Abstract
In this study, 2-amino-4-chloro-6-hydroxy-s-triazine (ACHT) was synthesized through controlled hydrolysis of 2-amino-4,6-dichloro-s-triazine (ADCT). A simple paddry-cure approach was employed to immobilize ACHT onto cellulosic fibrous materials. After treatment with diluted chlorine bleach, the covalently bound ACHT moieties were transformed into chloromelamines. The structures of the samples were fully characterized with NMR, UV/VIS, DSC, TG, iodometric titration and elemental analyses. The chloromelamine-based fibrous materials provided potent, durable, and rechargeable biocidal functions against bacteria (including multi-drug resistant species), yeasts, viruses, and bacterial spores. SEM studies demonstrated that the new fibrous materials could effectively prevent the formation of biofilms, and controlled release investigations in vitro suggested that the biocidal activities were bioresponsive. Biocidal mechanisms of the chloromelamine-based fibrous materials were further discussed.
1. Introduction
Fibrous materials, such as gowns, uniforms, face masks, bedding materials, drapes, pillows, mattresses, dishcloths, aprons, etc., are widely used in healthcare-, institutional- and household-settings. Unfortunately, like other polymeric materials, fibrous materials are susceptible to contamination of various microorganisms including pathogenic bacteria, viruses, yeasts and spores, with some species surviving for longer than 90 days [1-4]. The deposited microorganisms could be liberated and re-dispersed into the air [5], or transferred to the surrounding environments through direct or indirect contact [6-10]. Therefore, in real applications, contaminated fibrous materials can be important sources of cross-infections, which have already caused serious outbreaks of nosocomial infections in healthcare facilities [11-16]. As “one ounce of prevention equals a pound of cure” in dealing with the global concern of emerging and re-emerging infectious diseases [17,18], there is a clear need to control microbial contaminations on fibrous materials in order to reduce the incidence of infections.
One of the most effective approaches in controlling microbial contamination is to introduce biocidal functions into the target materials [19]. Following the pioneering work of Gagliardi [20], which described the principles and strategies for imparting antimicrobial activities into fibrous materials, various biocidal agents, including antibiotics [21-23], metal ions [24], quaternary ammonium salts [25-27], phosphonium compounds [27,28], N-halamines [29,30], etc., have been incorporated into fibrous materials. The antimicrobial activities and mechanisms of these agents differ considerably. Among them, organic N-halamines have been demonstrated to be a class of effective, durable and reachargeable antimicrobial agents with low toxicity and little environmental concern [31-38].
An N-halamine can be defined as a compound containing one or more nitrogen-halogen covalent bonds, in which the positive halogen provides antimicrobial properties [31]. Based on their chemical structures, organic N-halamines can be classified into three types: imide N-halamines, amide N-halamines, and amine N-halamines. Due to the differences in the localized chemical environments, their stabilities follow the order of imide N-halamines < amide N-halamines ⪡ amine N-halamine, and their antimicrobial activities have a trend of imide N-halamines > amide N-halamines ⪢ amine N-halamine [31]. In other words, the stability and antimicrobial activity of N-halamines are determined by opposite factors.
One of the research interests of this group is the development of N-halamine-based polymeric materials to achieve biocidal and biofilms-controlling functions [34,39-41]. Our most recent efforts have been focused on a class of unique N-halamines, chloromelamines, which have already been safely used as water and food disinfectants [42]. Structurally, chloromelamines belong to amine N-halamines. However, because of the strong electron withdrawing effect of the triazine rings, their chemical environments are similar to those of amide N-halamines. Therefore, it is expected that the biocidal activity and stability of chloromelamines may be between “normal” amine and amide N-halamine. This can be an attractive characteristic for biocidal treatments of polymeric materials in applications that require both strong biocidal activity and good stability.
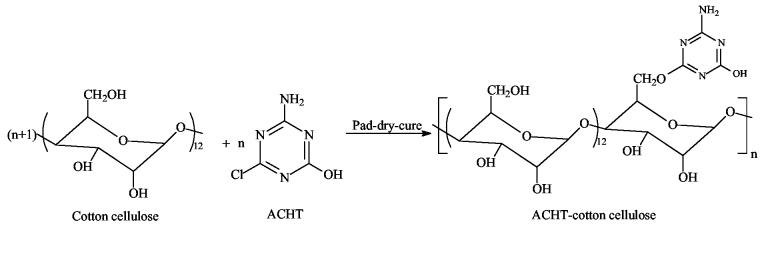
To provide detailed information about the performance of chloromelamine-based polymeric materials, in this study, 2-amino-4-chloro-6-hydroxy-s-triazine (ACHT) was synthesized through the hydrolysis of 2-amino-4,6-dichloro-s-triazine (ADCT), and then immobilized onto cotton cellulose using a pad-dry-cure approach. After chlorine bleach treatment, the immobilized ACHT moieties were transferred into chloromelamines. The resultant fibrous materials were challenged with bacteria, yeasts, viruses, and spores to determine the biocidal activities. Moreover, the biofilm-controlling functions and controlled release effects of the covalently bound chlorines were evaluated, and the biocidal mechanisms were discussed.
2. Materials and methods
2.1. Materials
Bleached cotton knit fabrics (catalog number: 489; weight: 175g/m2) were purchased from Testfabrics, Inc. (West Pittston, PA). Before immobilization reactions, the fabrics were treated with boiling acetone for 30 min to remove possible impurities. 2-amino-4,6-dichlorotriazine (ADCT) was provided by Monomer-Polymer & Dajac Labs, Inc. (Feasterville, PA), which was purified by two recrystallizations from ether and one from benzene. Other chemicals were obtained from Fisher Scientific (Fair Lawn, NJ) and used as received.
2.2. Synthesis of 2-amino-4-chloro-6-hydroxy-s-triazine (ACHT)
ACHT was synthesized through controlled hydrolysis of ADCT using a modified method reported previously [43], as shown in Scheme 1. In the current study, ADCT (16.5 g, 0.1 mol) was suspended in 250 ml of distilled water containing 4.4 g (0.11 mol) of sodium hydroxide. The mixture was stirred at room temperature for 15 h. After filtration, 7.32 g of un-reacted ADCT were removed. The clear, colorless filtrate was cooled to 0-5 °C and neutralized (pH 6.8-7.0) with glacial acetic acid. The white solid was collected by filtration, washed with cold water, dried in air, and recrystallized from hot water to yield 4.59 g of ACHT (Yield: 56.3%, based on the amount of reacted ADCT).
Scheme 1.
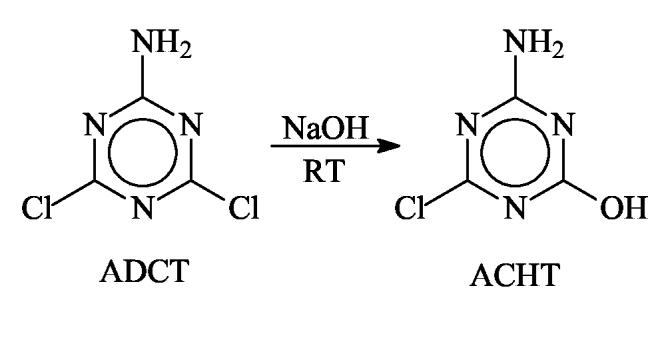
Synthesis of ACHT through controlled hydrolysis of ADCT
2.3. Immobilization of ACHT onto cotton cellulose
A known amount of ACHT was dissolved in distilled water containing 2 wt % of NaOH and 0.05 wt % of a nonionic wetting agent (TX-100) to form an aqueous solution containing 4 wt % of ACHT. A pad-dry-cure approach was used to immobilize ACHT onto cellulose. Our previous studies have demonstrated that this method could provide high reaction efficiency in a relatively short period of time [44]. In this treatment, cotton fabrics were dipped into the ACHT solution, padded through a laboratory wringer (Atlas Electric Devices Co., Chicago, IL) to 100% wet pickup (three repeats), wrapped in aluminum foil, and cured in an oven at 120 °C for 20 min. The fabrics were then washed thoroughly with a large amount of distilled water, dried at room temperature and stored in a desiccator to reach a constant weight.
