Abstract
The neurobiological doctrine governing the concept of neurogenesis has undergone a revolution in the past few years. What was once considered dubious is now well accepted: new neurons are born in the adult brain. Science fiction is quickly becoming a reality as scientists discover ways to convert skin, bone, or blood cells into neurons.
In the epilepsy arena, widespread interest has developed because of the evidence that neurogenesis increases after seizures, trauma, and other insults or injuries that alter seizure susceptibility.
This review discusses some of the initial studies in this field, and their often surprising functional implications. The emphasis will be on the granule cells of hippocampus, because they are perhaps more relevant to epilepsy than other areas in which neurogenesis occurs throughout life, the olfactory bulb and subventricular zone. In particular, the following questions will be addressed:
Do granule cells that are born in the adult brain become functional, and what are the limits of their function? Do they behave homogeneously? Results from our own laboratory have focused on cells that become established outside the normal boundaries of the granule cell layer, forming a group of “ectopic” granule cells in the hilar region.
Is increased neurogenesis beneficial, or might it actually exacerbate seizures? Evidence is presented that supports the hypothesis that new granule cells may not necessarily act to ameliorate seizures, and might even contribute to them. Furthermore, cognitive deficits following seizures might in part be due to new circuits that develop between new cells and the host brain.
How do the new cells interact with the host brain? Several changes occur in the dentate gyrus after seizures, and increased neurogenesis is only one of many. What is the interdependence of this multitude of changes, if any?
Is neurogenesis increased after seizures in man? Research suggests that the data from human epileptics are actually inconsistent with the studies in animal models of epilepsy, because there is little evidence of increased neurogenesis in epileptic tissue resected from intractable epileptics. Yet neurogenesis has been shown to occur in humans throughout adult life. What might be the reasons for these seemingly disparate results?
Introduction
Neurogenesis is the creation of nerve cells. New neurons normally develop from progenitor cells located in many areas of the immature nervous system during development. In the adult, it occurs primarily in three locations: the subgranular zone of the dentate gyrus, the olfactory bulb, and the subventricular zone lining the ventricular walls.
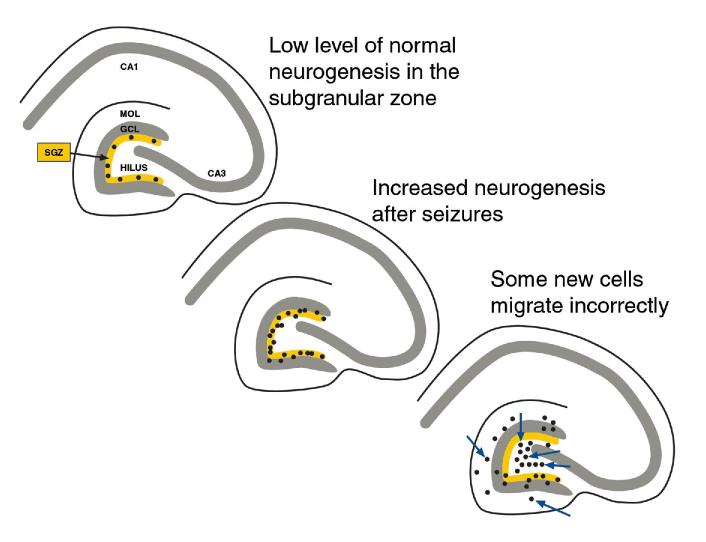
In the dentate gyrus, the subgranular zone is a thin layer that lies just beneath the granule cell layer (Fig. 1). Ordinarily, cells are born there at a modest rate throughout life, although this rate gradually decreases with age.56,76,90,116 Remarkably, seizures appear to increase the rate of neurogenesis. This appears to occur after brief seizures,9 kindling,60,71,92 and after status epilepticus induced by chemoconvulsants such as pilocarpine26,73 or kainic acid45,69 or status epilepticus induced by electrical stimulation.82 Seizures following electroconvulsive shock also increase neurogenesis.64,93 Other experimental insults that injure the brain cause increased neurogenesis also, such as experimental trauma or stroke.61,62,94 Importantly, a number of experiments have shown that various facets of the biological responses to such events (e.g., seizures, trauma) may contribute to the increase in neurogenesis, indicating that seizures per se may not be the only factor. For example, manipulations that increase neuronal activity, without necessarily leading to seizures, induce increased neurogenesis.11,20,21,29,42 Changes in stress also increase neurogenesis.42-44 Many of the neurons that are born in the dentate gyrus after seizures migrate correctly, that is, into the granule cell layer73 (Fig. 1). However, others apparently do not. Thus, new cells appear in the molecular layer and hilus after seizures73,87 (Fig. 1). Presumably, the new cells in the molecular layer were born in the subgranular zone and migrated into and past the cell layer. They may fail to stop because in the adult brain the signals for migration are weaker than in the developing animal, when virtually all cells know when to stop and the granule cell layer is a very well defined space. Regarding the granule cells that migrate into the hilus, the sites of origin may also be the subgranular zone, and in this case the cells may simply miss the signal that tells them the correct direction to travel. Instead, they may migrate in the opposite direction than they normally would move. What is extraordinary is that they apparently continue to migrate in this direction, about 180° from normal, and do not stop for hundreds of microns. Apparently they do stop eventually, because clusters of cells appear to accumulate at the border between the hilus and area CA3 (Fig. 2). Therefore, it is likely that the adult brain has a potent signal at that location that eliminates any chance that a dentate neuron would cross into area CA3.
Figure 1.
Schematic diagram of changes in neurogenesis of hippocampal granule cells after seizures. Top left: A low level of neurogenesis is normal in the adult dentate gyrus of the rat. A diagram of a transverse section of the rat hippocampus is shown. Neurogenesis normally occurs in a layer positioned immediately below the granule cell layer which is called the subgranular zone (SGZ). MOL = molecular layer, GCL = granule cell layer. Center: Increased neurogenesis occurs after seizures. This is thought to arise from the SGZ primarily. Lower right: After seizures induced by pilocarpine, some of the newly born cells appear to migrate from the SGZ incorrectly, entering the molecular layer and hilus (arrows).
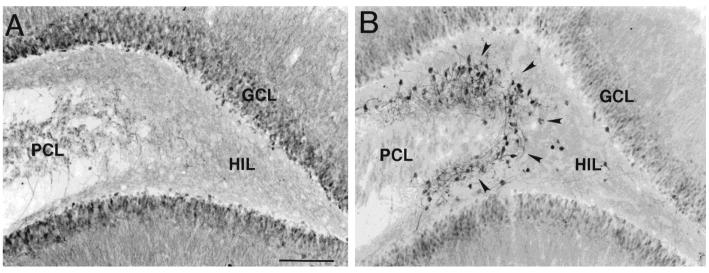
Figure 2.
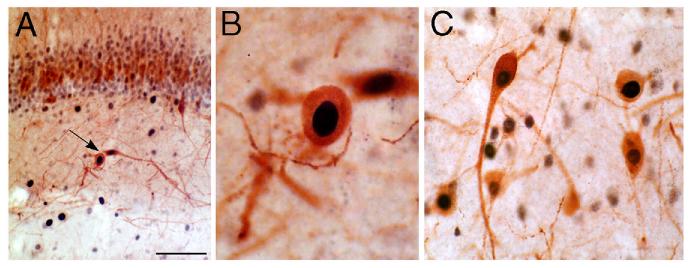
Ectopic hilar granule cells in the dentate gyrus after kainic acid-induced status epilepticus. A) A section from a saline-injected rat demonstrates calbindin-immunoreactivity predominantly in the cell bodies, dendrites and axons of dentate gyrus granule cells. PCL = pyramidal cell layer; HIL = hilus; GCL= granule cell layer. Calibration = 200 μm. B) A section from a kainic acid-injected rat, over 1 month after status epilepticus. Numerous calbindin-immunoreactive neurons surround the border of the hilus and pyramidal cell layer (arrows). Same calibration as A. Used with permission, ref. 87.
It is conceivable that the hilar granule cells actually don't arise from the subgranular zone. Instead, the site of origin may be the hilus itself, because this is an area that contributes, as late as postnatal day 10, to granule cells that are normally situated in the granule cell layer during adulthood;2,3 vestiges of this tertiary matrix might still exist thereafter. It is not entirely clear that this matrix converts completely to the subgranular zone at maturation. However, regardless of where in the hilus they are born, these cells still seem to migrate until the CA3/hilar border, as if they have migrated there and then must stop.
Recent evidence indicates that cells from the periphery can be transformed into cells with the characteristics of neurons.5,47,68,105 This raises an even more heretical possibility. Some of the new neurons that appear in the dentate gyrus could originate from the blood cells that enter the dentate gyrus through breaks in the blood-brain barrier during seizures. This would be consistent with the empirical observation that prolonged seizures (e.g., status epilepticus) lead to more neurogenesis than weak seizures, because severe seizures would be associated with greater breaks in the blood-brain barrier. In fact there does appear to be an increase in the endothelial cell staining of the hilus after pilocarpine-induced seizures (see chapter by Croll et al, this volume). But it is too soon to say whether these cells could become neurons.
Proving That Granule-Like Cells in the Hilus after Seizures Were Newly-Born Granule Cells
Our studies of granule-like cells in the hilus that appeared after seizures at first focused on whether in fact they were granule cells, and the evidence that they were born after seizures. The data were at first anatomical: multiple cells that were immunoreactive for a neuronal marker of granule cells, the calcium-binding protein calbindin D28K, were present in animals that had status epilepticus, but not saline-treated, age-matched controls.87 Our hypothesis, that these were newly-born granule cells that had somehow ended up in the hilus rather than the granule cell layer, clearly required proof. Our hypothesis stemmed from the work of Parent et al,73 who had already shown that some of the new granule cells that arose after seizures appeared to be in the hilus. Still, when we found very much larger numbers of calbindin-immunoreactive neurons that were quite far from the granule cell layer, actually on the border with area CA3 and the hilus (Fig. 2), it was not clear that these in fact were the same cells as shown in the study by Parent et al,73 which were much closer to the granule cell layer.
