Abstract
Background
Postcoital bleeding may be a symptom of cervical cancer. Guidance to aid a GP in determining whom to investigate or refer exists but recommendations vary. Women need to be involved in decisions about their care and this involves communicating risk and an exploration of the implications of the risk. Risk estimates of postcoital bleeding for cervical cancer are not available.
Aim
To provide an estimate of the positive predictive values of postcoital bleeding for cervical cancer to aid decision making in primary care about whom to investigate for cervical cancer.
Design of study
A systematic review.
Setting
Community, primary and secondary care.
Method
Six electronic databases were searched from the beginning of each of their time frames. Inclusion criteria were that the study was published in English and reported or contained enough data to calculate the prevalence or incidence of postcoital bleeding within the study population. No studies were excluded on issues of methodological quality.
Results
The search strategy identified 910 unique articles. The point prevalence of postcoital bleeding in the community ranged from 0.7 to 9% among women. One study reported an annual cumulative incidence of 6% of menstruating women. The prevalence of postcoital bleeding in women with cervical cancer ranged from 0.7 to 39%. Calculation of risk that a woman in the community developing postcoital bleeding has cervical cancer ranges from 1 in 44 000 at age 20–24 years to 1 in 2 400 aged 45–54 years. There was no information allowing the direct calculation of risk in women presenting to primary care.
Conclusion
The evidence base for management strategies of postcoital bleeding and calculations of risk for cervical cancer in women with postcoital bleeding are poor. Recommendations for clinical practice are made on the current evidence.
Keywords: cervical cancer, postcoital bleeding, uterine neoplasms
INTRODUCTION
GPs have been criticised for both too few and too many urgent referrals of patients with symptoms of possible cancer. This has given rise to debate concerning the reasons for the referral rates, and how the prognosis in patients with malignancy may be improved.1–7 There is agreement that research on common symptoms in primary care is lacking,8 that primary care clinicians have difficulty identifying people most at risk of cancer,7 and that the research base for published guidelines lies within secondary care.1 Cancer research is difficult within primary care due to the rarity with which the disease presents. In contrast the symptoms of possible cancer are often common.9 This produces a difficult decision for the primary care clinician about whom to investigate.
For the symptom of postcoital bleeding, guidance has been produced to identify those women whom GPs should refer for investigation. The content of this guidance varies, and includes recommending referral for all women,10,11 or women with non-defined ‘persistent’12,13 or ‘repeated’ postcoital bleeding,14 or postcoital bleeding that ‘persists for more than 4 weeks in women over 35 years of age’.14 Some recommendations have also combined the symptom of postcoital bleeding with specific abnormal cervical cytology.12,13
Patients have the right to accept or refuse whatever is proposed and in taking a decision as to whether or not to investigate for underlying malignancy, informed consent is required.15 The General Medical Council states that two of the duties of a doctor are to ‘give patients information in a way they can understand’ and ‘respect the rights of patients to be fully involved in decisions about their care’.16 Predictive values, expressed as natural frequencies, are the foundation for communicating risk17 and, used in conjunction with an adequate exploration of the implications of the risk, should allow the patient to make an informed decision. For cardiovascular disease it is known that the risks patients and doctors are prepared to take differ.18,19 Effective risk communication and shared decision making are dependent on several factors and include the style of the consultation, which may vary from doctor-centred to patient-centred,20 and the constraints of time.21 A number of aids and strategies are available which may promote shared decision making.22
The purpose of this study is to gain an estimate of positive predictive values of postcoital bleeding for cervical cancer to aid decision making in primary care about whom to investigate for cervical cancer. The method used was a systematic review of the literature on postcoital bleeding in women in the community, in primary and secondary care and in women with cervical cancer. By combining incidence rates of the symptom with the incidence of cervical cancer derived from national data, predictive values can be calculated.23
METHOD
Searching strategy
Six databases were searched from the start of each of their time frames to 11 August 2005. The databases, search strategy and terms used are given in Box 1.
Box 1. Search terms and strategy.
-
▸
Databases
Medline, EMBASE, CINAHL, British Nursing Index, AMED and PsycINFO
-
▸
Postcoital bleeding search terms
Text words: ((postcoital OR coital) NEXT bleeding) OR (bleeding NEXT intercourse)
-
▸
Cervical cancer and postcoital bleeding search terms
Subject heading: (Cervical cancer OR cervix neoplasms OR uterine neoplasms) AND Text word: bleed$ OR Text word: ((Cancer AND (cervix OR cervical)) AND bleed$) OR ((carcinoma AND (cervix OR cervical)) AND bleed$)
Inclusion and exclusion criteria
Studies of any design were included in the systematic review if they had been published in English in peer-reviewed journals. The article had to report or contain enough data to calculate the prevalence or incidence of postcoital bleeding within the study population. The purpose of the review was to provide predictive values for use in community populations or in general practice consultation, and so studies on treatment or treatment complications, diagnostic and screening techniques, unusual types of cervical cancer (that is, not squamous or adenocarcinoma) and cancer in pregnancy were excluded. Studies were also excluded if they were of less than 15 cases or non-gynaecological studies (such as cervical spine).
