Abstract
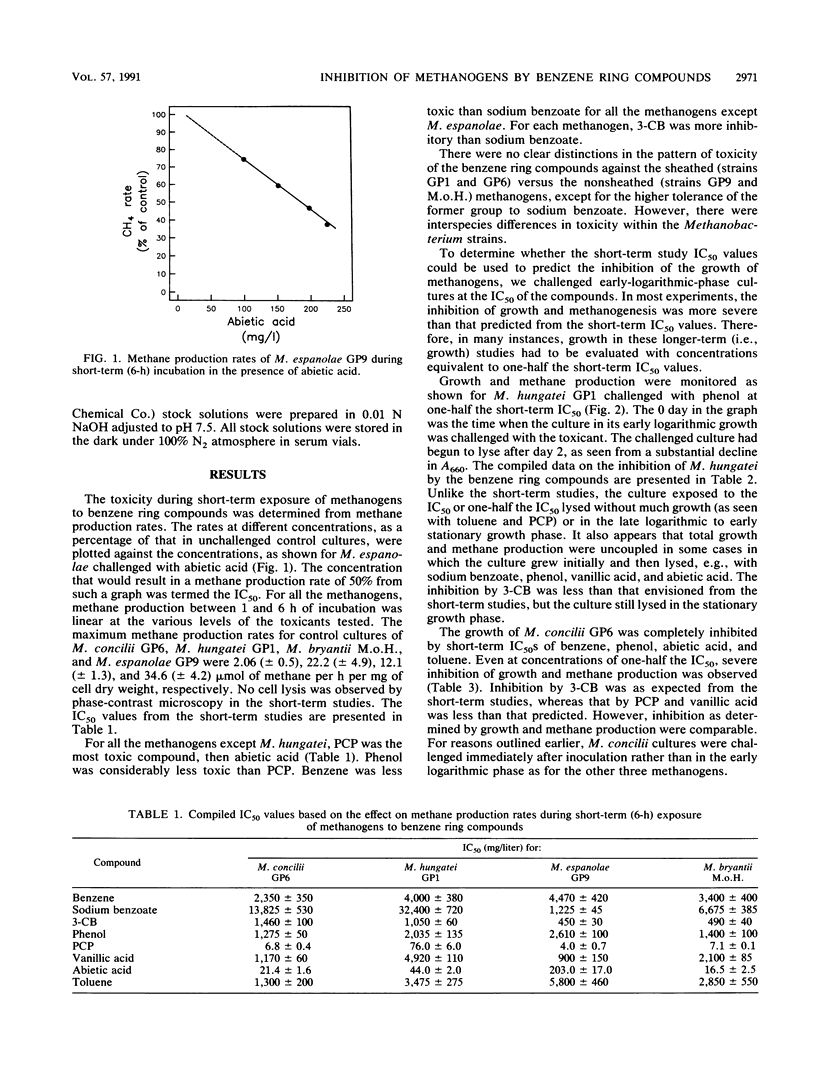
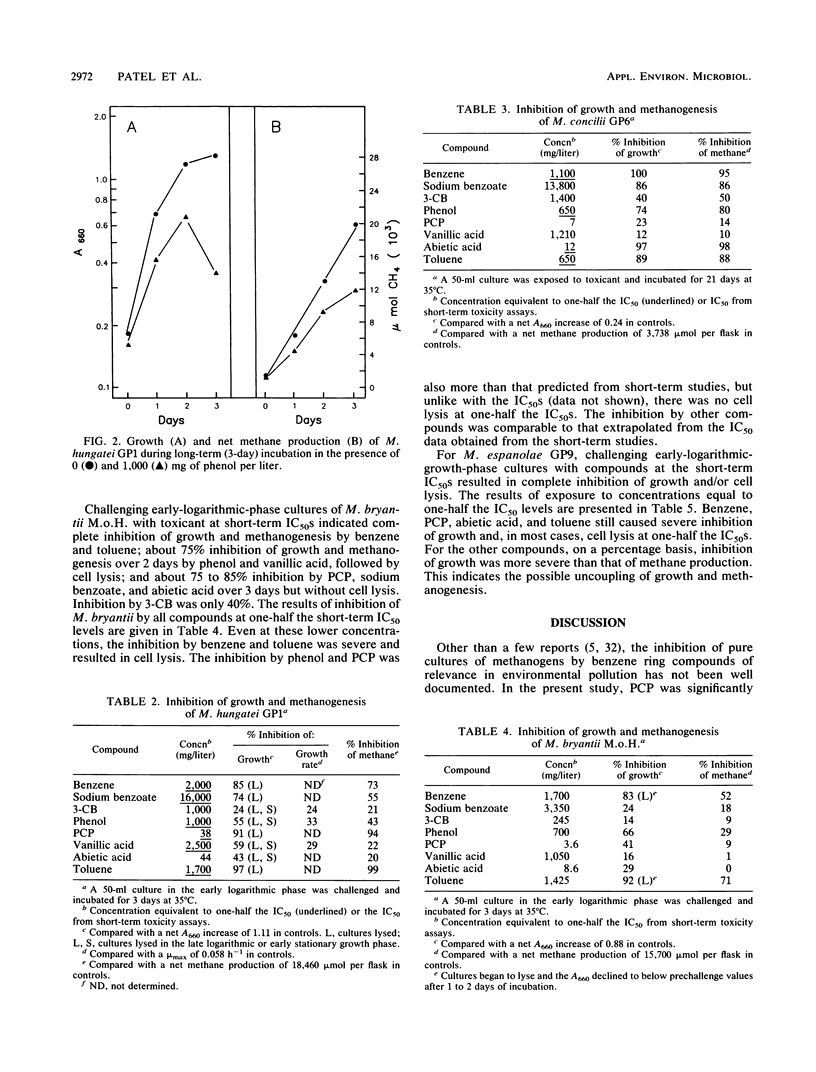
The inhibition of methane production by Methanosaeta concilii GP6, Methanospirillum hungatei GP1, Methanobacterium espanolae GP9, and Methanobacterium bryantii M.o.H. during short-term (6-h) exposure to eight benzene ring compounds was studied. The concentration that caused 50% inhibition of the methane production rate (IC50) was dependent on the species and the toxicant. Pentachlorophenol was the most toxic of the tested compounds, with an IC50 of less than 8 mg/liter for all species except M. hungatei. Abietic acid was the next most toxic compound for all the species, with an IC50 in the range of 21.4 to 203 mg/liter. Sodium benzoate was generally the least toxic, with an IC50 in the range of 1,225 to 32,400 mg/liter. 3-Chlorobenzoate was substantially more toxic (IC50, 450 to 1,460 mg/liter) than benzoate. The inhibition by benzene, phenol, vanillic acid, and toluene was intermediate to that of pentachlorophenol and benzoate. Long-term incubation (days) studies to determine effect on growth indicated that all eight compounds were usually much more toxic than predicted from the short-term data. In these latter studies, there was generally a good correlation in the observed inhibition as determined from growth and methane production.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battersby N. S., Wilson V. Survey of the anaerobic biodegradation potential of organic chemicals in digesting sludge. Appl Environ Microbiol. 1989 Feb;55(2):433–439. doi: 10.1128/aem.55.2.433-439.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuil C., Patel G. B. Composition of Methanospirillum hungatii GP1 during growth on different media. Can J Microbiol. 1980 May;26(5):577–582. doi: 10.1139/m80-102. [DOI] [PubMed] [Google Scholar]