One argument against the hypothesis was based on several papers which actually showed that some granule cells in the normal rat do exist in the hilus.38,67 However, the number of these “ectopic” granule cells is extremely small, and in fact in our own saline-control tissue, there were rarely any hilar neurons that were calbindin-immunoreactive (Fig. 2). After ischemia, there appear to be some ectopic hilar cells that have the general morphology of granule cells, but again only a few.52 This contrasted with our observations in rats that had status epilepticus and recurrent seizures, where in the average 50 μm section there were often as many as 100 cells (Fig. 2).
It is important to consider the validity of calbindin immunoreactivity to identify granule cells in this context. The immunoreactivity for calbindin appeared suitable to identify granule cells because, in saline-treated controls, granule cells were consistently immunoreactive for calbindin, as indeed many others have shown.6,48,77,97 Yet calbindin is not a consistent marker of granule cells after seizures. Thus, seizures lead to a decrease in calbindin immunoreactivity in some granule cells,7,106 and this was also evident in our epileptic tissue (Fig. 1 of ref. 89). Developing granule cells also have weak labeling for calbindin,32,41,77 so the cells that were recently born might not be immunoreactive. Therefore, calbindin may not be a faithful marker of granule cells after seizures. Thus, the number of granule neurons in the hilus after seizures is difficult to estimate based entirely on calbindin immunoreactivity, and it could be underestimated if many were recently born.
Based on the considerations above, it was highly unlikely that the neurons we found in pilocarpine-treated rats were simply normal granule cells that were in the wrong place. However, another possibility was that the hilar cells we surmised were granule cells because of their calbindin-immunoreactivity and general morphology were actually a type of hilar interneuron. The primary argument against this was the data from saline-treated controls, which did not show many neurons in the hilus that were immunoreactive for calbindin. However, calbindin-immunoreactive hilar neurons have been reported by others.95 Furthermore, some interneurons that normally have low levels of calbindin might increase expression after seizures. Indeed, this occurs for hilar neurons that express neuropeptide Y.91,108
Therefore, a series of anatomical experiments were conducted to address the hypothesis that the hilar neurons we suspected were granule cells were actually a type of interneuron. First, we found that markers of interneurons (parvalbumin, somatostatin, GABA, GAD, calretinin) did not coincide with the clusters of calbindin-immunoreactive, granule-like neurons in the hilus. Thus, in serial sections through the dentate gyrus, clusters of cells were present at the CA3/hilar border that were immunoreactive for calbindin, but adjacent sections failed to demonstrate hilar neurons in that location that stained for markers of GABAergic neurons. Interestingly, neuropeptide Y (NPY) did not label the hilar calbindin-immunoreactive plexus, although it would not have been surprising if it had, because NPY labels some granule cell axons after seizures, and sometimes there is immunoreactivity in their somata.91,108 Glial fibrillary acidic protein (GFAP), which labels adult glia, also did not label these clusters of calbindin-immunoreactive hilar neurons. Therefore, it was unlikely that the neurons in question were glia.
Although these results were useful, there still remained a possibility that the neurons we thought were newly-born granule cells were actually not. For example, perhaps they were VIP-or CCK-immunoreactive neurons; indeed there are so many possible types of interneurons it is difficult to completely test the spectrum of possibilities. Another possibility was that our antibodies performed differently in epileptic tissue than in control sections, or identified an odd cell type that had not previously been described. Knowing that the hilus contains a remarkable number of cell types,4 and many undergo seizure-induced changes in gene expression, questions still remained.
Perhaps the most convincing evidence came from the results of intracellular recordings from slices of pilocarpine-treated rats with chronic seizures. Normally in an adult rat, or in an aged saline control, it is quite rare to encounter neurons in the hilus with electrophysiological characteristics of granule cells. Most neurons are either similar to mossy cells or interneurons.84,85 Thus, granule cells have high resting membrane potential relative to other cell types (mossy cells, interneurons, and pyramidal cells). Strong spike frequency adaptation is another distinguishing feature. Granule cells also have an action potential duration that is consistent with a regular spiking neuron, the action potential has a high ratio of rate of rise to rate of decay (dv/dt ratio), and there is no significant inward rectification or “sag” in response to hyperpolarization from resting potential.84,85,87,103,104,113 Other cell types may have one or another of these characteristics, but not all of them. Therefore, it was striking when many healthy hilar neurons were encountered that had electrophysiological characteristics of granule cells.
Furthermore, after intracellular injection of Neurobiotin into the hilar cells with the electrophysiological “signature” of granule cells, the labeled neurons had the morphology of a granule cell. Thus, there was a small, ovoid soma, dendrites with many spines, and a mossy fiber axon. There were no thorny excrescences, consistent with the fact that these cells did not have electrophysiological characteristics of mossy cells or pyramidal neurons. Some of the hilar cells with electrophysiology like granule cells had a polarized dendritic tree, like a normal granule cell (Fig. 3). However, others had “basal” dendrites and their dendritic tree was therefore bipolar. This is actually consistent with the description of granule cells in the normal location (i.e., in the granule cell layer) in epileptic tissue, which may have basal dendrites.17,79,102,110 However, a very high percentage of hilar granule cells had basal dendrites relative to granule cells in the granule cell layer of epileptic tissue. This difference raises the possibility that there were fewer morphological constraints on the developing dendritic tree of the hilar “granule” cells as they differentiated, so they developed more basal dendrites. In summary, these correlative electrophysiological-anatomical data provided additional evidence that the calbindin-immunoreactive hilar neurons observed in fixed tissue sections were granule cells.
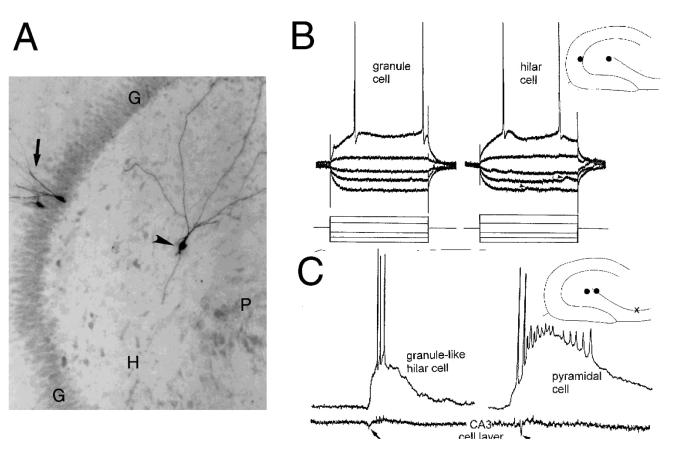
Figure 3.
Morphology and electrophysiology of ectopic hilar granule cells born after seizures. A) The morphology of a hilar granule cell from a pilocarpine-treated rat (arrowhead) is shown after intracellular injection of Neurobiotin. For comparison, two granule cells in the granule cell layer (arrow) were also injected. G = granule cell layer; H = hilus; P = pyramidal cell layer. Calibration = 100 μm. B) Physiology of hilar granule cells. Responses to direct current injection (rectangular current pulses, as shown at the bottom) are superimposed for a granule cell located in the granule cell layer (left, “granule cell”) and a granule-like neuron located in the hilus (right, “hilar cell”) of the same slice. This slice was from a pilocarpine-treated rat which had status epilepticus and recurrent seizures. A diagram of the location of these cells is shown at top right. G = granule cell layer, H = hilus, P = pyramidal cell layer. Arrowheads mark spontaneous synaptic potentials. Calibration = 20 mV, 50 msec. C) Comparison of pyramidal cell and ectopic granule cell activity. Simultaneous extracellular recordings from the pyramidal cell layer of CA3b (bottom) and intracellular recordings are shown for a hilar granule cell (left) and a pyramidal cell (right). This slice was from a pilocarpine-treated rat that had status epilepticus and recurrent seizures. A diagram of the location of the cells (dots) and extracellular recording (x) is shown at top right. The arrows point to the population spike of the spontaneous burst discharge recorded extracellularly. Calibration same as B. Used with permission, ref. 87.
Particularly important was the mossy fiber axon, given that some interneurons have small ovoid somata and can have spiny bipolar dendrites, somewhat similar to the bipolar hilar cells we suspected were granule cells. But interneurons do not have a mossy fiber axon. The classical meaning of the term “mossy fiber” is a granule cell axon that innervates stratum lucidum, where its major branch projects to CA2 in a fiber that runs approximately 100-200 μm and parallel to the pyramidal cell layer. Along its length there are giant boutons at regular intervals, and these innervate the proximal dendrites of CA3 neurons.1,13,24,36,37 There are also many collaterals in the hilus, where giant boutons innervate the excrescences of mossy cells, and in addition there are smaller, more conventional terminals that innervate dendrites of several types of hilar neurons. In epileptic tissue, mossy fiber axons develop additional collaterals that project to the inner molecular layer, referred to as “mossy fiber sprouting.” Sprouted axons contact the dendrites of granule cells and interneurons in the inner molecular layer.55,70,111 Notably, the cells impaled in the hilus of epileptic rats that had electrophysiological features of granule cells had an axon with the characteristics of mossy fibers. Because no other hippocampal neurons have such an axon, it was a strong argument that the hilar cells which were impaled indeed were a type of granule cell.
Thus, the combination of electrophysiology and morphology allowed us to easily distinguish granule cells from other cells in the hilus/CA3 region (see also refs. 84 85,86). Taken together, the morphology and electrophysiology made a compelling case that the granule-like cells in the hilus actually were granule cells (Fig. 3A,B; ref. 87).