How this fits in
Postcoital bleeding may be a symptom of cervical cancer. We do not know how useful this symptom is for predicting the presence of the disease. Systematic review evidence is gathered to calculate predictive values of postcoital bleeding in the community for cervical cancer. Recommendations for clinical practice are made on the current evidence.
The results of the electronic searches were reviewed by reading the titles and abstracts. Papers were retrieved if they contained information on symptoms of postcoital or coital bleeding or symptoms prior to diagnosis in patients with squamous cell or adenocarcinoma of the cervix. Reference lists of all articles retrieved were searched for additional articles.
Assessment of methodological quality
No studies were excluded on the basis of an assessment of methodological quality. Some early studies failed to report response or recruitment rates24,25 or only reported proportions26,27 but had the advantage of involving large populations. Later studies, which also failed to give this information, were included in the review as they were more likely to reflect current clinical activity.28–30 Only one study31 determined the prevalence or incidence of postcoital bleeding as the primary objective.
Data extraction and analysis
Relevant data was extracted from retrieved hard copies of eligible studies. Details were recorded of the study design and participants, definition of postcoital bleeding or cases, and the number or proportion of women reporting the symptom, with cancer and histological subtype. Summary prevalences were determined by summation of the original data from the studies and calculating the proportion with postcoital bleeding in the total study populations. Post-test probabilities (positive predictive values) were calculated as the estimated number of women who developed cervical cancer and presented with postcoital bleeding, expressed as a proportion of all women who developed postcoital bleeding.
QUORUM guidelines for reporting systematic reviews and meta-analyses were followed wherever possible.32
RESULTS
In total 910 unique articles were identified of which 38 reported or allowed the calculation of prevalence or incidence rates of postcoital bleeding in women in the community, women in primary or secondary care populations or women with cervical cancer as illustrated in Figure 1.
Figure 1.
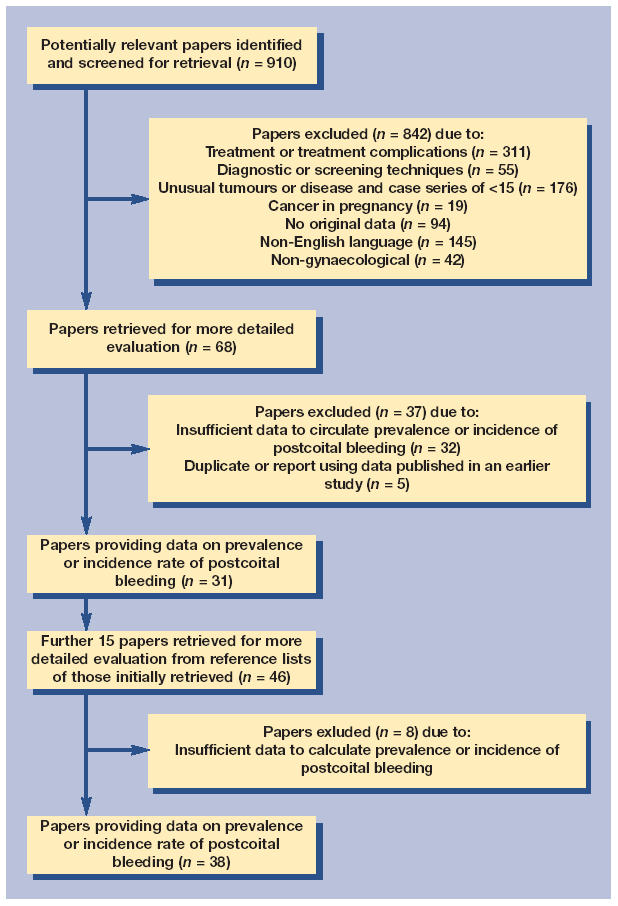
Flow diagram of inclusion process.
The prevalence and incidence of postcoital bleeding in the community and primary care
In total eight studies were identified in which rates of postcoital bleeding in the community were reported or could be calculated (Supplementary Table 1). The range in the point prevalence of postcoital bleeding was 0.7–9%. The large population studies on the Finnish mass cervical cytology screening programme27,33 and a study in Belgium concerning screening for chlamydia35 found a prevalence of coital bleeding of around 1%. In contrast the two studies performed in England28,31 on smaller populations and a Nigerian study in a hospital cytology clinic34 found a prevalence of around 5%. The disparities may be due to differences in definition of postcoital bleeding relating to time period covered (bleeding in the past month,36 2 months,27,33 3 months35 or 6 months31), frequency (ever in the time frame27,31,33 or frequently35), study populations (such as age, sexual activity, use of hormones, infection) and the effects of temporal trends.27 One study reported an annual cumulative incidence in menstruating women which was 6%.31
There were no studies of women consulting primary care. The proportion of women in the community with postcoital bleeding who consult primary care is not known.
The prevalence and incidence of postcoital bleeding in secondary care
There was only one study reporting rates in secondary care. A group in Holland performed chlamydial screening in new patients attending a hospital obstetrics and gynaecology outpatients department. Of the 1300 women responding ‘yes’ or ‘no’ on a questionnaire, the prevalence of ‘postcoital bleeding’ was 5% with 68 classified as ‘unknown’.37
The proportion of women presenting to primary care with postcoital bleeding who are referred to secondary care was not reported in any of the studies found. Neither was there any indication of the proportion of women attending secondary care with a predominant symptom such as menorrhagia who also had postcoital bleeding.