2.4. Chlorination of ACHT-immobilized cotton fabrics
The ACHT-immobilized cotton fabrics were immersed in diluted chlorine bleach (Clorox Company, Oakland, CA) solutions containing 3000 ppm of active chlorine and 0.05 wt % TX-100 at room temperature for 30 min under constant shaking. The bath ratio was kept at 30:1. After chlorination, the fabrics were washed with a large amount of distilled water (the washing water was tested with KI/starch to ensure that most of the free chlorines were removed), air-dried, and stored in a dessicator to reach constant weights.
The chlorine content of the treated fabrics was determined by iodimetric titration [44]. Briefly, about 0.5 g of the chlorinated ACHT-immobilized fabrics was cut into small pieces and then added into 40 ml of absolute ethanol containing 2 g KI. The mixture was vigorously stirred at room temperature for 60 min under N2 atmosphere. The iodine released during the oxidation reaction of KI by the covalently bound chloromelamine structures was titrated with 0.01 mol/l of sodium thiosulfate aqueous solution. The same amount of unchlorinated ACHT-immobilized fabrics was also titrated using the same method as the control. The chlorine content of the sample was calculated according to the following equation:
| (1) |
where [Cl] was the chlorine content of the sample (ppm); Vs and Vc were the volumes (ml) of the sodium thiosulfate aqueous solutions consumed in the titration of the sample and control, respectively; and Ws was the weight of the chlorinated ACHT-immobilized fabrics (g).
2.5. Characterization
Elemental analysis was conducted by Quantitative Technologies Inc. (Whitehouse, NJ). Thermal properties of the samples were tested on Shimadzu DSC-60 and Shimadzu TGA-50 (Shimadzu, Kyoto, Japan) at a heating rate of 10 °C/min under N2 atmosphere. UV/VIS spectra were recorded on a Beckman DU 520 General Purpose UV/VIS Spectrophotometer (Beckman Instruments Inc., Fullerton, CA) in N,N-dimethyl acetamide (DMAc) containing 8 wt% of lithium chloride (LiCl, EMD Chemicals Inc., NJ). The sample concentration was 1 mg/ml.
Because the ACHT-immobilized cotton fabrics were insoluble in common organic solvents, making it difficult for NMR analysis, an esterification approach was developed for NMR study of the samples. In this approach, 0.5 g of ACHT-immobilized cotton sample was dispersed in 50 ml of DMAc in the presence of 8 wt% of LiCl. The mixture was kept at 150 °C for 12 h. After cooling to 90 °C, 0.5 ml of pyridine was added and the solution was stirred for 1 h. 0.7 ml of acetyl chloride (Acros Organics, NJ) was dropped into the mixture in 30 min and the reaction was continued for 24 h. The esterified ACHT-immobilized cotton was precipitated out from distilled water, centrifuged, washed with absolute ethanol for three times, collected by filtration, and then dried under reduced pressure at room temperature for 24 h. After this esterification treatment, the samples could be readily dissolved in deuterized solvents. 13C NMR analysis of the esterified ACHT-immobilized cotton cellulose was carried out on a Varian Unity-300 NMR spectrometer (Palo Alto, CA) at ambient temperature in DMSO-d6 with 0.05% (v/v) of TMS as an internal standard. A solvent mixture containing 70% DMSO-d6, 15% D2O, and 15% sodium deuteroxide (NaOD) was used as the solvent in the NMR analysis of pure ACHT because of its low solubility in DMSO-d6.
2.6. Biocidal function assessment
The microorganisms tested in this study and the media used for their growth and incubation were listed in Table 1. All the microbial studies followed the guidelines provided by the U. S. Department of Health and Human Services to ensure lab safety [45]. The bacteria, multi-drug resistant bacteria, yeast and virus were purchased from American Type Culture Collection (ATCC, Manassas, VA). Among them, Escherichia coli (E. coli) 15597 and E. coli 29214 were used as typical examples of gram-negative bacteria, and Staphylococcus aureus (S. aureus) 6538 and S. aureus 14154 were examples of gram-positive bacteria. E. coli 29214 was resistant to sulfonamide, and S. aureus 14154 was resistant to tetracycline, penicillin, streptomycin, and erythromycin. Candida tropicalis (C. tropicalis) 62690 was employed to challenge the antifungal activities of the samples; and E. coli bacteriophage MS2 15597-B1 virus was used to represent viral species. The spore suspension (North American Science Associates, Northwood, OH) was a pure suspension of viable bacterial spores in distilled water within five passages from Bacillus atrophaeus (formerly Bacillus subtilis var. niger).
Table 1.
Microorganisms tested in this study and the media used for their growth and incubation
| Species | Bacteria | Multi-drug resistant bacteria | Yeast | Virus | Spore | ||
|---|---|---|---|---|---|---|---|
| E. coli 15597a) | S. aureus 6538b) | E. coli 29214a) | S. aureus 14154b) | C.tropicalis 62690 | MS2 15597-B1 | Bacillus atrophaeus spore | |
| Broth | LB brothc) | Tryptic soy brothd) | Nutrient brothd) | YM brothd) | EC Medium brothd) | N/A | |
| Agar | LB agarc) | Tryptic soy agard) | Nutrient agard) | YPD agard) | LB agarc) | Nutrient agard) | |
Gram-negative bacteria;
Gram-positive bacteria;
Purchased from Fisher Scientific (Fair Lawn, NJ);
Purchased from Difco Laboratories (Detroit, MI).
To prepare the bacteria or yeast suspensions, E. coli 15597, E. coli 29214, S. aureus 6538 and S. aureus 14154 were grown in the corresponding broth solutions (see Table 1) at 37 °C for 24 h, and C.tropicalis was grown in YM broth at 26 °C for 36 h. Cells were harvested by centrifuge, washed twice with sterile phosphate buffered saline (PBS, OmniPur®, NaCl, 8.0 g/l; KCl, 0.20 g/l; Na2HPO4, 1.42 g/l; KH2PO4, 1.36 g/l; pH 7.4), and then re-suspended in sterilized PBS to 107 CFU/ml. In the preparation of the viral suspensions, the freeze-dried bacteriophage MS2 virus was dispersed into Difco™ EC Medium broth containing 106-107 CFU/ml of 24h-old E. coli 15597 as hosts. The viral suspension was diluted with EC Medium broth to 107 plaque forming units (PFU)/ml. In the case of Bacillus atrophaeus spores, the original spore suspensions were diluted with sterile distilled water to known spore densities.
2.6.1. Waterborne biocidal effects
A modified AATCC (American Association of Textile Chemists and Colorists) Test Method 100-1999 was used to evaluate the biocidal efficacies of the chlorinated ACHT-immobilized fibrous materials. In this study, 1 ml of microorganism suspension was placed onto the surface of a stack of four fabric swatches (3 cm×3 cm, around 1 g) to simulate possible microbial challenges in real applications when microorganisms were suspended in water. After different periods of contact time, the samples were transferred into 100 ml of sterilized sodium thiosulfate (Na2S2O3) aqueous solution (0.03 wt%). The mixtures were vigorously shaken for 1 min and sonicated for 5 min to neutralize the active chlorines and detach adherent cells from the fabric surfaces. The resultant solutions were serially diluted, and 100 μl of each diluent were placed onto the corresponding agar plates (see Table 1). In the testing of MS2 virus, the diluent was placed onto LB agar plate overlaid with LB soft agar containing 24h-old E. coli 15597 as host, as suggested by ATCC. Pristine cotton and the unchlorinated ACHT-immobilized cotton fabrics were tested following the same procedure as controls. The viable microbial colonies or lysis on the corresponding agar plates were visually counted after incubation at 37 °C for 24 h (for the bacterial, viral and spore species) or at 26 °C for 36 h (in the testing of C. tropicalis 62690).