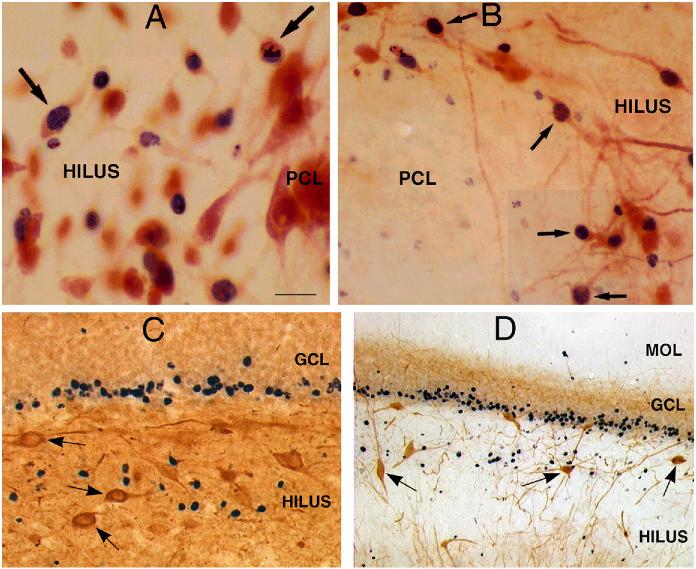
What was the evidence that these hilar granule cells were in fact born after seizures? To address this question, a marker of dividing cells, bromodeoxyuridine (BrdU), was used. BrdU was injected after status epilepticus using two different schedules (1 injection per day, 50 mg/kg, for 5 days, either days 3-8 after status or 4-11). In all cases, there were cells in the hilus that were immunoreactive for both BrdU, which stains the nucleus, and calbindin, which stains the cytoplasm (Fig. 4). Brdu/NeuN double-labeled cells were also present (Fig. 4). However, no BrdU profiles were double-labeled with markers of GABAergic neurons (parvalbumin, neuropeptide Y; Fig. 4). In addition, BrdU injections in the hours before status epilepticus, or in saline controls, have not provided evidence of new neurons in the hilar region. Furthermore, injection long after status (over one month) for 5 days (as described above, 1 injection per day, 50 mg/kg i.p.) did not result in labeled hilar neurons.
Figure 4.
Ectopic hilar granule cells are born after status epilepticus. A-B: Following injection of BrdU after status epilepticus, tissue sections were double-labeled for BrdU and either NeuN (A) or calbindin (B). The results showed numerous double-labeled neurons (arrows) at the border of the hilus and pyramidal cell layer. Calibration = 30 μm (A) and 60 μm (B). PCL = end of pyramidal cell layer. From ref. 87 with permission: C-D. In a different animal, double-labeling with BrdU and neuropeptide Y (C) or BrdU and parvalbumin (D) demonstrated no double-labeled cells, but numerous hilar neurons that were immunoreactive for neuropeptide Y or parvalbumin (arrows). Calibration (in A) = 60 μm (C) and 120 μm (D).
Do All the New Granule Neurons Become Functional? Do They Behave the Same?
Our experiments with the pilocarpine and kainic acid models suggested that hilar granule cells that are born after seizures survive for long periods of time and become functional. Evidence for their long term survival came from the studies of BrdU-labeled neurons in animals that were not killed until over 1 year had passed since BrdU injection. In these animals, numerous BrdU-labeled neurons were found in the dentate gyrus granule cell layer as well as the hilus, and they were double-labeled with NeuN or calbindin. Thus, neurons that divided after status appeared to survive as long as one year.
Regarding the development of neuronal function, intracellular recordings from hilar granule cells in slices demonstrated that they could mature into normal, functioning neurons with action potentials and other physiological characteristics of adult neurons. A recent study in normal adult rats has also identified that neurons which are born in the dentate gyrus in the adult animal can develop action potentials and membrane properties like normal adult granule cells.107 Therefore, in both the normal and pathological situation, granule cells that are born in the adult rat dentate gyrus develop functional characteristics of neurons, surviving at the very least to maturity.
Additional evidence from both in vitro and in vivo studies indicates that not only are these granule cells able to mature into functional neurons, but they also integrate into the host environment, so that they discharge in similar patterns as their neighbors.
In vivo studies used pilocarpine-treated rats at the stage when they displayed recurrent, spontaneous seizures. In these experiments, animals that had pilocarpine-induced status epilepticus were observed approximately 1-5 months after status, during the period of time when recurrent spontaneous seizures occur. Three hours after a spontaneous stage 5 limbic seizure (convulsion) was observed, each animal was perfused with fixative and compared to an animal that did not have a motor seizure in the last 8 hours, or a saline-treated, age-matched control, in which stage 5 seizures do not occur (and it is highly unlikely that subclinical seizure activity occurs). The immediate early gene c-fos was used as a marker of neural activity: we hypothesized that if the newly-born granule cells were active during a seizure, they would stain for c-fos, like other hippocampal neurons stain after a seizure. We thus used c-fos to mark a neuron that was active, regardless of the degree of activity, which c-fos immunoreactivity cannot describe. Indeed, in pilocarpine-treated rats with a recent seizure, there were numerous c-fos stained neurons in the hilus, and they could be double-labeled with markers of granule cells, such as calbindin and NeuN (see ref. 89; Fig. 5). There also were other cells in the hilus that were c-fos immunoreactive, and they could be demonstrated, for example, by double-labeling c-fos and parvalbumin or c-fos and NPY.89
Figure 5.
Ectopic granule cells in the hilus are active during a spontaneous seizure. Immunoreactivity for c-fos and calbindin in a pilocarpine-treated animal that had spontaneous seizures and was sacrificed 3 hours after a spontaneous seizure was observed. A-B) There are two double-labeled neurons in the hilus (arrow), enlarged in B. Calibration = 100 μm (A); 25 μm (B). C) Double-labeled hilar neurons in another animal are shown. Calibration (in A) = 50 μm. Used with permission, ref. 87.
Interestingly, at the 3-hour delay following a seizure, granule cells located in the granule cell layer were either not immunoreactive or less immunoreactive than the hilar granule cells, presumably because they become c-fos immunoreactive sooner and their immunoreactivity wanes by 3 hours.30,40,59,115 Thus, hilar granule cells seemed to adopt a pattern of activity similar to their immediate neighbors, the hilar neurons, rather than the normal granule cells in the granule cell layer. These data suggest that the development of seizure-related activity of newly-born granule cells is highly influenced by their neighbors and local environment, although the development of their intrinsic properties seems independent of their immediate surroundings. Thus, there is a dissociation in the development of the interaction with host neurons and the maturation of neuronal intrinsic properties. The data suggest that there is little that can interfere with the membrane properties of these cells, the axon distribution, and the general shape of the neuron (somata size and shape, dendritic patterns), but a great deal of plasticity is possible for other variables, such as their final location and circuit-related behavior.
Do all newly born granule cells become functional? It is certainly not known at the present time that they all become mature, functional neurons. Indeed it is most likely that some do not survive, because there are reports that more BrdU-labeled neurons are detected after seizures if the animal is treated with a caspase inhibitor.31 And even if they survive, whether all become functional or not is not clear. Some may develop to maturity, but others could have a different fate. Although there is little evidence at the present time, it is possible that some become glia, interneurons, or become dormant, perhaps retaining a capacity to differentiate at a future time.
Is Increased Neurogenesis Beneficial, or Might It Actually Increase Seizure Susceptibility?
Another line of evidence that the hilar granule cells function differently with respect to the host brain than normal granule cells came from in vitro studies. In these experiments, pilocarpine-treated rats that had status epilepticus and recurrent seizures were used to prepare hippocampal slices, and intracellular recordings were made from hilar granule cells as well as other neurons in the slice.
Although the hilar granule cells that were recorded in slices were remarkably similar to normal adult granule cells in their intrinsic properties (as described above), there was one aspect of their physiology that was quite different than the activity of a normal granule cell. Many of the hilar granule cells displayed spontaneous bursts of action potentials (Fig. 3C; see ref. 87). These bursts occurred at variable frequency from slice to slice (usually ∼1/10 sec), but within any given slice the burst frequency was consistent. Each burst was composed of a variable number of action potentials and arose on a large depolarization (Fig. 3C). The depolarization appeared to be a giant EPSP because it increased in amplitude with hyperpolarization and action potentials were triggered at its peak. We have also found subsequently that bursts are blocked by the AMPA receptor antagonists CNQX (10 μM) but are prolonged if exposed to the GABAA receptor antagonist bicuculline (25 μM).
Insight into the mechanism underlying these burst discharges was obtained when simultaneous recordings were made in the area CA3 pyramidal cell layer, either intracellularly or extracellularly (Fig. 3C). The bursts of ectopic hilar granule cells were synchronous with bursts of area CA3 neurons. Many of the recordings of extracellular CA3 bursts were small in amplitude, in all likelihood because there was some damage and loss of cells in the CA3 region due to status and chronic seizures. However, all CA3 pyramidal cells that were recorded intracellularly demonstrated epileptiform burst discharges that were synchronized with the small field bursts recorded from the CA3 cell layer, indicating it was a robust phenomenon. Interestingly, 54% of slices from animals that were examined less than 6 months after status epilepticus did not exhibit bursts in area CA3, but 90% did if slices were from animals sacrificed >6 months after status. The delay may be due to the time required for the mechanisms underlying these types of burst discharges to fully develop, perhaps because additional recurrent connections that are not already present must sprout and form functional synapses. Some of this new circuitry may require synapses between hilar granule cells and CA3 pyramidal cells. Indeed, it may be that the new synapses that are required are the new mossy fibers of the ectopic granule cells and the hilar collaterals from CA3 pyramidal neurons onto ectopic granule cells. The first would require time to form synapses on CA3 pyramidal cells, and the latter would also require time to “find” the new hilar granule cells and form functional synapses. Since CA3 axons normally innervate the hilar region and some hilar neurons are lost after seizures, the CA3 axons may be in a state that facilitates innervation of new hilar neurons.