The prevalence and incidence of postcoital bleeding in other specific populations
Four studies38–41 were identified which gave the prevalence of postcoital bleeding among populations who were HIV positive or at increased risk of HIV. The prevalence of postcoital bleeding ranged from 539 to 32%41 with three of the studies38,40,41 showing an association between HIV and postcoital bleeding.
A community study in Egypt and Jordan was identified which found a prevalence of postcoital bleeding in women with genital prolapse of 3%.42
The proportion of women with cervical cancer who report postcoital bleeding
Sixteen studies were identified that reported, or allowed the calculation of, the prevalence of postcoital bleeding among women with cervical cancer (Supplementary Table 2). The range of prevalence of postcoital bleeding in women with cervical cancer is 0.7–39%.
There has been a temporal change in developed countries with more women now presenting asymptomatically due to the widespread use of cervical screening30,45,55 and a temporal change in the histological type of tumour with an increase in the proportion of women presenting with adenocarcinoma as opposed to squamous cell carcinoma.30,52,56 Some authors feel that adenocarcinoma is less likely to cause bleeding than squamous cell carcinoma.27 There is a large geographical variation in the rate of cervical cancer with Indian districts and African countries having higher rates than developed countries57 because of variations in the aetiology of the disease and the presence or absence of screening programmes. We calculated an estimate relevant to a British general practice population, by using studies from developed countries which did not select by tumour histology; this gave a summary prevalence of postcoital bleeding in women with cervical cancer of 11%.24,30,43,45,49
The proportion of women with postcoital bleeding who on screening or investigation have cancer
Only one community-based study was identified that allowed the calculation of a positive predictive value of postcoital bleeding in the community for cervical cancer. The study on the Finnish mass-screening programme identified 2648 women with coital bleeding of whom 12 had invasive cervical cancer33 at baseline, giving a positive predictive value on cross-sectional analysis of 0.5%. The relative risk for invasive carcinoma was 6.3 for bleeding on sexual intercourse compared to those without the symptom. A subsequent follow-up study58 on the same cohort, after the first smear, showed that women with coital bleeding and a negative screen had up to a 15-fold risk of late invasive cervical cancer compared to those without bleeding symptoms, although 93% of all cervical cancers in the cohort were in those without coital bleeding. A subsequent study by Viikki et al,27 again in Finland and using the same methodology, reported that this risk had fallen over the intervening 23 years to threefold. The authors concluded this was due to changes in the prevalence of symptoms and incidence of cancer, although the data on which this statement was based was not presented.
Eight studies in secondary care concerning the investigation of women with postcoital bleeding for cancer were identified (Supplementary Table 3). It appears that even in secondary care postcoital bleeding is a poor predictor of cervical cancer. In the largest English study of women with postcoital bleeding13 only 4% were diagnosed as having invasive cancer (including vaginal and endometrial). The hospital colposcopy clinic accepted referrals from both primary and secondary care and reported that only 0.6% of women attending with postcoital bleeding, and having a normal smear and cervix, had invasive cancer of the cervix. In the studies in secondary care that report the number of women with postcoital bleeding13,61–63 the summary figure for the proportion with cervical cancer is 2% and gynaecological malignancy 3%.
There are no studies determining the proportion of women presenting to primary care with postcoital bleeding in whom a diagnosis of cervical cancer is made.
Non-malignant causes of postcoital bleeding
Few studies were identified concerning the many other postulated causes of postcoital bleeding. Cervical erosion is often given as a cause of postcoital bleeding but Goldacre et al28 found no association. Hospital studies reporting causes of postcoital bleeding give cervical polyps,13,63 endometrial polyps63 and cervical ectopy63 as diagnoses but the studies lack controls to measure any association and suffer from selection bias. Some studies have shown an association between postcoital bleeding and HIV or risk of HIV38,40,41 and postcoital bleeding and chlamydial infection.35–37
Cervical dyskaryosis and cervical intraepithelial neoplasia are felt to be asymptomatic64,65 although Rosenthal et al13 found a statistically non-significant association with postcoital bleeding. Selo-Ojeme et al63 in their series reported that 5% of women with postcoital bleeding had cervical intraepithelial neoplasia, suggesting an association, although the study was in a selected population with no control group. Analysis of the data presented by Hakama et al33 on the Finnish screening programme gives an odds ratio for women with coital bleeding for histological cervical dysplasia (including carcinoma in situ) of 2.0 (95% confidence interval [CI] = 1.3 to 3.0) compared to women without. There is some evidence for an association between a cervix that bleeds on touch and cytological cervical dysplasia.66
There are no studies determining the proportion of women presenting to primary care with postcoital bleeding in whom a specific diagnosis is made.