2.6.2. Airborne biocidal effect
The airborne tests were performed according to a method reported by Tiller and colleagues [46,47] to determine the biocidal activities of the treated fabrics against microorganisms that were in air, or, from the coughing/sneezing of infected humans/animals. In this study, E. coli 15597 (gram-negative) and S. aureus 6538 (gram-positive) species were grown and harvested as described above. 100 μl of the bacterial suspension (107 CFU/ml) were sprayed onto the surface of a chlorinated ACHT-immobilized cotton swatch (3 cm ×3 cm, around 0.25 g) using a commercial chromatography sprayer (VWR Scientific) (spray rate: 10 ml/min) in a biosafety cabinet. After different periods of contact time, the fabrics were transferred into 10 ml of Na2S2O3 aqueous solution (0.03 wt%). The mixtures were shaken and sonicated, the resultant solutions were serially diluted, and 100 μl of each diluent were placed onto LB agar plates (for E. coli) or Tryptic soy agar plates (for S. aureus), as described above. Pristine cotton and unchlorinated ACHT-immobilized cotton fabrics were tested following the same procedure as controls. The viable bacterial colonies on the agar plates were visually counted after incubation at 37 °C for 24 h.
2.7. Biofilm-controlling function
Scanning Electron Microscope (SEM) was used to evaluate the biofilm-controlling function of the chlorinated ACHT-immobilized cotton fabrics. In this study, 100 μl of 107 CFU/ml of E. coli (ATCC 15597) LB broth suspension were placed onto the surface of a fabric swatch (1.0 cm × 1.0 cm) in a sterile Petri dish. The sample was allowed to stand for 1 h at 37 °C, and 5 ml of sterile distilled water were added into the Petri dish to ensure that the fabric was fully submerged. The Petri dish was then sealed and incubated at 37 °C for 24 h. At the end of the incubation, the fabrics were gently washed with PBS and immersed in 2.5 vol% of glutaraldehyde PBS solution at 4 °C for 24 h. The glutaraldehyde solution was then removed and the fabrics were washed with PBS, followed by step dehydration with 25%, 50%, 70%, 95% and 100% of ethanol (10 min at each concentration) [48]. The fabrics were then dried, sputter-coated with a thin layer of gold, and examined with a LEO 1530 SEM. The residual distilled water in the Petri dish was serially diluted, and 100 μl of each diluent were placed onto LB agar plates and incubated at 37 °C for 24 h to determine the density of recoverable bacteria. Unchlorinated ACHT-immobilized cotton fabrics were tested following the same procedure as controls.
2.8. Controlled release characteristic of the chlorinated ACHT-immobilized cotton fabrics
The controlled release of biocidal agents (chlorine) from the chlorinated ACHT-immobilized cotton fabrics was examined in terms of zone of inhibition; E. coli 15597 was used as the test organism. In this study, the surfaces of a series of LB agar plates were overlaid individually with 1 ml of LB broth containing 107 CFU/ml of E. coli. The plates were then allowed to stand at 37 °C for 4 h. Two pieces of chlorinated ACHT-cotton fabrics (each was 1.5 × 1.5 cm), one was relatively “dry” (conditioned at 23 ± 2 °C and 70 ± 5% RH for 24 h) and the other one was wetted with 100 μl of sterile distilled water, were placed onto the surface of the bacteria-containing agar. The fabrics were gently pressed to ensure full contact between the fabrics and the agar. The same procedure was also applied to the unchlorinated ACHT-immobilized cotton fabrics as controls. After incubation at 37 °C for 24 h, the zone of inhibition around the fabrics (if any) was measured.
After zone of inhibition measurement, the corresponding fabrics were individually transferred into 10 ml of sterile Na2S2O3 aqueous solution (0.03%) to quench the active chlorine. The mixtures were vigorously shaken, sonicated for 5 min, and the resultant solutions were serially diluted, incubated, and recoverable bacteria from the fabrics were determined, as described above.
A quantitative evaluation of the controlled release of chlorine from the fabrics was carried out in vitro. In each test, 4 pieces of fabrics (3×3 cm, around 1 g) were submerged in 20 ml of freshly distilled water in a closed container. After different periods of releasing time, 10 ml of the water were transferred into a 25-ml Erlenmeyer flask, which contained about 0.5 g of potassium iodide. After stirring under nitrogen atmosphere for 30 min, the solution was titrated with 0.001 mol/l Na2S2O3 aqueous solution, and the chlorine content of the distilled water was calculated according to Equation 2:
| (2) |
where [C]cl was the concentration of the active chlorine in the solution (μg/ml), and ΔV was the volume of Na2S2O3 aqueous solution consumed in the titration (ml).
3. Results and discussion
3.1. Immobilization of ACHT onto cotton cellulose
In the current study, a pad-dry-cure approach was used to immobilize ACHT moieties onto cotton cellulose. Because the ACHT-immobilized cotton fabrics were insoluble in common organic solvent that could be used for NMR analysis, a post-esterification treatment was employed to transfer part of the residual hydroxyl groups into acetates. After esterification, the resultant samples could be readily dissolved in DMSO-d6 for NMR analysis. The 13C NMR spectrum of the esterified ACHT-immobilized cotton cellulose was given in Fig. 1. The resonance peaks of the carbon atoms in the anhydroglucose units were in the ranged of 70 ppm to 105 ppm, and they were assigned as: C1 at 102 ppm, C2 at 72 ppm, C3 at 73 ppm, C4 at 80 ppm, and C5 at 75 ppm, respectively, in good agreement with the literature data [49,50]. Two intensive peaks at 20 ppm and 170 ppm could be detected, and they were caused by the -CH3 and -C=O of the acetyl groups, respectively. Both of them were single peak, implying that only one type of hydroxyl group (hydroxyl groups at C2, C3, or C6) of the anhydroglucose units was esterified. On the other hand, two signals, one at 60 ppm and the other one at 63 ppm, could be observed. These two peaks were related to the C6 atom: the former could be caused by C6 atoms that connected with unsubstituted hydroxyl groups (CH2-OH), and the latter could be related to C6 atoms that were bound to acetyl groups (CH2-OCOCH3) [49]. These findings suggested that the esterification reactions mainly took place at the primary hydroxyl groups at C6. This was reasonable because the hydroxyl group at C6 was much more reactive than those at C2 and C3 due to steric effects [51-53].
Fig. 1.
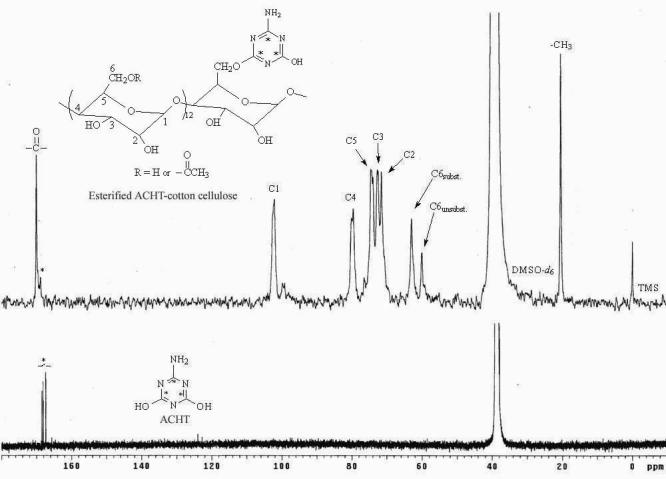
13C NMR spectra of esterified ACHT-immobilized cotton cellulose and pure ACHT (Solvents used were DMSO-d6 for the former and 70% DMSO-d6 + 15% D2O + 15% NaOD for the latter.)
