M, et al. Treatment of hypertension with ascorbic acid. Lancet. 1999;354(9195):2048–2049. doi: 10.1016/s0140-6736(99)04410-4. [DOI] [PubMed] [Google Scholar]
- 17.Witteman JC, Grobbee DE, Derkx FH, et al. Reduction of blood pressure with oral magnesium supplementation in women with mild to moderate hypertension. Am J Clin Nutr. 1994;60(1):129–135. doi: 10.1093/ajcn/60.1.129. [DOI] [PubMed] [Google Scholar]
- 18.Mangoni AA, Sherwood RA, Swift CG, et al. Folic acid enhances endothelial function and reduces blood pressure in smokers: a randomized controlled trial. J Intern Med. 2002;252(6):497–503. doi: 10.1046/j.1365-2796.2002.01059.x. [DOI] [PubMed] [Google Scholar]
- 19.de Wardener HE, He FJ, MacGregor GA. Plasma sodium and hypertension. Kidney Int. 2004;66(6):2454–2466. doi: 10.1111/j.1523-1755.2004.66018.x. [DOI] [PubMed] [Google Scholar]
- 20.Resnick LM, Barbagallo M, Dominguez LJ, et al. Relation of cellular potassium to other mineral ions in hypertension and diabetes. Hypertension. 2001;38(3 Pt 2):709–712. doi: 10.1161/01.hyp.38.3.709. [DOI] [PubMed] [Google Scholar]
- 21.Mihailovic MB, Avramovic DM, Jovanovic IB, et al. Blood and plasma selenium levels and GSH-Px activities in patients with arterial hypertension and chronic heart disease. J Environ Pathol Toxicol Oncol. 1998;17(3-4):285–289. [PubMed] [Google Scholar]
- 22.Department of Health. Report of health and social subjects No. 41. Dietary reference values for food energy and nutrients for the United Kingdom. London: HMSO; 1991. [PubMed] [Google Scholar]
- 23.Joffres MR, Reed DM, Yano K. Relationship of magnesium intake and other dietary factors to blood pressure: the Honolulu heart study. Am J Clin Nutr. 1987;45(2):469–475. doi: 10.1093/ajcn/45.2.469. [DOI] [PubMed] [Google Scholar]
- 24.Conlin PR, Chow D, Miller ER, III, et al. The effect of dietary patterns on blood pressure control in hypertensive patients: results from the Dietary Approaches to Stop Hypertension (DASH) trial. Am J Hypertens. 2000;13(9):949–955. doi: 10.1016/s0895-7061(99)00284-8. [DOI] [PubMed] [Google Scholar]
- 25.Farvid MS, Jalali M, Siassi F, et al. The impact of vitamins and/or mineral supplementation on blood pressure in type 2 diabetes. J Am Coll Nutr. 2004;23(3):272–279. doi: 10.1080/07315724.2004.10719370. [DOI] [PubMed] [Google Scholar]
- 26.Bonnefont-Rousselot D. The role of antioxidant micronutrients in the prevention of diabetic complications. Treat Endocrinol. 2004;3(1):41–52. doi: 10.2165/00024677-200403010-00005. [DOI] [PubMed] [Google Scholar]
- 27.Barringer TA, Kirk JK, Santaniello AC, et al. Effect of a multivitamin and mineral supplement on infection and quality of life. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138(5):365–371. doi: 10.7326/0003-4819-138-5-200303040-00005. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt U, Kuhn U, Ploch M, et al. Efficacy of the hawthorn (Crataegus) preparation LI 132 in 78 patients with chronic congestive heart failure defined as NYHA functional class II. Phytomedicine. 1994;1(1):17–24. doi: 10.1016/S0944-7113(11)80018-8. [DOI] [PubMed] [Google Scholar]