The incidence of cervical cancer
In England carcinoma of the cervix is rare below the age of 20 years.67 In 2001 the incidence ranged from a rate of 2.6 per 100 000 population per year in the age 20–24 years to plateau in the age group 30–49 years with a peak of 16.3 in the age group 40–44 years. It was 10.6 in the age group 55–59 years, with a peak of 18.5 in the age group 75–79 years. The incidence of the disease fell by 42% in England and Wales between 1988 and 199768 probably as a consequence of the National Cervical Screening Programme.69,70
The predictive value of postcoital bleeding for cervical cancer
The study on the Finnish mass-screening programme33 gave a positive predictive value of 1 in 220 of postcoital bleeding for cervical cancer. However, in the intervening 30 years the epidemiology of postcoital bleeding and cervical cancer has changed. Bleeding symptoms have become more frequent27 and the incidence of cervical cancer lower68 altering the conditional probabilities. Using the annual incidence figures for postcoital bleeding by age bands given by Shapley et al,31 and combining them with the annual incidence of cervical cancer by age bands in England for the year 2001,67 and an estimate that 11%24,30,43,45,49 of women with cervical cancer will present with postcoital bleeding, risks can be calculated as given in Table 1.
Table 1.
The probability that a woman developing postcoital bleeding in the community has cervical cancer.
| Age (years) | Annual cumulative incidence of postcoital bleeding per 100 women31 | Women with cervical cancer who present with postcoital bleeding (%) | Annual incidence of cervical cancer in England per 100 000 women67 | Probability that a woman developing postcoital bleeding in the community has cervical cancer |
|---|---|---|---|---|
| 20–24 | 12.6 | 11 | 2.6 | 1 in 44 000 |
| 25–34 | 7.2 | 11 | 11.7 | 1 in 5600 |
| 35–44 | 4.8 | 11 | 15.8 | 1 in 2800 |
| 45–54 | 3.4 | 11 | 12.7 | 1 in 2400 |
Direct estimates of predictive values in primary and secondary care populations cannot be calculated due to the absence of information concerning the incidence of postcoital bleeding in primary care and referred populations.
DISCUSSION
Methodological issues
A number of methodological problems need to be considered before attempting to use the derived predictive values in the community and primary care.
Definition of denominator population
The conditional probabilities of predictive values, sensitivity and specificity depend on definition of symptom, disease and denominator population. Women with postcoital bleeding are a subgroup of women from the total female population who are sexually active and therefore at risk of developing the symptom. Unlike the situation for other possible symptoms of cancer, the total female population, in the case of postcoital bleeding and cervical cancer, is at risk of the disease but not all are at risk of developing the symptom. When a clinician is presented with the symptom of postcoital bleeding, the woman is by definition sexually active. Using the incidence of postcoital bleeding in all women to calculate the conditional probabilities of postcoital bleeding for cervical cancer will overestimate the risk while using the prevalence in all women with cervical cancer who present with postcoital bleeding will underestimate the risk.
The prevalence of sexual activity varies between populations. Few studies indicate the proportion of women studied who are at risk of developing postcoital bleeding. The exceptions have been studies involving screening sexually active women for chlamydia35,36 and studies concerning postcoital bleeding and HIV.38–40 Two studies have been in community contraception clinics28,29 where most, but not all women are sexually active.29
In studies on women in the community there is also an additional inconsistency in the definition of the denominator population as some studies use all women and others menstruating women (Supplementary Table 1).
Setting
The predictive value of a symptom for a disease varies with the prevalence of the symptom and disease within the populations studied. This is in contrast to sensitivity and specificity, which are independent of the prevalence of disease. There are three populations outside hospital where interventions may take place to promote the early detection of cancer. These populations represent the target groups for health education programmes, for opportunistic intervention and for decisions to investigate for possible malignancy. These settings are, respectively, people within the community, people who consult primary care with an unrelated symptom, about an administrative issue or for a screening procedure (such as cervical cytology), and patients who consult with a symptom of possible cancer. Selection as women move up this iceberg of symptoms and illness23,71,72 on to secondary care alters the prevalence of disease within the populations and hence predictive values.
Definition of the symptom of postcoital bleeding
Definitions of postcoital bleeding vary between studies (Supplementary Tables 1 and 2) and may lack detail on certain characteristics (such as frequency or quantity). In studies on women with cervical cancer, symptoms are often not sub-divided (such as ‘abnormal bleeding’) and thus postcoital bleeding may cluster within a symptom group, such as postmenopausal bleeding. This is in sharp contrast to the detail given of the morphology and histology of tumours.73 Only the studies concerning HIV specifically report vaginal postcoital bleeding.38–40
Measurement of the symptom of postcoital bleeding
The methodology used in most studies on women with cervical cancer is a retrospective review of hospital records of women who have been diagnosed as having cervical cancer. In one study a review of records and telephone interview with survivors was used.30 Only one study was prospective in which women were asked to complete a questionnaire prior to screening for cervical cancer.43 Methodology using a review of medical records is subject to recall bias by the women at the time of presentation to secondary care, and observer bias, both at the time of recording the symptom and when extracting information from the records. None of the studies concerning symptoms in women with carcinoma of the cervix appeared to conceal patient identity during data extraction from medical records or interview. Recall bias may be in favour of the symptom (such as knowledge about the classical presentation of the disease or prompting by clinicians) or against it (such as embarrassment or guilt about not presenting earlier). Recall bias is also an issue in studies using population surveys.23 The studies concerning women with cervical cancer do not always state if the reported symptoms are at presentation or diagnosis. In some studies multiple symptoms for individuals are reported, while in others only one appears (Supplementary Table 2).
There is a time delay between presentation from primary to secondary care and thus symptoms at secondary care may not have been present at initial consultation with primary care.