Adjacent to the -C=O peak at 170 ppm, a weak resonance at 169 ppm designated as C* could be detected in the 13C NMR spectrum of the esterified ACHT-immobilized cellulose. Comparing with the 13C NMR spectrum of pure ACHT (Fig. 1), in which the carbon atoms of the triazine rings showed similar signals around 169 ppm, the resonance at 169 ppm in the spectrum of esterified ACHT-immobilized cotton cellulose could be attributed to the carbon atoms of the immobilized ACHT moieties, suggesting that ACHT molecules were incorporated into cellulose structures.
The ACHT content of the immobilized cotton fabrics was calculated to be 5.21% based on elemental analysis data (N%), indicating that on average, approximately every 13 anhydroglucose units (C6H10O5) of cotton celluloses were bound with one ACHT molecule. As mentioned earlier, although each anhydroglucose unit contains three hydroxyl groups, the ACHT molecules might mainly bind to the primary hydroxyl groups at C6 because of the difference in reactivity of the hydroxyl groups [53]. Based on these results, the preparation and probable structure of ACHT-immobilized cotton cellulose could be illustrated in Scheme 2.
Scheme 2.
Preparation and probable structure of ACHT-immobilized cotton cellulose
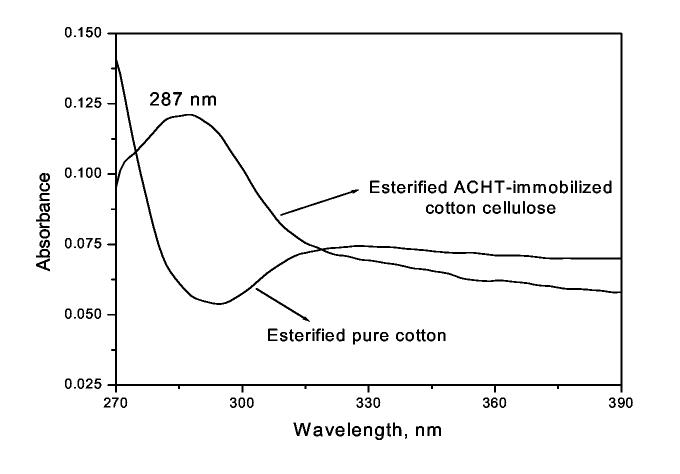
The immobilization of ACTH onto cotton cellulose was further confirmed by UV/VIS study, and the absorption curves were presented in Fig. 2. While pristine cotton cellulose did not show any UV adsorption in the range of 270-390 nm, a broad and intensive peak around 287 nm could be observed in the spectrum of ACHT-immobilized cotton cellulose, and this must be caused by the conjugated triazine ring structure of the immobilized ACHT moieties [43].
Fig. 2.
UV-VIS spectra of pristine cotton and ACHT-immobilized cotton (Sample concentration was 1 mg/ml in DMAc containing 8 wt% of LiCl)
3.2. Chlorination of ACHT-immobilized cotton fabrics
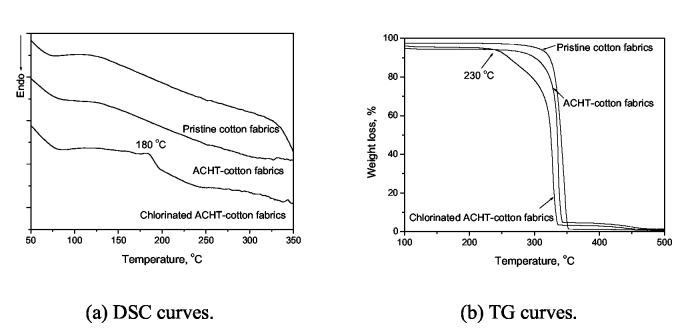
To transform the immobilized ACHT moieties into chloromelamines to provide biocidal functions, the fabrics were treated with diluted bleach containing 3000 ppm of active chlorine for 30 min at room temperature. Iodimetric titration showed that the chlorinated fabrics contained 7007 ppm of active chlorine. The “ACHT → chloromelamine” transformation was confirmed by DSC and TG analyses, as shown in Fig. 3. Fig. 3(a) displayed the DSC curves of pristine cotton, ACHT-immobilized cotton, and chlorinated ACHT-immobilized cotton fabrics. The pristine cotton and ACHT-immobilized cotton showed very similar DSC curves. In the DSC curve of chlorinated ACHT-immobilized cotton, however, a new exothermic peak around 180 °C could be clearly observed. This was a characteristic peak of organic N-halamines, which was caused by the thermal decomposition of the N-Cl bond [34,40]. In addition, in the TG studies (Fig. 3(b)), although pristine cotton and unchlorinated ACHT-immobilized cotton did not show any significant weight loss in 100-250 °C, the chlorinated ACHT-immobilized cotton had a dramatic weight decrease starting form 230 °C. This quick weight loss must be caused by the presence of covalently bound chlorines in the chlorinated ACHT-immobilized cotton: due to the relatively low chlorine content (7007 ppm by titration), the weight loss caused by the decomposition of the N-Cl bond at 180 °C was difficult to determine. However, this decomposition most likely had accelerating effects on the thermal decomposition of the cellulosic polymer backbones, leading to quick weight loss of the sample at higher than 230 °C.
Fig. 3.
Thermal properties of pristine cotton, ACHT-immobilized cotton and chlorinated ACHT-immobilized cotton fabrics
3.3. Biocidal effects
3.3.1. Waterborne biocidal effects
The biocidal efficacies of the chlorinated ACHT-immobilized cotton fibrous materials were evaluated against bacteria, multi-drug resistant bacteria, yeasts and viruses in the waterborne studies. The minimum contact time for a total kill of the test microorganisms was listed in Table 2. Although neither the pristine cotton nor the unchlorinated ACHT-immobilized cotton fabrics showed any biocidal effects, the chlorinated ACHT-immobilized cotton fabrics (chlorine content: 7007 ppm) were highly effective against the bacteria and yeast species: a total kill of 107 CFU/ml of multi-drug resistant bacteria (E. coli 29214 and S. aureus 14154) and a 100% of reduction of common bacteria (E. coli 15597 and S. aureus 6538) as well as yeast (C. tropicalis 62690) were achieved in 0.5 min - 1 min. The virus (E. coli bacteriophage MS2), which has been widely used as surrogate of enteric viral pathogens [54], was relatively difficult to inactivate, and it took 30 min to achieve a total kill. Similar results on the resistance of MS2 virus to different biocidal agents have also been reported by other researchers [55-57].
Table 2.
Minimum contact time of chlorinated ACHT-immobilized cotton for a total kill of bacteria, yeast, and virus.*
| Microorganisms | Minimum contact time for a total kill (min) | |
|---|---|---|
| Common bacteria | E. coli 15597 | 1.0 |
| S. aureus 6538 | 1.0 | |
| Multi-drug resistant bacteria | E. coli 29214 | 0.5 |
| S. aureus 14154 | 0.5 | |
| Yeast | C. tropicalis 62690 | 1.0 |
| Virus | E. coli bacteriophage MS2 15597-B1 | 30 |
The microbial concentration was 107 CFU (or PFU for MS2 virus)/ml; the biocidal tests followed AATCC Test Method 100-1999.