This of course assumes that the circuitry required for burst discharges to develop are chemical synapses, but one cannot rule out the potential role of gap junctions. The main argument for a role of chemical synapses lies in the relative timing of the bursts of CA3 neurons and the ectopic hilar granule cells. The peak of the first action potential of a given CA3 burst began at least 1 millisecond before the onset of the depolarization of a simultaneously-recorded hilar granule cell, and often there was a delay far greater than 1 millisecond; if there was no delay, gap junctions would be the more likely candidate. It is possible that ephaptic interactions are a factor because the clusters of ectopic granule cells can be quite dense, and there may be decreased efficacy in clearing extracellular potassium in epileptic tissue.27 However, there seems to be quite a bit of damage in the CA3 cell layer in pilocarpine-treated rats, because it is very difficult to obtain a healthy intracellular impalement outside of CA3b, and the field potentials are quite small relative to control slices. This argues that ephaptic interactions would be less likely, at least in the CA3 cell layer.
These data address the long-standing controversy that seizures beget more seizures, a question that has clear implications for treating individuals who have just had their first seizure. Especially when the individual is a child, it is not clear that anticonvulsant drugs are needed to prevent another seizure, or desirable, given their side effects. It appears, at least in the pilocarpine model in adult rats, that the pathophysiology in the epileptic brain can indeed get “worse” as new circuits develop between excitatory neurons, in this case pyramidal cells and newly-born granule cells. Therefore, intervention to block this recurrent excitatory circuitry could be prudent. A major argument against this interpretation is that irradiation to block neurogenesis after pilocarpine-induced status epilepticus did not block seizures.72 However, this may simply reflect that there are many potential foci after pilocarpine-induced status, and that neurogenesis does not explain seizures and epilepsy completely.
Thus, the hilar-CA3 region may contribute to seizures as one of many epileptic foci. After status, the development of ectopic hilar granule cells would occur, and this would be followed, hypothetically, by the formation of circuitry between the new cells and surviving CA3 neurons. This would ultimately lead to repetitive “interictal” types of burst discharges. It could directly foster a transition between status and subsequent seizures.
Notably, this “focus” may actually include other cells as well, such as the hilar mossy cells that survive repeated seizures.88 These neurons are glutamatergic hilar neurons that discharge synchronously with pyramidal cells in slices of pilocarpine-treated rats. Although we cannot be sure at the present time what percentage of hilar mossy cells survive status and chronic seizures, and what subset participates in pyramidal cell burst discharges, it is clear that at least some do.88
The implications would be slight if in fact these cell types, area CA3 pyramidal cells, ectopic hilar granule cells, and hilar mossy cells, did not have such substantial projections to other excitatory neurons. However, the opposite is actually the case. CA3 projections include adjacent CA3 neurons, hilar neurons and CA1 pyramidal cells in the normal rat, and may include other cell types in the epileptic rat if sprouting of the CA3 axon occurs. CA3 also projects to the contralateral hippocampus. Ectopic hilar granule cells have, at least, projections to the inner molecular layer and CA3 pyramidal cells, and may also innervate hilar neurons because of the substantial collateralization of their axons in the hilus. These collaterals have numerous varicosities, indicating potential synapses. The inner molecular layer innervation may include granule cell dendrites, but it might also include processes of GABAergic neurons, analogous to the projections of sprouted mossy fibers from granule cells located in the granule cell layer. Mossy cells project to granule cells in the normal rat, both proximally and distally, ipsilateral and contralateral. They also innervate interneurons. Therefore, the initial focal discharges between relatively small populations of pyramidal cells, ectopic granule cells and mossy cells could have substantial impact on other neuronal cell types, and have potential to exit the hippocampus.
Figure 6 shows schematically how bursts within the CA3 region, ectopic granule cells and mossy cells could lead to seizure-like activity in the hippocampus. Empirical observations suggest CA3 bursts would occur first because simultaneous recordings showed that the first action potential of a spontaneous burst in a CA3 pyramidal cell always occurred before the first action potential of a simultaneously recorded ectopic hilar granule cell or mossy cell. Second, ectopic granule cells or mossy cells could excite the granule cells located in the granule cell layer because both project to the inner molecular layer. This would be particularly effective in depolarizing granule cells if the ectopic neurons and mossy cells were synchronized; asynchronous depolarizations may not reach threshold in a granule cell, because granule cells are quite hyperpolarized normally. However, synchronous release of glutamate, particularly on the proximal portion of the granule cell dendritic tree, where both the terminals of mossy cells and ectopic hilar granule cells are located, would be likely to depolarize a granule cell above its threshold. Action potentials in even a few granule cells would be likely to be amplified throughout the population because of recurrent excitatory connections within the sprouted network. Indeed, this activity may serve as a trigger for the sprouted network that normally appears to be silent. Of course, one would expect that at least some of this excitatory activity would be limited by concurrent activation of GABAergic neurons, but if interneurons are damaged or lost after seizures, as indeed appears to be the case,16,18,50 GABAergic neurons might not limit the glutamatergic activity enough to stop its progression. The balance of excitation and inhibition may shift as neuroactive peptides in the hippocampus wax and wane with the circadian rhythm.10,15,23 Indeed, there may be several times of day when GABAergic inhibition would not balance the effect of glutamatergic neurons on the sprouted granule cell network. Thus, GABAergic inhibition could perhaps keep the network in control for the majority of the time, but at certain times inhibition might decrease, leading to periodic large discharges and possibly seizures.
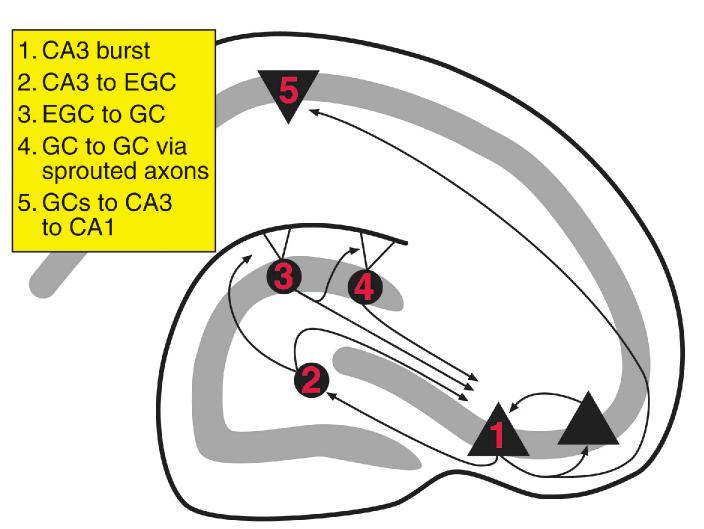
Figure 6.
Schematic illustration of the development of widespread seizure activity from initial synchronized burst discharges among CA3 pyramidal cells and ectopic hilar granule cells. 1. CA3 burst. Initially, activity that is synchronized in area CA3 neurons develops after status epilepticus. The reasons may include increased recurrent excitatory activity due to loss of interneurons, as well as sprouting among residual pyramidal cells. 2. CA3 to hilus. Based on empirical findings in pilocarpine-treated rats, synchronized burst discharges develop among pyramidal cells, newly-born hilar ectopic granule cells (EGCs) and hilar mossy cells (MCs) several months after status epilepticus. This is likely to be due to the normal projection of pyramidal cells to hilar neurons, and the mossy fiber axon that develops in EGCs. 3. hilus to GC. Because EGCs have mossy fiber collaterals that contribute to the sprouted fiber plexus in the inner molecular layer87, and MCs also project there, the bursts can potentially lead to activation of granule cells located in the granule cell layer. This would be limited by whatever interneurons are innervated by EGCs and MCs. 4. GC to GC. GC activation may be amplified because they are interconnected by sprouted fibers. However, this also would be limited by the extent that sprouted fibers activate GABAergic neurons. 5. GCs to CA3 to CA1. Strong excitatory activity that develops in the granule cell layer could potentially exit the hippocampus and trigger a limbic seizure by the trisynaptic circuit.
Thus, the focal burst discharges in CA3-hilus could generate a larger degree of reverberatory activity, and this might eventually leave the hippocampus via area CA3 neuronal projections to CA1. Based on the findings thus far, the results support the hypothesis that new granule cells may not necessarily act to ameliorate seizures, but might even contribute to them.
These circuit considerations also may have implications for the cognitive deficits following seizures. Such deficits might in part be due to new circuits that develop between new hilar granule cells and the host brain. These new circuits would be likely to disrupt the trisynaptic circuit, and thus potentially interfere with normal learning and memory (Fig. 7A).
Figure 7.
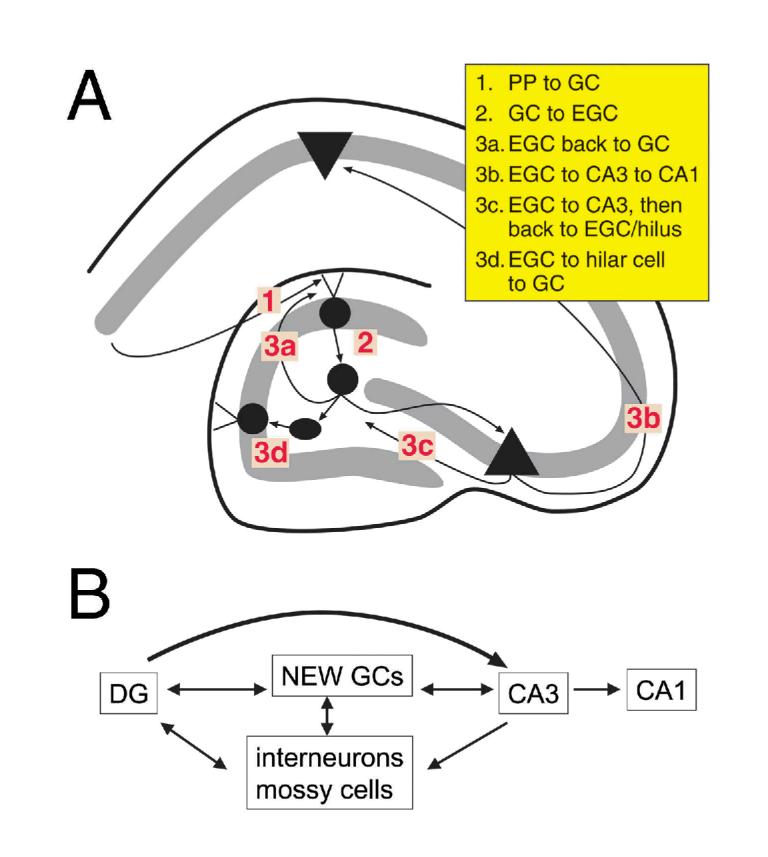
Effects of ectopic hilar granule cells on normal signal processing in the trisynaptic circuit. It is proposed that the development of ectopic hilar granule cells could contribute to cognitive deficits in epileptics by interfering with the normal trisynaptic circuit. A) The trisynaptic circuit is schematized as perforant path axons innervating distal granule cell dendrites (1), granule cell mossy fibers innervating proximal CA3 pyramidal cell dendrites (2), and CA3 pyramidal cell projections to apical dendrites of CA1 pyramidal cells (3). The accumulation of hilar ectopic granule cells with axons that innervate pyramidal cells, hilar neurons, and the inner molecular layer, suggests a multitude of complex pathways that would potentially interfere with normal transmission along the trisynaptic pathway, as shown diagrammatically in B.