Strengths and limitations of the calculated probability
The positive predictive value for the diagnosis of cervical cancer in women with coital bleeding in the Finnish mass screening programme33 is 10 times higher than that calculated using contemporary data. This may be due to changes in the definition of coital/postcoital bleeding (time frame of 2 months versus 6 months), an increase in the incidence of postcoital bleeding (such as due to infection, use of hormones or sexual activity), differences in age, the falling incidence of cervical cancer and the effect of the screening programme.
The study used to determine the incidence of postcoital bleeding was based in an urban population within a single town in England.31 There is a geographical variation in the incidence of postcoital bleeding and the estimate of incidence may not be applicable to other populations. The incidence is expressed in terms of all menstruating women and it is not known if the incidence is the same in women who are amenorrhoeic. It was not determined if the women were sexually active and therefore at risk of the symptom.
The risk estimates are not individualised and true risk will depend on the presence or absence of other risk factors such as smoking and human papilloma virus infection.74
The risk estimates only apply to women in the community and do not apply to women who consult primary care and present with postcoital bleeding or women in whom the symptom is discovered opportunistically. With regard to overt blood loss per rectum and colorectal malignancy, risk estimates increase at least 20-fold as the calculation moves its denominator from community to primary care.23
Conclusion and implications
Evidence for the predictive value of postcoital bleeding for cervical cancer is poor but this should be considered against the background of the difficulties of undertaking research in primary care on the relationship between symptoms and comparatively rare outcomes such as cancer. It is unlikely that better evidence will be available in the near future. Given the high prevalence and incidence of postcoital bleeding27–29,33–36 and the physical, psychological and economic cost of colposcopy, it would appear to be inappropriate to investigate all women with postcoital bleeding for cervical cancer. The need for colposcopy in women who present to primary care cannot be determined precisely as there are no studies concerning the prevalence of postcoital bleeding in populations consulting primary care.
There is evidence of the need for a cervical smear to be performed at the recommended times according to the national screening programme33 although there is no evidence for a role for cervical cytology in the assessment of a woman with postcoital bleeding if a cervical smear is not due.
There is no evidence on the value of visual and manual inspection of the cervix in women with postcoital bleeding who present to primary care although this is currently accepted as good practice.75
Genital swabs, urine and/or blood should be used to detect sexually transmitted disease in a woman who presents with postcoital bleeding35–38,40,41 but it is unclear if assessment is necessary in all women with postcoital bleeding or only in those whose medical history or physical examination suggests a risk.
We suggest that in a patient-centred consultation, an estimate of the risk of cervical cancer, together with the poor evidence base and the technique of colposcopy, should be discussed. In all consultations the woman, in conjunction with the clinician, should decide on the need for referral to secondary care for investigation and treatment.15 This should take place if the woman requests it, if the cervix is clinically suspicious of cancer, if the smear shows cervical dyskaryosis12,13 or if symptoms persist and interfere with the woman's life in the broadest sense, including psychological distress.
There is a need for more robust studies on the epidemiology of symptoms of possible gynaecological malignancy in the community and primary care. The effectiveness of community-based education programmes, aimed at the detection of cervical carcinoma, informing women to consult a doctor if they have ‘bleeding after sex or between periods’ should be reviewed.76,77 The role of cytology and microbiology in the assessment of a woman with postcoital bleeding in primary care should be evaluated.
Supplementary Material
Acknowledgments
We wish to thank Dr Kelvin Jordan and Mr Charles Redman who kindly reviewed the manuscript and provided advice. We acknowledge the work of administrative staff at the Primary Care Sciences Research Centre, Keele University.
Supplementary information
Additional information accompanies this paper at http://www.rcgp.org.uk/Default.aspx?page=2482
Funding body
We are grateful for funding from the NHS(E) West Midlands R&D office and the North Staffordshire Primary Care R&D Consortium
Competing interests
The authors have stated that there are none
REFERENCES