In addition to antibacterial, antifungal and antiviral functions, the anti-spore activities of the chlorinated ACHT-immobilized fibrous materials were of significant research interests. It has been well established that bacterial spores are highly resistant to disinfection [58-60]. A number of chemical disinfectants (phenolics, quaternary ammonium compounds, alcohols, etc.) are effective antibacterial agents, but they have little or no sporicidal activity. Other disinfectants, such as glutaraldehyde, chlorine, and iodine, can inactivate both bacteria and spores, but the sporcidal effect often requires much higher disinfectant concentrations and much longer contact time. The resistance of bacterial spores to chemical agents has been attributed to the spore coat and cortex, which act as effective barriers that prevent the access of disinfectants to the underlying spore protoplast [58]. Although the sporicidal efficacies and inactivation mechanisms of monomeric disinfectants have been reported [58-60], investigations concerning the sporicidal activities of polymeric biocides, particularly fibrous biocidal materials, are still lacking.
Since sporicidal efficacies are regarded as a direct indication of the biocidal power of biocidal polymers, in the present study, the chlorinated ACHT-immobilized cotton fabrics were challenged with Bacillus atrophaeus (ATCC 9372) spores, which have been used as biological indicators in sterilizations as well as surrogates of anthrax spores because of their high resistance. Results of the sporcidial studies were summarized in Table 3. The chlorinated ACHT-immobilized cotton fabrics were highly effective against bacterial spores, and the samples inactivated 99% of the spores within 5 min for all spore solutions with different spore densities (103-105 cells/ml). A total kill was achieved at longer periods of contact time, and these were concentration-dependant: 120 min for a total kill when challenged with 2×103 cells/ml of spores, 240 min for 2×104 cells/ml of spores, and 360 min for a 100% reduction of 7×105 cells/ml of spores. These sporicidal activities are similar to or even more potent than those of many monomeric disinfectants [58-60].
Table 3.
Sporicidal efficacies of chlorinated ACHT-immobilized cotton fabrics against B. atrophaeus spores.*
| Contact time | Anti-spore activity at different original spore concentration (% reduction compared with the control) | ||
|---|---|---|---|
| 2×103 cells/ml | 2×104 cells/ml | 7×105 cells/ml | |
| 5 min | 99% | 99% | 99% |
| 15 min | 99% | 99.50% | 99.43% |
| 30 min | 99.35% | 99.75% | 99.86% |
| 120 min | 100% | 99.95% | 99.99% |
| 240 min | 100% | 100% | 99.99% |
| 360 min | 100% | 100% | 100% |
The sporicidal tests followed AATCC Test Method 100-1999.
3.3.2. Airborne bactericidal effects
The airborne bactericidal efficacies of the chlorinated ACHT-immobilized cotton fabrics were challenged with E. coli (15597) and S. aureus (6538). To simulate the deposition of airborne bacteria, a common route of spreading infectious agents generated, for example, by talking, sneezing, coughing, or just breathing, a commercial chromatography sprayer was used [46, 47] to spray the test organisms onto the chlorinated ACHT-immobilized cotton fabrics. Fig. 4 showed the level of recoverable bacteria from the fabric samples after different periods of contact time. Similar to the waterborne test, the controls (pristine cotton and unchlorinated ACHT-immobilized cotton fabrics) did not show any bactericidal effect (data not shown), but the chlorinated ACHT-immobilized cotton fabrics provided powerful bactericidal activities: the viable bacterial colonies showed 6-log reduction for E. coli and 5-log reduction for S. aureus in less than 30 seconds. After that, a total kill was achieved within 2 minutes for E. coli and 3 minutes for S. aureus.
Fig. 4.
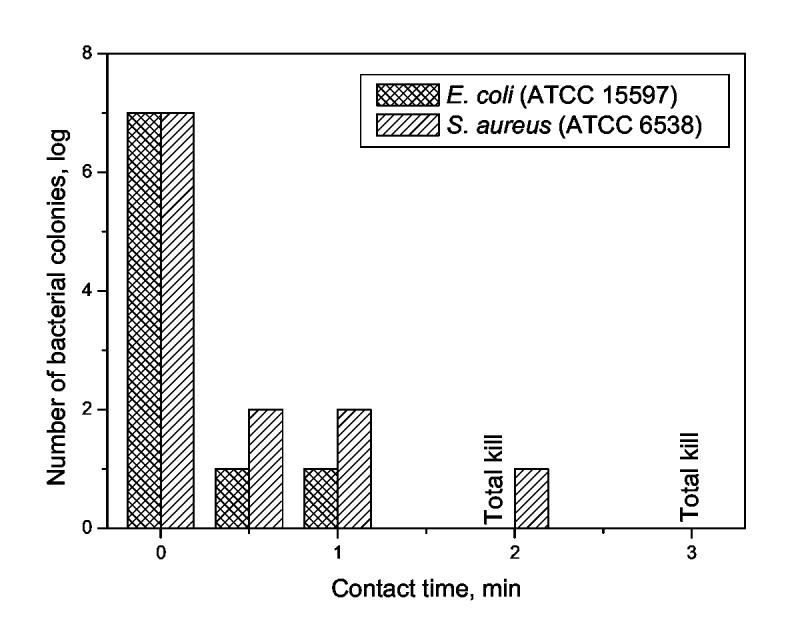
Bactericidal effects of chlorinated ACHT-immobilized cotton fabrics against E. coli 15597 and S. aureus 6538 in airborne bactericidal tests
3.4. Biofilm-controlling functions
It has been well recognized that bacteria can adhere to solid surfaces and form firmly-fixed slimy, slippery biofilms. Once biofilms are formed, bacteria embedded inside are hundreds to thousands times more resistant to biocides and the host defense system than their nonattached individual planktonic counterparts [61-67]. Moreover, bacterial populations living in biofilms can remain quiet for a long period of time, but they will continuously produce planktonic cells that contaminate the ambient microenvironment when the surrounding conditions allow them overgrow. Therefore, the formation and development of bacterial biofilms can cause serious environmental and industrial problems [68,69] as well as infectious diseases. According to the National Institutes of Health, more than 80% of all microbial infections in the body are caused by biofilms [65,70].
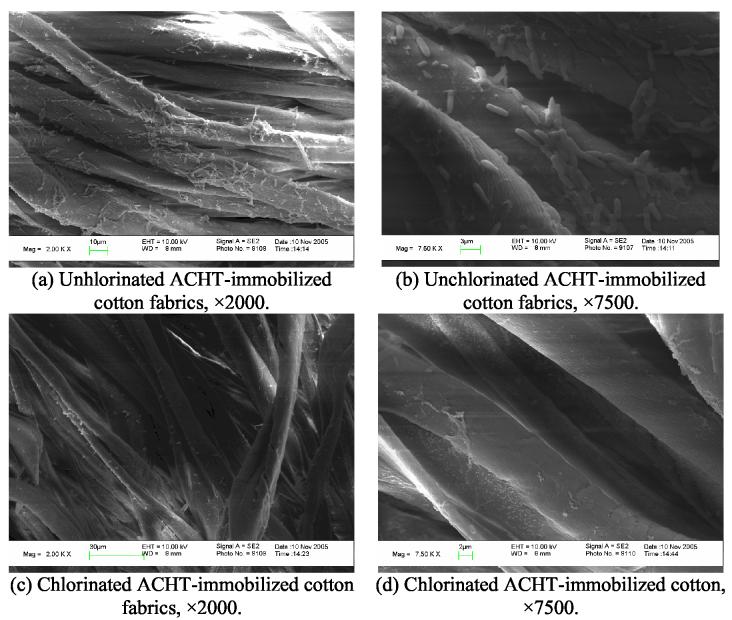
Because the chlorinated ACHT-immobilized fibrous materials were able to effectively inactivate microbes, it was highly possible that they could prevent the formation and development of biofilms. To evaluate this effect, 100 μl of 107 CFU/mL of E. coli 15597 suspensions were placed onto the fabrics samples. The samples were submerged in 5 ml of sterile distilled water in sealed Petri dishes, which were incubated at 37 °C for 24 h. After washing, fixation, and drying, the surfaces of the fabrics were observed with SEM. The results were presented in Fig. 5.