In Figure 7A, the trisynaptic circuit is illustrated schematically as: 1) the synapse of the perforant path onto granule cell outer dendrites; 2) the synapse of granule cell mossy fibers onto CA3 pyramidal proximal dendrites; and 3) the synapse of CA3 pyramidal Schaffer collaterals onto CA1 pyramidal apical dendrites. The activity along the trisynaptic pathway would clearly be more circuitous by the addition of hilar granule cells, particularly given the evidence that these new neurons have perforant path input, pyramidal cell input, and their own mossy fiber axons.87 Thus, information from the perforant path that would normally pass through the trisynaptic circuit would be likely to pass through hilar granule cells. Information could become "stalled" in a circular nest of intermediary pathways within the CA3-dentate region (Fig. 7B).
How Do the New Cells Interact with the Host Brain?
This question actually can be addressed at a number of levels. One is the functional relationship of the new neurons and surrounding adult neurons or “host environment,” once the new neurons have been born, developed, and matured. This is discussed above.
A second issue is how the new cells and host interact as the new cells are developing. In other words, do the mature cells influence those that are newly-born, and vice-versa?
Influence of the Host Environment on Newly-Born Cells
Clearly the host environment normally is quite influential on new neurons. Studies in culture have shown repeatedly that addition of specific factors to the culture medium can have a striking influence on neurodevelopment. In addition, transplantation of neurons into a new location often leads to the transformation of those neurons into those with characteristics of the new environment.
How might the characteristics of the epileptic “host” dentate gyrus influence cells that have recently divided in the subgranular zone? One effect may be a spatial influence, because in the epileptic dentate gyrus there are fewer cells in the hilar region than normal,50 and this might provide an impetus to move into the hilus because there would be less competition for space there. Thus, hilar cell loss after seizures may facilitate the entry of new cells to the hilar region. In the normal adult hippocampus, the situation may be quite different, and indeed, newly-born granule cells are found in the hilus under very few conditions other than those following seizures. Furthermore, seizures without cell loss result in few new granule cells in the hilus (Scharfman et al, unpublished results).
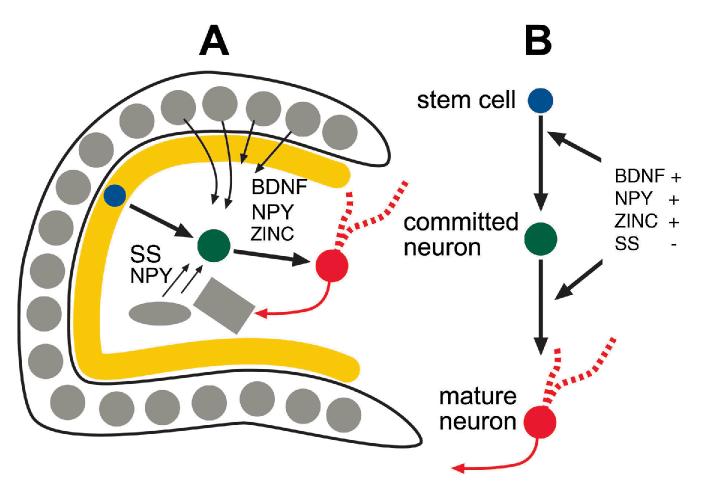
Besides spatial cues, there is a likely chemical influence of numerous peptides, growth factors, and other substances in the host dentate gyrus (Fig. 8). These may provide chemoattractive/repulsive forces as well as supportive, proliferative and developmental influence on developing neurons of the subgranular zone. Indeed, one common question of past studies about the dentate gyrus has been the reason why so many substances that specifically influence development are present in adult dentate neurons. The answer may lie in the fact that ongoing neurogenesis occurs in the adult. Thus, some of the substances in adult neurons may aid growth and development of newly-born granule cells. These substances are even more likely to influence newly-born neurons after seizures because they appear to increase their expression after seizures. Thus, although many neuroactive substances in the adult dentate gyrus modulate adult neurotransmission, they could also have another function: to modulate neurogenesis.
Figure 8.
Interaction of newly-born granule cells and the host environment. A-B) A schematic illustrates the potential interactions between developing granule cells born after seizures and the adjacent adult (host) neurons. After seizures, new cells are born in the subgranular zone, and at a similar time, there is altered expression of various proteins in the surrounding adult granule cells and adult hilar neurons. The fact that these two phenomena occur at a similar time may be no coincidence, because the proteins may enhance growth, survival, and proliferation of newly-born cells. Examples include BDNF, zinc, neuropeptide Y and somatostatin. BDNF and zinc are normally present in adult granule cells, and BDNF expression increases in granule cells after seizures. NPY and somatostatin are present in a subset of dentate gyrus GABAergic neurons. After seizures, some of these cells increase their expression of NPY, and many somatostatin-containing neurons die. BDNF, NPY, and zinc appear to have a positive influence and somatostatin a negative influence on processes relating to cell proliferation, so the increase in BDNF, NPY and decrease in somatostatin may foster phenomena associated with neurogenesis. Interestingly, these same substances also have effects on adult synaptic transmission, making their overall influence on the epileptic dentate gyrus network difficult to predict. For further discussion, see text.
The neurotrophin BDNF is one example. BDNF is normally synthesized in granule cells at much greater concentration than other neurons in the brain,25,117 and until now the reason for this has been unclear. In addition, the expression of BDNF increases after seizures.12,39 Why should granule cells possess high concentrations of BDNF? And why should this increase after seizures? It may be due to the need for BDNF in the environment of the subgranular zone, where newly-born cells arise. Indeed, BDNF is localized in granule cell axons, not their dendrites or soma, and the axons of course collateralize in the hilar region, where the subgranular zone exists. One might ask how BDNF is released so that it can affect newly-born cells, and this is not clear at the present time. Although it is likely that BDNF can be released using mechanisms common to peptide release at synapses,25 whether it can be secreted in a non-vesicular manner or transported in some other way to specifically affect newly-born cells is not presently known.
Neuropeptide Y is another example. This peptide has been shown to promote neuroproliferation outside the hippocampus,46 and it may do so in the dentate gyrus as well.51 It is localized to a subset of hilar GABAergic neurons normally, and after seizures its expression increases in those neurons.100 It also appears in granule cell axons of the hilus after seizures.66 For many years the physiological role of NPY has been unclear, and recently it has been associated with depression of synaptic transmission.109 These effects have been attributed to Y2 and Y5 receptors, mostly.109 Yet there are also Y1 receptors in hippocampus, and specifically in the subgranular zone as well as the molecular layer.109 What role might NPY play at its Y1 receptor? The answer may be related to neurogenesis, because NPY's proliferative effects appear to be mediated by this receptor.46,51 The new data make it seem likely that NPY has effects on synaptic transmission as well as a role in neuroproliferation in the dentate gyrus.
Similar arguments may be possible with other peptides in interneurons of the dentate hilus besides NPY, such as somatostatin, which appears to influence proliferation in peripheral tissues and tumor growth.57,78,83 Interestingly, somatostatin appears to influence proliferation negatively, and has been suggested as a potential therapy to treat cancer. Therefore, it may be no coincidence that, of all the peptidergic neurons in the dentate gyrus, the neurons which synthesize somatostatin are the ones that are most vulnerable to seizures.80,108 As a result, neuroproliferation after seizures may be positively modulated, i.e., by the loss of somatostatin.
Another player could very well be zinc, long known to be a native constituent of granule cells both before and after seizures. It has often been asked why granule cells require zinc, and moreover, so much more zinc than other neurons. A potential purpose is to influence proliferation. The basis for this hypothesis comes from studies of the immune system, where zinc stimulates proliferation of T cells by effects on DNA synthesis75 and the cell cycle.28 Given that zinc is proliferative in the periphery, perhaps zinc also has a role in proliferation in the dentate gyrus. Zinc could potentially influence neuroproliferation, or zinc could play a role in glial changes, since some glia arise from the same lineage as some immune cells. Thus, analogous to NPY and BDNF, zinc may not only influence dentate gyrus physiology,19,63,96,112 but also have effects on proliferation.
Finally, GABA is a potential substance that might influence newly-born granule cells of the dentate gyrus. It is normally present in hilar interneurons and, to a lesser extent, granule cells.35,49,81 After seizures, GABA increases in both the interneurons and the granule cells34 (see also Sperk et al, this volume). GABA has many actions that are trophic and influence development.8,65
Thus, many substances that are made in neurons of the dentate gyrus, substances previously associated with an influence only on neurotransmission of adult neurons, may have an important role in the development and synaptogenesis of newly-born granule cells. This role may explain the remarkable number of substances ordinarily synthesized by neurons in the dentate gyrus, and why they appear to increase expression after seizures.