- 1.Summerton N. Symptoms of possible oncological significance: separating the wheat from the chaff. BMJ. 2002;325:1254–1255. doi: 10.1136/bmj.325.7375.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eccersley AJ, Wilson EM, Makris A, Novell JR. Referral guidelines for colorectal cancer—do they work? Ann R Coll Surg Engl. 2003;85:107–110. doi: 10.1308/003588403321219885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pengiran Tengah DSNA, Byrne PO, Wills AJ. Urgent 2-week referrals for CNS/Brain tumours: a retrospective audit. Clin Oncol (R Coll Radiol) 2003;15:7–9. doi: 10.1053/clon.2002.0133. [DOI] [PubMed] [Google Scholar]
- 4.Morrison J, Gillespie S, MacKenzie IZ. ‘Two week wait’ standards for suspected gynaecological malignancy. On target but missing the point? Journal of the British Menopause Society. 2003;(December):170–172. doi: 10.1258/136218003323010629. [DOI] [PubMed] [Google Scholar]
- 5.Jiwa M, Hamilton W. Referral of suspected colorectal cancer: have guidelines made a difference? Br J Gen Pract. 2004;54:608–610. [PMC free article] [PubMed] [Google Scholar]
- 6.Panter SJ, Bramble MG, O'Flanagan H, Hungin APS. Urgent cancer referral guidelines: a retrospective cohort study of referrals for upper gastrointestinal adenocarcinoma. Br J Gen Pract. 2004;54:611–613. [PMC free article] [PubMed] [Google Scholar]
- 7.National Audit Office. Tackling cancer in England: saving more lives. London: The Stationary Office; 2004. [Google Scholar]
- 8.Medical Research Council. Topic review: primary health care. London: Medical Research Council; 1997. [Google Scholar]
- 9.Hamilton WT, Round A, Sharp D, Peters T. GPs can separate oncological wheat from chaff [letter] BMJ. 2003;326:397. doi: 10.1136/bmj.326.7385.397/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rees M, Purdie DW. The management of the menopause: an integrated healthcare pathway for the menopausal woman in primary care. 3rd edn. London: The British Menopause Society; 2002. [Google Scholar]
- 11.Jha S, Sabharwal S. Outcome of colposcopy in women presenting with postcoital bleeding and negative or no cytology — results of a 1-year audit. J Obstet Gynaecol. 2002;22:299–301. doi: 10.1080/01443610220130625. [DOI] [PubMed] [Google Scholar]
- 12.Fraser IS, Petrucco OM. Management of intermenstrual and postcoital bleeding, and an appreciation of the issues arising out of the recent case of O'Shea versus Sullivan and Macquarie Pathology. Aust NZ J Obstet Gynaecol. 1996;36:67–73. doi: 10.1111/j.1479-828x.1996.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal AN, Panoskaltsis, Smith T, Soutter WP. The frequency of significant pathology in women attending a general gynaecology service for postcoital bleeding. BJOG. 2001;108:103–106. doi: 10.1111/j.1471-0528.2001.00008.x. [DOI] [PubMed] [Google Scholar]
- 14.NHS Executive. Referral guidelines for suspected cancer. London: Department of Health; 2000. [Google Scholar]
- 15.Mazur D. Influence of the law on risk and informed consent. BMJ. 2003;327:731–736. doi: 10.1136/bmj.327.7417.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.General Medical Council. Duties of a doctor. www.gmc-uk.org/guidance/library/duties_of_a_doctor.asp (accessed 12 May 2006)
- 17.Gigerenzer G, Edwards A. Simple tools for understanding risks: from innumeracy to insight. BMJ. 2003;327:741–744. doi: 10.1136/bmj.327.7417.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steel N. Thresholds for taking antihypertensive drugs in different professional and lay groups: questionnaire survey. BMJ. 2000;320:1446–1447. doi: 10.1136/bmj.320.7247.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devereaux PJ, Anderson DR, Gardner MJ, et al. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. BMJ. 2001;323:1218–1222. doi: 10.1136/bmj.323.7323.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrne PS, Long BEL. Doctors talking to patients. Exmouth: Royal College of General Practitioners; 1989. [Google Scholar]
- 21.Edwards A, Elwyn G, Wood F, et al. Shared decision making and risk communication in practice. Br J Gen Pract. 2005;55:6–13. [PMC free article] [PubMed] [Google Scholar]
- 22.O'Connor AM, Legare F, Stacey D. Risk communication in practice: the contribution of decision aids. BMJ. 2003;327:736–740. doi: 10.1136/bmj.327.7417.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fijten GH, Blijham GH, Knottnerus JA. Occurrence and clinical significance of overt blood loss per rectum in the general population and in medical practice. Br J Gen Pract. 1994;44:320–325. [PMC free article] [PubMed] [Google Scholar]
- 24.Truelsen F. Cancer of the uterine cervix. Copenhagen: Rosenkilde and Bagger; 1949. [Google Scholar]
- 25.Dass A, Mookerjea G. A statistical survey of cervical cancer. J Obstet Gynaecol India. 1961;12:52–57. [Google Scholar]
- 26.Subhadra Devi N, Prabhavathi R. Statistical review of 2046 cases of carcinoma of cervix. J Obstet Gynaecol India. 1961;12:55–61. [Google Scholar]