Fig. 5.
SEM micrographs of unchlorinated and chlorinated ACHT-immobilized cotton fabrics in biofilms-controlling studies (Bacteria species: E. coli, ATCC 15597).
As shown in Fig. 5(a), numerous E. coli bacteria adhered to the surface of the control (unchlorinated ACHT-immobilized cotton fabrics). Some bacteria were aligned one by one, but most of the cells aggregated together, forming microcolonies. Detailed morphologies of the bacteria and microcolonies were examined at higher magnification, as given in Fig. 5(b). It could be seen that single E. coli cell was 2 to 3 μm in length and about 0.5 μm in diameter. These cells appeared to be actively growing since some cells were in the process of cell division. Some of them seemed to be ready to separate to form the “one by one structure” and others appeared to just begin to form their “cross wall”. Cells in the bacterial microcolonies bound to each other with fiber-like slimes (exopolysaccharide glycocalyx polymers), which could facilitate surface attachment, microcolony formation, and protect the organisms within the microcolonies [63-67]. These results suggested that during this test, the bacteria in the culture medium first attached to the fiber surfaces because bacteria prefer to grow on solid surfaces rather than in the surrounding aqueous phase [71,72]. Over time, individual cells attached to the surface multiplied and formed microcolonies that ultimately coalesced to form a continuous bacterial biofilms protected by the glycocalyx polymers. These findings indicated that the unchlorinated ACHT-immobilized cotton fabrics (and pure cotton fabrics, too; figures not shown) were good templates for the proliferation of microorganisms, and biofilm would occur readily on such materials.
Evidently different from the control samples, the chlorinated ACHT-immobilized cotton fabrics showed a much clearer surface under SEM observation (Fig. 5(c)). Although bacterial residues could still be detected on the sample surface at higher magnification, as shown in Fig. 5(d), the size of the bacterial cell was much smaller than those observed in Fig. 5(b), and the cells in Fig. 5(d) seemed to be dead because the morphology and structure did not show any characteristics of active growing. These observations strongly suggested that the chloromelamine-based fibrous materials could effectively prevent bacterial adhesion and the subsequent biofilm formation.
The biofilms-controlling functions of the chloromelamine-based fibrous materials were further confirmed by examining the viable cells in the aqueous suspensions in which the fabrics were immersed during the incubation period. The initial concentration of E. coli suspension was 106-7 CFU/ml. After 24 h of incubation, 108-9 CFU/ml of viable cells were recovered from the control suspension, while no cells could be retrieved form the sample suspension, indicating that the biofilm-controlling function of the chloromelamine-based fibrous materials was provided by their potent biocidal effects, i.e., the bacterial cells were totally killed before or during the biofilm formation process.
3.5. Controlled release characteristics
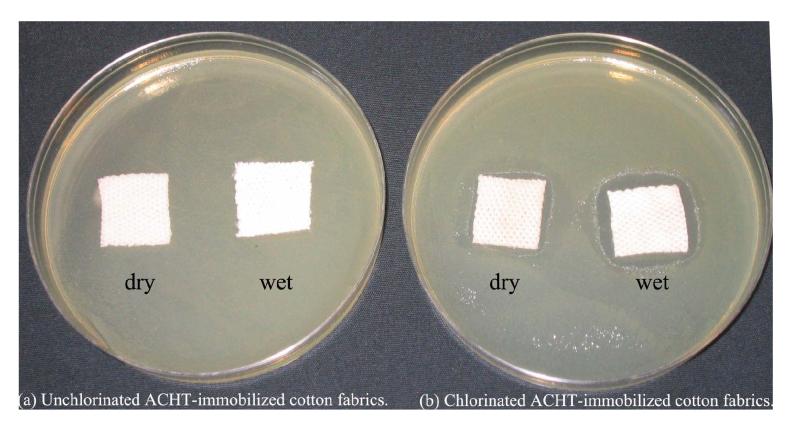
To provide further information about the biocidal performance of the chlorinated ACHT-immobilized cotton fabrics, zone of inhibition of the samples was measured after incubation at 37 °C for 24 h, using unchlorinated ACHT-immobilized cotton fabrics as controls. As shown in Fig. 6, neither “dry” nor “wet” controls could provide any inhibition zone. However, clear zones of inhibition around the chlorinated ACHT-immobilized samples were observed: when the sample was “dry” (conditioned at 23 ± 2 °C and 70 ± 5% RH for 24 h), 1.2 mm of inhibition zone was formed. If the sample was first wetted with 100 μl of sterile distilled water and then tested, the inhibition zone increased to 3 mm. Further, immediately after zone size measurement, the fabric samples were sonicated in 10 ml of sterile Na2S2O3 (0.03wt%). The resultant solution was diluted and incubated to determine levels of recoverable microbial cells from the fiber surfaces. In this test, 107 CFU/ml of viable E. coli cells were recovered from the unchlorinated ACHT-immobilized cotton (moisture content did not affect the bacterial level). On the chlorinated ACHT-immobilized cotton, when the sample was “dry”, the recovery rate was 102 CFU/ml (5-log reduction compared with the controls); when the sample was “wet”, no viable cells could be recovered from the fibrous materials.
Fig. 6.
Zone of inhibition of unchlorinated and chlorinated ACHT-immobilized cotton fabrics. Before this test, the “dry” samples were conditioned at 23 ± 2 °C and 70 ± 5% RH for 24 h, and the “wet” samples were wetted with 100 μl of sterile distilled water.
These findings suggested that during the tests, at least some of the biocidal agents diffused away from the fibrous materials to kill the bacteria. According to the previous studies on the antimicrobial properties of N-halamines [31,73,74], it was believed that the positive chlorines generated from the dissociation of N-chloramine in aqueous solution were the effective biocidal agents that diffused away, as shown in Scheme 3.
Scheme 3.
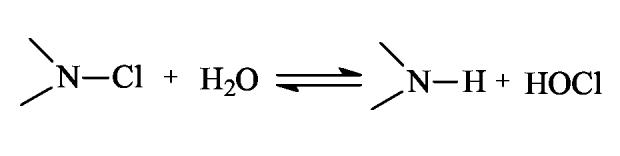
Dissociation of N-chloramine in aqueous solution.