An important element that is unknown presently is how the substances would be released into the extracellular milieu from the axons of granule cells and other hilar neurons so that they could influence newly-born cells; one would predict that this would be critical if they were to exert effects on immature neurons located in the hilus.
It is important to consider that some of the relevant factors may be released from astrocytes, which are known to influence neurogenesis, although the exact mechanisms are as yet unclear.99 Glia also have important roles in synaptic transmission in hippocampus.22,53,54 After seizures, these actions could become more potent as glia become increased in the hilar region. In addition, there is a transformation of many glia into reactive microglia, which may have additional functions besides those of normal glia.
Influence of New Cells on Host Neurons
One cannot rule out the potential influence of the developing neurons in the hilus or granule cell layer on the host hippocampus, even before they are matured completely. This is because immature granule cells have a few characteristics that may affect their neighbors. One is a high incidence of electrical coupling. A second is a chloride reversal potential that produces depolarizing responses to GABA rather than hyperpolarizations (see Staley, this volume). These two characteristics are interesting in light of the fact that granule cells and interneurons of the dentate gyrus increase synthesis of GAD and GABA after seizures. In the case of the granule cells, it is not altogether clear when GABA is released, but it may not be through conventional chemical neurotransmission, since the granule cells may not have the appropriate vesicular transporter.58,101 Instead, GABA could be released by reverse transport (see Richerson and Wu, this volume, see also refs. 74, 114). In any case, one would predict that release of GABA onto the immature hilar granule cells that are networked by gap junctions would lead to their synchronous depolarization. If this were to happen at a time when the synapses of the immature cells had formed on host neurons, such as pyramidal cells or granule cells, it might lead to synchronous activation and stimulate epileptiform activity. Indeed, it may be no coincidence that the maturation of the new granule cells in the hilus is similar in length to the so-called latent period, i.e., the time between status epilepticus and the first spontaneous seizure.
Is Neurogenesis Increased after Seizures in Man?
As described in part above, new data now exist that suggest that neurogenesis after seizures may play an important role in epilepsy. However, one of the areas of research that has been puzzling is that there has been minimal evidence for increased neurogenesis in human epileptics. Thus, Blumcke et al14 showed that only the tissue from pediatric cases provided some evidence consistent with laboratory animals, that neurogenesis might increase after seizures. These data are surprising in light of the strong evidence in non-epileptics that neurogenesis increases throughout life.33 One explanation could be that there is a difference between the stages of epileptogenesis. Although acute seizures or status epilepticus increase neurogenesis in a non-epileptic brain, after repetitive seizures or the chronic condition there may actually be a decreased rate of neurogenesis. Thus, if tissue is examined at a chronic stage of the disease, there may be little evidence of neurons that were recently-born. Another hypothesis is that the tissue examined thus far comes from a select population of pharmacologically refractory epileptics who may not be representative of all individuals. It is also possible that the patients examined by Blumcke et al14 were not representative even of medically-intractable cases. Indeed, a different study of calbindin-immunoreactivity in human epileptics demonstrated neurons in the hilus that had the general morphology of granule cells.98
Yet even if the phenomenon of dentate neurogenesis is not as important to human epilepsy as it appears to be in the rodent, there are other aspects of these findings that may be quite relevant to the clinical condition. Indeed the possible abnormalities that arise from misplaced neurons in the dentate gyrus underscore that in perhaps many of the idiopathic epilepsies there are small areas of abnormal circuits. Each might be too small for current imaging techniques to recognize, hence the use of the term idiopathic. Thus, a large subset of the idiopathic epilepsies are actually epileptics with small migrational disorders. Whether the abnormal neurons were born prenatally or born in the adult brain, it points out the potential importance of understanding every step in development. Indeed, the importance of understanding the molecular basis of neuronal development has become a major emphasis in epilepsy research.
Summary
In this review, the focus has been on the remarkable changes in neurogenesis and function of adult rats after status epilepticus. A number of robust alterations of structure and function occur, independent of the plethora of changes already demonstrated and discussed concerning seizure-induced gene expression and mossy fiber sprouting. Our challenge will be to identify which changes are associated with epileptogenesis and which simply exemplify the remarkable plasticity of the epileptic brain. If this can be done, it may be possible to construct new strategies that are tailored to block epileptogenesis and epilepsy.
Acknowledgements
I thank Annamaria Vezzani and Devin Binder for their comments on the manuscript and Chris Hough for discussions about zinc. This work was supported by NS 37562, 38285, and the Human Frontiers Science Program.
References
- 1.Acsady L, Kamondi A, Sik A, et al. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during perinatal and postnatal periods. J Comp Neurol. 1990;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Bayer SA. Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J Comp Neurol. 1990;301:325–342. doi: 10.1002/cne.903010302. [DOI] [PubMed] [Google Scholar]
- 4.Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182:851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- 5.Azizi SA. Exploiting nonneuronal cells to rebuild the nervous system: from bone marrow to brain. The Neuroscientist. 2000;6:353–361. [Google Scholar]
- 6.Baimbridge KG, Miller JJ. Immunohistochemical localization of calcium binding proteins in hippocampus, cerebellum and olfactory bulb of the rat. Brain Res. 1982;245:223–229. doi: 10.1016/0006-8993(82)90804-6. [DOI] [PubMed] [Google Scholar]
- 7.Baimbridge KG, Mody I, Miller JJ. Reduction of rat hippocampal calcium-binding protein following commissural, amygdala, septal, perforant path, and olfactory bulb kindling. Epilepsia. 1985;26:460–465. doi: 10.1111/j.1528-1157.1985.tb05681.x. [DOI] [PubMed] [Google Scholar]
- 8.Behar TN, Schaffner AE, Colton CA, et al. GABA-induced chemokinesis and NGF-induced chemotaxis of embryonic spinal cord neurons. J Neurosci. 1994;14:29–38. doi: 10.1523/JNEUROSCI.14-01-00029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bengzon J, Kokaia Z, Elmer E, et al. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci USA. 1997;94:10432–10437. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berchtold NC, Oliff HS, Isackson P, et al. Hippocampal BDNF mRNA shows a diurnal regulation, primarily in the exon III transcript. Brain Res Mol Brain Res. 1999;71:11–22. doi: 10.1016/s0169-328x(99)00137-0. [DOI] [PubMed] [Google Scholar]
- 11.Bernabeu R, Sharp FR. NMDA and AMPA/kainate glutamate receptors modulate dentate neurogenesis and CA3 synapsin-I in normal and ischemic hippocampus. J Cereb Blood Flow Metab. 2000;20:1669–1680. doi: 10.1097/00004647-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Binder DK, Croll SD, Gall CM, et al. BDNF and epilepsy: too much of a good thing? Trends Neurosci. 2001;24:47–53. doi: 10.1016/s0166-2236(00)01682-9. [DOI] [PubMed] [Google Scholar]
- 13.Blackstad TW, Brink K, Hem J, et al. Distribution of hippocampal mossy fibers in the rat. An experimental study with silver impregnation methods. J Comp Neurol. 1970;138:433–449. doi: 10.1002/cne.901380404. [DOI] [PubMed] [Google Scholar]
- 14.Blumcke I, Schewe J-C, Normann S, et al. Increase of nestin-immunoreactive neural precursor cells in the dentate gyrus of pediatric patients with early-onset temporal lobe epilepsy. Hippocampus. 2001;11:311–321. doi: 10.1002/hipo.1045. [DOI] [PubMed] [Google Scholar]
- 15.Bova R, Micheli MR, Qualadrucci P, et al. BDNF and trkB mRNAs oscillate in rat brain during the light-dark cycle. Brain Res Mol Brain Res. 1998;57:321–324. doi: 10.1016/s0169-328x(98)00092-8. [DOI] [PubMed] [Google Scholar]
- 16.Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- 17.Buckmaster PS, Dudek FE. In vivo intracellular analysis of granule cell axon reorganization in epileptic rats. J Neurophysiol. 1999;81:712–721. doi: 10.1152/jn.1999.81.2.712. [DOI] [PubMed] [Google Scholar]
- 18.Buckmaster PS, Jongen-Relo AL. Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainate-induced epileptic rats. J Neurosci. 1999;19:9519–9529. doi: 10.1523/JNEUROSCI.19-21-09519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science. 1996;271:369–373. doi: 10.1126/science.271.5247.369. [DOI] [PubMed] [Google Scholar]
- 20.Cameron HA, Hazel TG, McKay RDG. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- 21.Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castonguay A, Levesque S, Robitaille R. Glial cells as active partners in synaptic functions. Prog Brain Res. 2001;132:227–240. doi: 10.1016/S0079-6123(01)32079-4. [DOI] [PubMed] [Google Scholar]