- 27.Viikki M, Pukkala E, Hakama M. Bleeding symptoms and subsequent risk of gynecological and other cancers. Acta Obstet Gynecol Scand. 1998;77:564–569. [PubMed] [Google Scholar]
- 28.Goldacre MJ, Loudon N, Watt B, et al. Epidemiology and clinical significance of cervical erosion in women attending a family planning clinic. BMJ. 1978;1:748–750. doi: 10.1136/bmj.1.6115.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindner LE, Geerling S, Nettum JA, et al. Clinical characteristics of women with chlamydial cervicitis. J Reprod Med. 1988;33:684–690. [PubMed] [Google Scholar]
- 30.Pretorius R, Semrad N, Watring W, Fotheringham N. Presentation of cervical cancer. Gynecol Oncol. 1991;42:48–53. doi: 10.1016/0090-8258(91)90229-x. [DOI] [PubMed] [Google Scholar]
- 31.Shapley M, Jordan K, Croft PR. An epidemiological survey of symptoms of menstrual loss in the community. Br J Gen Pract. 2004;54:359–363. [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 33.Hakama M, Joutsenlahti U, Virtanen A, Rasanen U. Mass screenings for cervical cancer in Finland 1963–1971. Ann Clin Res. 1975;7:101–111. [PubMed] [Google Scholar]
- 34.Anorlu RI, Abdul-kareem FB, Abudu OO, Oyekan TO. Cervical cytology in an urban population in Lagos, Nigeria. J Obstet Gynae. 2003;23:285–288. doi: 10.1080/01443610310000100114. [DOI] [PubMed] [Google Scholar]
- 35.Verhoeven V, Avonts D, Meheus A, et al. Chlamydial infection: an accurate model for opportunistic screening in general practice. Sex Transm Infect. 2003;79:313–317. doi: 10.1136/sti.79.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Götz HM, van Bergen JEAM, Veldhuijzen IK, et al. A prediction rule for selective screening of Chlamydia trachomatis infection. Sex Transm Infect. 2005;81:24–30. doi: 10.1136/sti.2004.010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bax CJ, Oostvogel PM, Mutsaers JAEM, et al. Clinical characteristics of chlamydia trachomatis infections in a general outpatient department of obstetrics and gynaecology in the Netherlands. Sex Transm Infect. 2002;78:e6. doi: 10.1136/sti.78.6.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padian NS, Abrams J, Skurnick JH, et al. Risk factors for postcoital bleeding among women with or at risk for infection with human immunodeficiency virus. J Infect Dis. 1995;172:1084–1087. doi: 10.1093/infdis/172.4.1084. [DOI] [PubMed] [Google Scholar]
- 39.Ellerbrock TV, Wright TC, Bush TJ, et al. Characteristics of menstruation in women infected with human immunodeficiency virus. Obstet Gynecol. 1996;87:1030–1034. doi: 10.1016/0029-7844(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 40.Guimaraes MDC, Vlahov D, Castilho EA. Postcoital vaginal bleeding as a risk factor for transmission of the human immunodeficiency virus in a heterosexual partner study in Brazil. Arch Intern Med. 1997;157:1362–1369. doi: 10.1001/archinte.1997.00440330102012. [DOI] [PubMed] [Google Scholar]
- 41.Seidlin M, Vogler M, Lee E, et al. Heterosexual transmission of HIV in a cohort of couples in New York City. AIDS. 1993;7:1247–1254. doi: 10.1097/00002030-199309000-00015. [DOI] [PubMed] [Google Scholar]
- 42.Mawajdeh SM, Al-Qutob RJ, Farag AM. Prevalence and risk factors of genital prolapse. Saudi Med J. 2003;24:161–165. [PubMed] [Google Scholar]
- 43.Kauraniemi T. Gynaecological health screening by means of questionnaire and cytology. Acta Obstet Gyn Scand. 1969;4:48. [PubMed] [Google Scholar]
- 44.Das RK. Cancer cervix in Assam. J Obstet Gynaecol India. 1970;20:234–239. [PubMed] [Google Scholar]
- 45.Pardanani NS, Tischler LP, Brown WH, De Feo E. Carcinoma of cervix. Evaluation of treatment in community hospital. N Y State J Med. 1975;(June):1018–1021. [PubMed] [Google Scholar]
- 46.Adelusi B. Carcinoma of the cervix uteri in Ibadan: clinico-pathologic features. Niger Med J. 1978;8:129–132. [PubMed] [Google Scholar]
- 47.Nnatu SNN, Durosinmi-Etti FA. The problems with the management of carcinoma of the cervix in Nigeria-Lagos experience. East Afr Med J. 1985;62:347–354. [PubMed] [Google Scholar]
- 48.Saigo PE, Cain JM, Kim WS, et al. Prognostic factors in adenocarcinoma of the uterine cervix. Cancer. 1986;57:1584–1593. doi: 10.1002/1097-0142(19860415)57:8<1584::aid-cncr2820570825>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz PE, Merino MJ, McCrea Curnen MG. Clinical management of patients with invasive cervical cancer following a negative pap smear. Yale J Biol Med. 1988;61:327–338. [PMC free article] [PubMed] [Google Scholar]
- 50.Emembolu JO, Ekwempu CC. Carcinoma of the cervix uteri in Zaria: etiological factors. Int J Gynecol Obstet. 1988;26:265–269. doi: 10.1016/0020-7292(88)90272-x. [DOI] [PubMed] [Google Scholar]
- 51.Machoki JMN, Rogo KO. Knowledge and attitudinal study of Kenyan women in relation to cervical carcinoma. Int J Gynecol Obstet. 1990;34:55–59. doi: 10.1016/0020-7292(91)90539-h. [DOI] [PubMed] [Google Scholar]
- 52.Angel C, DuBeshter B, Lin JY. Clinical presentation and management of stage 1 cervical adenocarcinoma: a 25 year experience. Gynecol Oncol. 1992;44:71–78. doi: 10.1016/0090-8258(92)90015-b. [DOI] [PubMed] [Google Scholar]