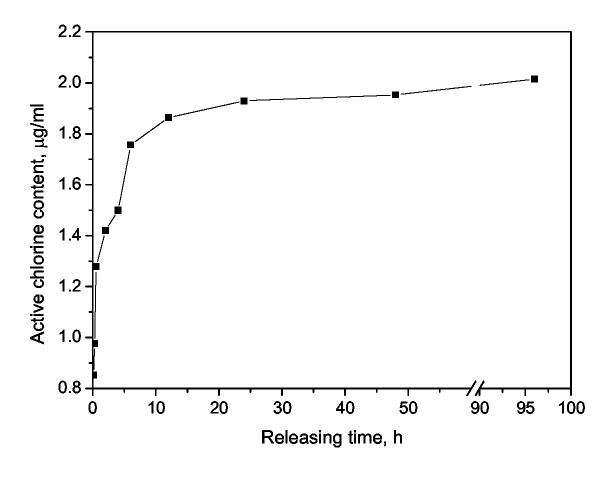
Generally speaking, the release rate of a specific antimicrobial agent from polymers has two opposite effects: for biocidal action, a fast release is preferred because it will lead to powerful and instant efficacies. On the other hand, however, the environmental impacts posed by releasing antimicrobial agents and the short-term effectiveness due to the exhaustion of these agents are also very important factors that should be taken into account in real applications. To test whether and to what extent the biocidal agent (positive chlorine) could leach from the chloramelamine-based fabrics, a quantitative evaluation of the release of the positive chlorines from the samples was conducted in vitro (see Section 2.8 for experimental details). Fig. 7 presented the positive chlorine content in the solution as a function of releasing time. It was found that in the initial stage (5 min to 6 h), the positive chlorine content increased dramatically; after that, when the equilibrium of the dissociation of the N-Cl bond was achieved (see Scheme 3), the chlorine content in the solution was kept constant at about 2.0 μg/ml (2 ppm). In other words, although the chlorinated ACHT-immobilized cotton fabrics contained 7007 ppm of active chlorine, only 2.0 μg/ml of positive chlorine was released from the fabric to the solution. These results suggested that the -N-Cl bonds in the chloromelamines were quite stable. That is also the reason that the chloromelamine-based fibrous materials have almost no detectable chlorine odor when exposed to the ambient conditions.
Fig. 7.
Positive chlorine content in the releasing solution (The initial chlorine content of the chlorinated ACHT-immobilized cotton fabrics was 7007 ppm.)
3.6. Biocidal mechanisms
N-halamines, including amine N-halamines, amide N-halamines and imide N-halamines, can be regarded as chlorine-releasing agents (CRAs), which exert the antimicrobial functions by releasing positive chlorine into the environment, and the positive chlorines react with appropriate receptors in the cells to achieve biocidal functions [75-77]. In their comprehensive review on halamines [31], Worley and Williams reported that 0.2-5.0 μg/ml of total chlorine in distilled water was sufficient to provide 8-log reduction of gram-positive and gram-negative bacteria at a contact time of 10 min. The zone of inhibition study conducted in our investigation showed that chlorine release was crucial for the biocidal efficacies of the chloromelamine-based fibrous materials, and this was also supported by the fact that the contact time for a total kill of waterborne bacteria is shorter than that for airborne bacteria (1 min vs. 3 min).
On the other hand, however, the chlorine releasing mechanism alone may not be potent enough for the anti-spore functions of the chlorinated ACHT-immobilized cotton fabrics. At equilibrium (after > 10 h of releasing; see Fig. 7), the chloromelamine-based fibrous materials could only release around 2 μg/ml of active chlorine, which might be too low for sporicidal activities. To confirm this, we tested the sporicidal effect of sodium hypochlorite (NaOCl), a widely used chlorine releasing agent, against 103-4 cells/ml of B. atrophaeus spores. It was found that with 2 μg/ml of active chlorine, NaOCl could only provide < 90% reduction of the spore after 30 min of contact. Similar results of the anti-spore functions of NaOCl were also reported by other authors [78]. However, as shown in Table 3, the chloromelamine-based fibrous materials developed in this study achieved 99% reduction in 5 min, and provided 99.35%-99.75% of reduction of the same spore in the same concentration range in 30 min.
Therefore, we suggest that there could be two action mechanisms responsible for the biocidal efficacies of the chloromelamine-based fibrous materials. First, the positive chlorine resulting from the dissociation of N-Cl bonds might be partially or completely transferred to the appropriate acceptors in the cell, leading to expiration of the organisms. This effect could be the predominant mechanism, because 2 ppm of positive chlorines could be high enough to provide a total kill of bacterial species [31]. Besides, the intact chloromelamine moieties could also have biocidal effect, which might remain bound to cell membranes after partial penetration into the cells [76,79]. This action might further contribute to the observed inhibitory effect particularly if the quantity of free chlorines released to the wet environment was not sufficient enough for inactivating some tough species (e.g., spores). Once inside or bind to the cell membrane, various enzymes or enzyme systems in the cell could be subjected to inactivation or inhibition. In the case of bacterial spores, the spore coat material and the spore cortex could be removed and degraded by the interaction with the hypochlorite ion [58,80-82], which could facilitate the access of chlorinated ACHT moieties to their sites of action on or in the spore protoplast.
3.7. Other properties of the chloromelamine-based fibrous materials
Many antibiotic-treated biomaterials release antimicrobials continuously at high dose levels, irrespective of whether infectious agents are presented or not. With the chloromelamine-based fibrous materials, however, when microorganisms were absent, the chlorinated ACHT moieties were covalently bound to the fibrous materials, and only a very small amount of positive chlorine (e.g., 2.0 μg/ml at equilibrium when the fibrous materials were immersed in water) was released into the surrounding environment, ensuring long-term biocidal effectiveness and minimizing concerns of the side effects of the released chlorines. On the other hand, the zone of inhibition study and the waterborne and airborne microbial tests revealed that, when the chlorinated ACHT-immobilized fibrous materials were brought into contact with microorganisms, the released active chlorines would react with the microbes to provide biocidal functions. As shown in Scheme 3, this effect would disturb the equilibrium, and the consumption of hypochlorous acid by microbes would shift the reaction to the right side of the equation, leading to more chorines being released, and thus, potent biocidal effects. Therefore, in the new chloromelamine-based fibrous materials, the release rate of biocidal agents (active chlorines) is bioresponsive; and this can be an important characteristic to combine excellent storage stability (when microbes are not presented) and fast biocidal action (when microbes are presented) into one system.
When used in real applications, infectious agents can be presented in blood and/or body fluid. To preliminarily test the performance of the chloromelamine-based fibrous materials under these circumstances, the bactericidal activities of the fabrics were evaluated against 7×107 CFU/ml of gram-negative (E. coli, 15597) and gram-positive (S. aureus, 6538) bacteria suspensions, using the same procedures as described in the waterborne biocidal tests. The bacterial suspensions contained 10 vol% of human serum (SeraCare Life Sciences, Inc., Suite F Oceanside, CA) to simulate the micro-environments of human body fluid. It was found that although the human serum acted as a denaturant and decreased the bactericidal efficacies, a total kill of E. coli and a 3-log reduction of S. aureus were achieved within 5 min of contact time, further indicating that the new fibrous materials are attractive candidates for a number of real applications.
Durability and rechargeability are very important features of N-halamine-based biocidal materials. After standing at 23 ± 2 °C and 70 ± 5% RH for 6 months, more than 93.5% of the original chlorine content of the chlorinated ACHT-immobilized cotton fabric was retained (7007 ppm vs. 6552 ppm), suggesting good durability, which agreed well with the controlled release studies. After chlorination, the fabrics were immerged in 0.03 wt% of Na2S2O3 aqueous solution to quench the active chlorine, and then rechlorinated with diluted bleach containing 3000 ppm of chlorine. After 50 cycles of the “chlorinating-quenching” treatment, the chlorine content was essentially unchanged, indicating that the biocidal activities were fully rechargeable.
4. Conclusions
In this study, a simple pad-dry-cure approach was used to immobilize ACHT moieties onto cellulosic fibrous materials. The structures of the samples were fully characterized with NMR, UV/VIS, DSC, TG, iodimetric titration, and elemental analyses. An esterification approach was successfully developed to facilitate the NMR characterization of the ACHT-immobilized cellulose. After chlorination, the immobilized fibrous materials provided powerful, durable and rechargeable biocidal activities against gram-positive and gram-negative bacteria, multi-drug resistant bacteria, yeast, virus, and spores, and they effectively inhibited the formation of bacterial biofilms. It is believed that both the released positive chlorines and the covalently bound chloromelamine molecules contributed to the biocidal effects. Thanks to the long-term effectiveness, sustained-release of biocidal agent, and the bioresponsive releasing nature, the chloromelamine-based fibrous materials may have great potential for a broad range of biocidal and biofilm-controlling applications.