- 23.Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–321. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- 24.Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- 25.Conner JM, Lauterborn JC, Yan Q, et al. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Covolan L, Ribeiro LTC, Longo BM. Cell damage and neurogenesis in the dentate granule cell layer of adult rats after pilocarpine-or kainate-induced status epilepticus. Hippocampus. 2000;10:169–180. doi: 10.1002/(SICI)1098-1063(2000)10:2<169::AID-HIPO6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 27.D'Ambrosio R, Maris DO, Grady MS, et al. Impaired K(+) homeostasis and altered electrophysiological properties of post-traumatic hippocampal glia. J Neurosci. 1999;19:8152–8162. doi: 10.1523/JNEUROSCI.19-18-08152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dardenne M. Zinc and immune function. Eur J Clin Nutr. 2002;56(Suppl 3):S20–23. doi: 10.1038/sj.ejcn.1601479. [DOI] [PubMed] [Google Scholar]
- 29.Derrick BE, York AD, Martinez JL., Jr Increased granule cell neurogenesis in the adult dentate gyrus following mossy fiber stimulation sufficient to induce long-term potentiation. Brain Res. 2000;857:300–307. doi: 10.1016/s0006-8993(99)02464-6. [DOI] [PubMed] [Google Scholar]
- 30.Dragunow M, Yamada N, Bilkey DK, et al. Induction of immediate-early gene proteins in dentate granule cells and somatostatin interneurons after hippocampal seizures. Brain Res Mol Brain Res. 1992;13:119–126. doi: 10.1016/0169-328x(92)90051-c. [DOI] [PubMed] [Google Scholar]
- 31.Ekdahl CT, Mohapel P, Elmer E, et al. Caspase inhibitors increase short-term survival of progenitor-cell progeny in the adult rat dentate gyrus following status epilepticus. Eur J Neurosci. 2001;14:937–945. doi: 10.1046/j.0953-816x.2001.01713.x. [DOI] [PubMed] [Google Scholar]
- 32.Enderlin S, Norman AW, Celio MR. Ontogeny of the calcium binding protein calbindin D-28k in the rat nervous system. Anat Embryol (Berl) 1987;177:15–28. doi: 10.1007/BF00325286. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 34.Esclapez M, Houser CR. Up-regulation of GAD65 and GAD67 in remaining hippocampal GABA neurons in a model of temporal lobe epilepsy. J Comp Neurol. 1999;412:488–505. [PubMed] [Google Scholar]
- 35.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 36.Gaarskjaer FB. Organization of the mossy fiber system of the rat studied in extended hippocampi. I. Terminal area related to number of granule and pyramidal cells. J Comp Neurol. 1978;178:49–72. doi: 10.1002/cne.901780104. [DOI] [PubMed] [Google Scholar]
- 37.Gaarskjaer FB. The hippocampal mossy fiber system of the rat studied with retrograde tracing techniques. Correlation between topographic organization and neurogenetic gradients. J Comp Neurol. 1981;203:717–735. doi: 10.1002/cne.902030409. [DOI] [PubMed] [Google Scholar]
- 38.Gaarskjaer FB, Laurberg S. Ectopic granule cells of hilus fasciae dentatae projecting to the ipsilateral regio inferior of the rat hippocampus. Brain Res. 1983;274:11–16. doi: 10.1016/0006-8993(83)90516-4. [DOI] [PubMed] [Google Scholar]
- 39.Gall CM. Seizure-induced changes in neurotrophin expression: implications for epilepsy. Exp Neurol. 1993;124:150–166. doi: 10.1006/exnr.1993.1186. [DOI] [PubMed] [Google Scholar]
- 40.Gass P, Herdegen T, Bravo R, et al. Spatiotemporal induction of immediate early genese in the rat brain after limbic seizures: effects of NMDA receptor antagonist MK-801. Eur J Neurosci. 1993;5:933–943. doi: 10.1111/j.1460-9568.1993.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 41.Goodman JH, Wasterlain CG, Massarweh WF, et al. Calbindin-D28k immunoreactivity and selective vulnerability to ischemia in the dentate gyrus of the developing rat. Brain Res. 1993;606:309–314. doi: 10.1016/0006-8993(93)90999-4. [DOI] [PubMed] [Google Scholar]
- 42.Gould E, McEwen BS, Tanapat P, et al. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 44.Gould E, Tanapat P, McEwen BS, et al. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray WP, Sundstrom LE. Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Res. 1998;790:52–59. doi: 10.1016/s0006-8993(98)00030-4. [DOI] [PubMed] [Google Scholar]
- 46.Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410:940–944. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- 47.Hess DC, Hill WD, Martin-Studdard A, et al. Bone marrow as a source of endothelial cells and NeuN-expressing cells after stroke. Stroke. 2002;33:1362–1368. doi: 10.1161/01.str.0000014925.09415.c3. [DOI] [PubMed] [Google Scholar]
- 48.Holm IE, Geneser FA, Zimmer J, et al. Immunocytochemical demonstration of the calcium-binding proteins calbindin-D 28k and parvalbumin in the subiculum, hippocampus and dentate area of the domestic pig. Prog Brain Res. 1990;83:85–97. doi: 10.1016/s0079-6123(08)61243-1. [DOI] [PubMed] [Google Scholar]
- 49.Houser CR, Esclapez M. Localization of mRNAs encoding two forms of glutamic acid decarboxylase in the rat hippocampal formation. Hippocampus. 1994;4:530–545. doi: 10.1002/hipo.450040503. [DOI] [PubMed] [Google Scholar]
- 50.Houser CR, Esclapez M. Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res. 1996;26:207–218. doi: 10.1016/s0920-1211(96)00054-x. [DOI] [PubMed] [Google Scholar]
- 51.Howell OW, Scharfman HE, Beck-Sickinger AG, et al. Neuropeptide Y is neuroproliferative for hippocampal stem cells and neuroblasts. J Neurochem. 2003;86:646–659. doi: 10.1046/j.1471-4159.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- 52.Hsu M, Buzsaki G. Vulnerability of mossy fiber targets in the rat hippocampus to forebrain ischemia. J Neurosci. 1993;13:3964–3979. doi: 10.1523/JNEUROSCI.13-09-03964.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang J, Jiang L, Goldman SA, et al. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- 54.Keyser DO, Pellmar TC. Synaptic transmission in the hippocampus: critical role for glial cells. Glia. 1994;10:237–243. doi: 10.1002/glia.440100402. [DOI] [PubMed] [Google Scholar]
- 55.Kotti T, Riekkinen PJ, Sr., Miettinen R. Characterization of target cells for aberrant mossy fiber collaterals in the dentate gyrus of epileptic rat. Exp Neurol. 1997;146:323–330. doi: 10.1006/exnr.1997.6553. [DOI] [PubMed] [Google Scholar]
- 56.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kunert-Radek J, Stepien H, Radek A, et al. Somatostatin suppression of meningioma cell proliferation in vitro. Acta Neurol Scand. 1987;75:434–436. doi: 10.1111/j.1600-0404.1987.tb05474.x. [DOI] [PubMed] [Google Scholar]
- 58.Lamas M, Gomez-Lira G, Gutierrez R. Vesicular GABA transporter mRNA expression in the dentate gyrus and in mossy fiber synaptosomes. Mol Brain Res. 2001;93:209–214. doi: 10.1016/s0169-328x(01)00202-9. [DOI] [PubMed] [Google Scholar]
- 59.LeGallaSalle G. Long-lasting and sequential increase of c-fos oncoprotein expression in kainic acid-induced status epilepticus. Neurosci Lett. 1988;88:127–130. doi: 10.1016/0304-3940(88)90112-7. [DOI] [PubMed] [Google Scholar]
- 60.Liptakova S, Jacobi H, Sperber EF. Kindling increases dentate granule neurogenesis in immature rats. Epilepsia. 1999;40:13. doi: 10.1111/j.1528-1157.2000.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 61.Liu J, Bernabeu R, Lu A, et al. Neurogenesis and gliogenesis in the postischemic brain. The Neuroscientist. 2000;6:362–370. [Google Scholar]
- 62.Liu J, Solway K, Messing RO, et al. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacDonald RL, Kapur J. Pharmacological properties of recombinant and hippocampal dentate granule cell GABAA receptors. Adv Neurol. 1999;79:979–990. [PubMed] [Google Scholar]
- 64.Madsen TM, Treschow A, Bengzon J, et al. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 65.Maric D, Liu QY, Maric I, et al. GABA expression dominates neuronal lineage progression in the embryonic rat neocortex and facilitates neurite outgrowth via GABAA autoreceptor/Cl− channels. J Neurosci. 2001;21:2343–2360. doi: 10.1523/JNEUROSCI.21-07-02343.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marksteiner J, Ortler M, Bellmann R, et al. Neuropeptide Y biosynthesis is markedly induced in mossy fibers during temporal lobe epilepsy of the rat. Neurosci Lett. 1990;112:143–148. doi: 10.1016/0304-3940(90)90193-d. [DOI] [PubMed] [Google Scholar]
- 67.Marti-Subirana A, Garcia-Verdugo JM. Morphological aspects of the ectopic granule-like cellular populations in the albino rat hippocampal formation: a Golgi study. J Anat. 1986;144:31–47. [PMC free article] [PubMed] [Google Scholar]
- 68.Mezey E, Chandross KJ, Harta G, et al. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 69.Nakagawa E, Aimi Y, Yasuhara O, et al. Enhancement of progenitor cell division in the dentate gyrus triggered by initial limbic seizures in rat models of epilepsy. Epilepsia. 2000;41:10–18. doi: 10.1111/j.1528-1157.2000.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 70.Okazaki MM, Evenson DA, Nadler JV. Hippocampal mossy fiber sprouting and synapse formation after status epilepticus in rats: visualization after retrograde transport of biocytin. J Comp Neurol. 1995;352:515–534. doi: 10.1002/cne.903520404. [DOI] [PubMed] [Google Scholar]
- 71.Parent JM, Janumpalli S, McNamara JO, et al. Increased dentate granule cell neurogenesis following amygdala kindling in the adult rat. Neurosci Lett. 1998;247:9–12. doi: 10.1016/s0304-3940(98)00269-9. [DOI] [PubMed] [Google Scholar]