- Dolfing J., Tiedje J. M. Acetate inhibition of methanogenic, syntrophic benzoate degradation. Appl Environ Microbiol. 1988 Jul;54(7):1871–1873. doi: 10.1128/aem.54.7.1871-1873.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C. Biochemistry of the bacterial catabolism of aromatic compounds in anaerobic environments. Nature. 1977 Nov 3;270(5632):17–22. doi: 10.1038/270017a0. [DOI] [PubMed] [Google Scholar]
- Godsy E. M., Goerlitz D. F., Ehrlich G. G. Effects of pentachlorophenol on methanogenic fermentation of phenol. Bull Environ Contam Toxicol. 1986 Feb;36(2):271–277. doi: 10.1007/BF01623507. [DOI] [PubMed] [Google Scholar]
- Jarrell K. F., Colvin J. R., Sprott G. D. Spontaneous protoplast formation in Methanobacterium bryantii. J Bacteriol. 1982 Jan;149(1):346–353. doi: 10.1128/jb.149.1.346-353.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. J., Nagle D. P., Jr, Whitman W. B. Methanogens and the diversity of archaebacteria. Microbiol Rev. 1987 Mar;51(1):135–177. doi: 10.1128/mr.51.1.135-177.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel G. B., Roth L. A., van den Berg L., Clark D. S. Characterization of a strain of Methanospirillum hungatti. Can J Microbiol. 1976 Sep;22(9):1404–1410. doi: 10.1139/m76-208. [DOI] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Microbial degradation of haloaromatics. Annu Rev Microbiol. 1988;42:263–287. doi: 10.1146/annurev.mi.42.100188.001403. [DOI] [PubMed] [Google Scholar]
- Roberton A. M., Wolfe R. S. Adenosine triphosphate pools in Methanobacterium. J Bacteriol. 1970 Apr;102(1):43–51. doi: 10.1128/jb.102.1.43-51.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic Acid. Appl Environ Microbiol. 1984 Oct;48(4):840–848. doi: 10.1128/aem.48.4.840-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleat R., Robinson J. P. The bacteriology of anaerobic degradation of aromatic compounds. J Appl Bacteriol. 1984 Dec;57(3):381–394. doi: 10.1111/j.1365-2672.1984.tb01404.x. [DOI] [PubMed] [Google Scholar]