- 53.Onwudiegwu U, Bako A, Oyewumi A. Cervical cancer — a neglected health tragedy. J Obstet Gynae. 1999;19:61–64. doi: 10.1080/01443619966001. [DOI] [PubMed] [Google Scholar]
- 54.Ijaiya MA, Aboyeji PA, Buhari MO. Cancer of the cervix in Ilorin, Nigeria. WAJM. 2004;23:319–322. doi: 10.4314/wajm.v23i4.28148. [DOI] [PubMed] [Google Scholar]
- 55.Slater DN. Multifactorial audit of invasive cervical cancer: key lessons for the national screening programme. J Clin Pathol. 1995;48:405–407. doi: 10.1136/jcp.48.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nieminen P, Kallio M, Hakama M. The effect of mass screening on incidence and mortality of squamous and adenocarcinoma of the cervix uteri. Obstet Gynecol. 1995;85:1017–1021. doi: 10.1016/0029-7844(95)00063-W. [DOI] [PubMed] [Google Scholar]
- 57.Parkin DM, Whelan SL, Ferlay J, et al., editors. Cancer incidence in five continents. Volume VIII. Lyon, France: International Agency for Cancer Research; 2002. [scientific publication 155]. [Google Scholar]
- 58.Hakama M, Pukkala E. Selective screening for cervical cancer. Experience of the Finnish mass screening system. Br J Prev Soc Med. 1977;31:238–244. doi: 10.1136/jech.31.4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mackenzie IZ, Bibby JG. Critical assessment of dilatation and curettage in 1029 women. Lancet. 1978;9:566–568. doi: 10.1016/s0140-6736(78)92895-7. [DOI] [PubMed] [Google Scholar]
- 60.Allen DG, Correy JF, Marsden DE. Abnormal uterine bleeding and cancer of the genital tract. Aust NZ J Obstet Gynaecol. 1990;30:81–83. doi: 10.1111/j.1479-828x.1990.tb03204.x. [DOI] [PubMed] [Google Scholar]
- 61.Shalini R, Amita S, Neera MA. How alarming is post-coital bleeding—a cytologic, colposcopic and histopathologic evaluation. Gynecol Obstet Invest. 1998;45:205–208. doi: 10.1159/000009957. [DOI] [PubMed] [Google Scholar]
- 62.Decloedt JF, Fenton DW. Outpatient hysteroscopy: indications and hysteroscopic findings in pre- and postmenopausal patients. Gynaecological Endoscopy. 1999;8:137–141. [Google Scholar]
- 63.Selo-Ojeme DO, Dayoud N, Patel A, Metha M. A clinico-pathological study of postcoital bleeding. Arch Gynecol Obstet. 2004;270:34–36. doi: 10.1007/s00404-002-0457-6. [DOI] [PubMed] [Google Scholar]
- 64.West Midlands Cervical Screening Programme. Factsheet 5. Information sheet for health professionals. Unscheduled cervical screening. Birmingham: West Midlands Cervical Screening Modernisation Strategy Group; 2004. [Google Scholar]
- 65.National Institute for Health and Clinical Excellence. Guidance on the use of liquid-based cytology for cervical screening. London: National Institute for Health and Clinical Excellence; 2003. Technology Appraisal 69. [Google Scholar]
- 66.Misra JS, Das K, Chandrawati Results of clinically downstaging cervical cancer in a cytological screening programme. Diagn Cytopathol. 1998;19:344–348. doi: 10.1002/(sici)1097-0339(199811)19:5<344::aid-dc6>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 67.National Statistics. Cancer statistics: registration. London: Office for National Statistics; 2001. http://www.statistics.gov.uk/downloads/theme_health/MB1_32/MB1_32.pdf (accessed 12 May 06) [Google Scholar]
- 68.Quinn M, Babb P, Kirby EA, Brock A. Report: Registration of cancer diagnosed in 1971–2000, England and Wales. Health Stat Q. 2000;7:82. [Google Scholar]
- 69.Quinn M, Babb P, Jones J, Allen E. Effect of screening on incidence of and mortality from cancer of the cervix in England: evaluation based on routinely collected statistics. BMJ. 1999;318:904–908. doi: 10.1136/bmj.318.7188.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet. 2004;364:249–256. doi: 10.1016/S0140-6736(04)16674-9. [DOI] [PubMed] [Google Scholar]
- 71.Pearse IH, Crocker LH. The Peckham experiment. London: Allen and Urwin; 1943. [Google Scholar]
- 72.Hannay DR. The ‘iceberg’ of illness and ‘trivial’ consultations. J R Coll Gen Pract. 1980;30:551–554. [PMC free article] [PubMed] [Google Scholar]
- 73.Matthews CM, Burke TW, Tornos C, et al. Stage 1 cervical adenocarcinoma: prognostic evaluation of surgically treated patients. Gynecol Oncol. 1993;49:19–23. doi: 10.1006/gyno.1993.1079. [DOI] [PubMed] [Google Scholar]
- 74.Kjaer SK, van den Brule AJC, Paull G, et al. Human papillomavirus—the most significant risk determinant of cervical intraepithelial neoplasm. Int J Cancer. 1996;65:601–606. doi: 10.1002/(SICI)1097-0215(19960301)65:5<601::AID-IJC8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 75.National Institute for Health and Clinical Excellence. Referral for suspected cancer. London: National Institute for Health and Clinical Excellence; 2005. [Google Scholar]
- 76.NHS. Cervical cytology recall letter. London: Department of Health; 2004. [Google Scholar]
- 77.NHS Cancer Screening Programme. Cervical screening: the facts. London: Department of Health; 2002. (updated March 2004) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