- 72.Parent JM, Tada E, Fike JR, et al. Inhibition of dentate granule cell neurogenesis with brain irradiation does not prevent seizure-induced mossy fiber synaptic reorganization in the rat. J Neurosci. 1999;19:4508–4519. doi: 10.1523/JNEUROSCI.19-11-04508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parent JM, Yu TW, et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patrylo PR, Spencer DD, Williamson A. GABA uptake and heterotransport are impaired in the dentate gyrus of epileptic rats and humans with temporal lobe sclerosis. J Neurophysiol. 2001;85:1533–1542. doi: 10.1152/jn.2001.85.4.1533. [DOI] [PubMed] [Google Scholar]
- 75.Prasad AS, Beck FW, Endre L, et al. Zinc deficiency affects cell cycle and deoxythymidine kinase gene expression in HUT-78 cells. J Lab Clin Med. 1996;128:51–60. doi: 10.1016/s0022-2143(96)90113-4. [DOI] [PubMed] [Google Scholar]
- 76.Rakic P, Nowakowski RS. The time of origin of neurons in the hippocampal region of the rhesus monkey. J Comp Neurol. 1981;196:99–128. doi: 10.1002/cne.901960109. [DOI] [PubMed] [Google Scholar]
- 77.Rami A, Brehier A, Thomasset M, et al. The comparative immunocytochemical distribution of 28 kDa cholecalcin (CaBP) in the hippocampus of rat, guinea pig and hedgehog. Brain Res. 1987;422:149–153. doi: 10.1016/0006-8993(87)90549-x. [DOI] [PubMed] [Google Scholar]
- 78.Reisine T. Somatostatin. Cell Mol Neurobiol. 1995;15:597–614. doi: 10.1007/BF02071127. [DOI] [PubMed] [Google Scholar]
- 79.Ribak CE, Tran PH, et al. Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J Comp Neurol. 2000;428:240–253. doi: 10.1002/1096-9861(20001211)428:2<240::aid-cne4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 80.Riekkinen PJ, Pitkanen A. Somatostatin and epilepsy. Metabolism. 1990;39:112–115. doi: 10.1016/0026-0495(90)90225-2. [DOI] [PubMed] [Google Scholar]
- 81.Sandler R, Smith AD. Coexistence of GABA and glutamate in mossy fiber terminals of the primate hippocampus: an ultrastructural study. J Comp Neurol. 1991;303:177–192. doi: 10.1002/cne.903030202. [DOI] [PubMed] [Google Scholar]
- 82.Sankar R, Shin D, Liu H, et al. Granule cell neurogenesis after status epilepticus in the immature rat brain. Epilepsia. 2000;40(S6):134–143. doi: 10.1111/j.1528-1157.2000.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 83.Sara VR, Rutherford R, Smythe GA. The influence of maternal somatostatin administration on fetal brain cell proliferation and its relationship to serum growth hormone and brain trophin activity. Horm Metab Res. 1979;11:147–149. doi: 10.1055/s-0028-1092697. [DOI] [PubMed] [Google Scholar]
- 84.Scharfman HE. Differentiation of rat dentate neurons by morphology and electrophysiology in hippocampal slices: granule cells, spiny hilar cells and aspiny ‘fast-spiking’ cells. Epilepsy Res Suppl. 1992;7:93–109. [PMC free article] [PubMed] [Google Scholar]
- 85.Scharfman HE. Electrophysiological diversity of pyramidal-shaped neurons at the granule cell layer/hilus border of the rat dentate gyrus recorded in vitro. Hippocampus. 1995;5:287–305. doi: 10.1002/hipo.450050403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scharfman HE. The role of nonprincipal cells in dentate gyrus excitability and its relevance to animal models of epilepsy and temporal lobe epilepsy. Adv Neurol. 1999;79:805–820. [PubMed] [Google Scholar]
- 87.Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scharfman HE, Smith KL, Goodman JH, et al. Survival of dentate hilar mossy cells after pilocarpine-induced seizures and their synchronized burst discharges with area CA3 pyramidal cells. Neuroscience. 2001;104:741–759. doi: 10.1016/s0306-4522(01)00132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scharfman HE, Sollas AL, Goodman JH. Spontaneous recurrent seizures after pilocarpine-induced status epilepticus activate calbindin-immunoreactive hilar cells of the rat dentate gyrus. Neuroscience. 2002;111:71–81. doi: 10.1016/s0306-4522(01)00599-1. [DOI] [PubMed] [Google Scholar]
- 90.Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975;159:149–176. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- 91.Schwarzer C, Sperk G, Samanin R, et al. Neuropeptides-immunoreactivity and their mRNA expression in kindling: functional implications for limbic epileptogenesis. Brain Res Brain Res Rev. 1996;22:27–50. [PubMed] [Google Scholar]
- 92.Scott BW, Wang S, Burnham WM, et al. Kindling-induced neurogenesis in the dentate gyrus of the rat. Neurosci Lett. 1998;248:73–76. doi: 10.1016/s0304-3940(98)00355-3. [DOI] [PubMed] [Google Scholar]
- 93.Scott BW, Wojtowicz JM, McIntyre-Burnham W. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol. 2000;165:231–236. doi: 10.1006/exnr.2000.7458. [DOI] [PubMed] [Google Scholar]
- 94.Sharp FR, Liu J, Bernabeu R. Neurogenesis following brain ischemia. Dev Brain Res. 2002;134:23–30. doi: 10.1016/s0165-3806(01)00286-3. [DOI] [PubMed] [Google Scholar]
- 95.Shetty AK, Turner DA. Hippocampal interneurons expressing glutamic acid decarboxylase and calcium-binding proteins decrease with aging in Fischer 344 rats. J Comp Neurol. 1998;394:252–269. [PubMed] [Google Scholar]
- 96.Shumate MD, Lin DD, Gibbs JW, 3rd, et al. GABAA receptor function in epileptic human dentate granule cells: comparison to epileptic and control rat. Epilepsy Res. 1998;32:114–128. doi: 10.1016/s0920-1211(98)00045-x. [DOI] [PubMed] [Google Scholar]
- 97.Sloviter RS. Calcium-binding protein (calbindin-D28k) and parvalbumin immunocytochemistry: localization in the rat hippocampus with specific reference to the selective vulnerability of hippocampal neurons to seizure activity. J Comp Neurol. 1989;280:183–196. doi: 10.1002/cne.902800203. [DOI] [PubMed] [Google Scholar]
- 98.Sloviter RS, Sollas AL, Barbaro NM, et al. Calcium-binding protein (calbindin-D28K) and parvalbumin immunocytochemistry in the normal and epileptic human hippocampus. J Comp Neurol. 1991;308:381–396. doi: 10.1002/cne.903080306. [DOI] [PubMed] [Google Scholar]
- 99.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 100.Sperk G, Marksteiner J, Gruber B, et al. Functional changes in neuropeptide Y-and somatostatin-containing neurons induced by limbic seizures in the rat. Neuroscience. 1992;50:831–846. doi: 10.1016/0306-4522(92)90207-i. [DOI] [PubMed] [Google Scholar]
- 101.Sperk G, Schwarzer C, Hellman J. Membrane and vesicular GABA transporters after kainate-induced seizures. Soc Neurosci Abs. 2001:551–553. [Google Scholar]
- 102.Spigelman I, Yan XX, Obenaus A, et al. Dentate granule cells form novel basal dendrites in a rat model of temporal lobe epilepsy. Neuroscience. 1998;86:109–120. doi: 10.1016/s0306-4522(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 103.John JL, Rosene DL, Luebke JI. Morphology and electrophysiology of dentate granule cells in the rhesus monkey: comparison with the rat. J Comp Neurol. 1997;387:136–147. doi: 10.1002/(sici)1096-9861(19971013)387:1<136::aid-cne11>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 104.Staley KJ, Otis TS, Mody I. Membrane properties of dentate gyrus granule cells: comparison of sharp microelectrode and whole-cell recordings. J Neurophysiol. 1992;67:1346–1358. doi: 10.1152/jn.1992.67.5.1346. [DOI] [PubMed] [Google Scholar]
- 105.Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 106.Tonder N, Kragh J, Bolwig T, et al. Transient decrease in calbindin immunoreactivity of the rat fascia dentata granule cells after repeated electroconvulsive shocks. Hippocampus. 1994;4:79–83. doi: 10.1002/hipo.450040110. [DOI] [PubMed] [Google Scholar]
- 107.van Praag H, Schinder AF, Christie BR, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vezzani A, Schwarzer C, Lothman EW, et al. Functional changes in somatostatin and neuropeptide Y containing neurons in the rat hippocampus in chronic models of limbic seizures. Epilepsy Res. 1996;26:267–279. doi: 10.1016/s0920-1211(96)00059-9. [DOI] [PubMed] [Google Scholar]
- 109.Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- 110.Wenzel HJ, Robbins CA, Tsai LH, et al. Abnormal morphological and functional organization of the hippocampus in a p35 mutant model of cortical dysplasia associated with spontaneous seizures. J Neurosci. 2001;21:983–998. doi: 10.1523/JNEUROSCI.21-03-00983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wenzel HJ, Woolley CS, Robbins CA, et al. Kainic acid-induced mossy fiber sprouting and synapse formation in the dentate gyrus of rats. Hippocampus. 2000;10:244–260. doi: 10.1002/1098-1063(2000)10:3<244::AID-HIPO5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 112.Williamson A, Spencer D. Zinc reduces dentate granule cell hyperexcitability in epileptic humans. Neuroreport. 1995;6:1562–1564. doi: 10.1097/00001756-199507310-00024. [DOI] [PubMed] [Google Scholar]
- 113.Williamson A, Spencer DD, Shepherd GM. Comparison between the membrane and synaptic properties of human and rodent dentate granule cells. Brain Res. 1993;622:194–202. doi: 10.1016/0006-8993(93)90819-9. [DOI] [PubMed] [Google Scholar]
- 114.Williamson A, Telfeian AE, Spencer DD. Prolonged GABA responses in dentate granule cells in slices isolated from patients with temporal lobe sclerosis. J Neurophysiol. 1995;74:378–387. doi: 10.1152/jn.1995.74.1.378. [DOI] [PubMed] [Google Scholar]
- 115.Woldbye DPD, Greisen MH, Bolwig TG, et al. Prolonged induction of c-fos in neuropeptide Y and somatstatin-immunoreactive neurons of the rat dentate gyrus after electroconvulsive stimulation. Brain Res. 1996;720:111–119. doi: 10.1016/0006-8993(96)00158-8. [DOI] [PubMed] [Google Scholar]
- 116.Yackel JW, Puri PS. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- 117.Yan Q, Rosenfeld RD, Matheson CR, et al. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78:431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]