Acknowledgement
This work is supported by the National Institute for Occupational Safety and Health (grant number: OH008354-01) and the National Institutes of Health (grant number: DE01640301A1).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Litsky W. Wanted: plastics with antimicrobial properties. Am J Public Health. 1990;80:13–15. doi: 10.2105/ajph.80.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wendt C, Wiesenthal E, Dietz E, Rüden H. Survival of vancomycin-resistant and vancomycin-susceptible Enterococci on dry surfaces. J Clin Microbiol. 1998;36:3734–3736. doi: 10.1128/jcm.36.12.3734-3736.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neely AN, Maley MP. Survival of Enterococci and Staphylococci on hospital fabrics and plastic. J Clin Microbiol. 2000;38:724–726. doi: 10.1128/jcm.38.2.724-726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott E, Bloomfield SF, Barlow CG. An investigation of microbial contamination in the home. J Hyg Camb. 1982;89:279–293. doi: 10.1017/s0022172400070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubbo SD, Saunders J. Liberation of organisms from contaminated textiles. J Hyg Camb. 1963;61:507–513. doi: 10.1017/s0022172400021136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott E, Bloomfield SF. The survival and transfer of microbial contamination via cloths, hands and utensils. J App Bacteriol. 1990;68:271–278. doi: 10.1111/j.1365-2672.1990.tb02574.x. [DOI] [PubMed] [Google Scholar]
- 7.Sattar SA, Springthorpe S, Mani S, Gallant M, Nair RC, Scott E. Transfer of bacteria from fabrics to hands and other fabrics: development and application of a quantitative method using Staphylococcus aureus as a model. J Appl Microbiol. 2001;90:962–970. doi: 10.1046/j.1365-2672.2001.01347.x. [DOI] [PubMed] [Google Scholar]
- 8.Mackintosh CA, Hoffman PN. An extended model for transfer of micro-organisms via the hands: differences between organisms and the effect of alcohol disinfection. J Hyg Camb. 1984;82:345–355. doi: 10.1017/s0022172400064561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNeil E. Dissemination of microorganisms by fabrics and leather. Develop Ind Microbiol. 1964;5:30–35. [Google Scholar]
- 10.Whyte W. The role of clothing and drapes in the operating room. J Hosp Infect. 1988;11(Suppl C):2–17. doi: 10.1016/0195-6701(88)90019-9. [DOI] [PubMed] [Google Scholar]
- 11.Marples RR, Towers AG. A laboratory model for the investigation of contact transfer of micro-organisms. J Hyg Camb. 1979;82:237–248. doi: 10.1017/s0022172400025651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita K, Lilly HA, Kidson A, Ayliffe GA. Gentamicin-resistant Pseudomonas aeruginosa infection from mattresses in a burns unit. Br Med J. 1981;283:219–220. doi: 10.1136/bmj.283.6285.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherertz RJ, Sullivan ML. An outbreak of infections with Acinetobacter calcoaceticus in burn patients: contamination of patients' mattress. J Infect Dis. 1985;151:252–258. doi: 10.1093/infdis/151.2.252. [DOI] [PubMed] [Google Scholar]
- 14.Weernink SWPJ, Tjernberg I, Dijkshoorn L. Pillows, an unexpected source of Acinetobacter. J Hosp Infect. 1995;29:189–199. doi: 10.1016/0195-6701(95)90328-3. [DOI] [PubMed] [Google Scholar]
- 15.Oliphant JW, Gordon DA, Meis A, Parker RR. Q fever in laundry workers, presumably transmitted from contaminated clothing. Am J Hyg. 1949;49:76–82. doi: 10.1093/oxfordjournals.aje.a119261. [DOI] [PubMed] [Google Scholar]
- 16.Standaert SM, Hutcheson RH, Schaffner W. Nosocomial transmission of Salmonella gastroenteritis to laundry workers in a nursing home. Infect Contr Hosp Epidemiol. 1994;15:22–26. doi: 10.1086/646813. [DOI] [PubMed] [Google Scholar]
- 17.Binder S, Levitt AM, Sacks JJ, Hughes JM. Emerging infectious diseases: Public health issues for the 21st century. Science. 1999;284:1311–1313. doi: 10.1126/science.284.5418.1311. [DOI] [PubMed] [Google Scholar]
- 18.Lederberg J. Infectious history. Science. 2000;288:287–293. doi: 10.1126/science.288.5464.287. [DOI] [PubMed] [Google Scholar]
- 19.Vigo TL. Advances in antimicrobial polymers and materials. In: Gebelein C, Carraher C, editors. Biotechnology and Bioactive Polymers. Plenum Press; New York: 1994. pp. 225–237. [Google Scholar]
- 20.Gagliardi DD. Antibacterial finishes. Am Dyestuff Reptr. 1962;51:31–40. [Google Scholar]
- 21.Schierholz JM, Steinhauser H, Rump AFE, Berkels R, Pulverer G. Controlled release of antibiotics from biomedical polyurethanes: morphological and structural features. Biomaterials. 1997;18:839–844. doi: 10.1016/s0142-9612(96)00199-8. [DOI] [PubMed] [Google Scholar]
- 22.Phaneuf MD, Bide MJ, Hannel SL, Platek MJ, Monahan TS, Contreras MA, Phaneuf TM, LoGerfo FW. Development of an infection-resistant, bioactive wound dressing surface. J Biomed Mater Res. 2005;74A:666–676. doi: 10.1002/jbm.a.30347. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal P, Phaneuf MD, Bide MJ, Sousa KA, LoGerfo FW. Development of an infection-resistant bifunctionalized Dacron biomaterial. J Biomed Mater Res. 2005;75A:224–231. doi: 10.1002/jbm.a.30427. [DOI] [PubMed] [Google Scholar]
- 24.Klueh U, Wagner V, Kelly S, Johnson A, Bryers JD. Efficacy of silver-coated fabric to prevent bacterial colonization and subsequent device-based biofilm formation. J Biomed Mater Res. 2000;53:621–631. doi: 10.1002/1097-4636(2000)53:6<621::aid-jbm2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Bottenbos B, van der Mei HC, Klatter F, Nieuwenhuis P, Busscher HJ. In vitro and in vivo antimicrobial activity of covalently coupled quaternary ammonium silane coatings on silicone rubber. Biomaterials. 2002;23:1417–1423. doi: 10.1016/s0142-9612(01)00263-0. [DOI] [PubMed] [Google Scholar]
- 26.Liang J, Chen Y, Barnes K, Wu R, Worley SD, Huang TS. N-halamine/quat siloxane copolymers for use in biocidal coatings. Biomaterials. 2006;27:2495–2501. doi: 10.1016/j.biomaterials.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Wehner W, Grade R. Canadian Patent. No. CA2082994 Preparation of ammonium and phosphonium salts as biocides in the protection of a variety of materials and water systems. 1993
- 28.Kobase S, Takerchi H, Sumita M. Japanese Patent. No. JP2000316701 Antimicrobial mats containing phosphonium compounds. 2000
- 29.Sun G, Xu XJ. Durable and regenerable antibacterial finishing of fabrics: biocidal properties. Textile Chemist and Colorist. 1998;30:26–30. [Google Scholar]
- 30.Sun YY, Sun G. Novel refreshable N-halamine polymeric biocides: grafting hydantoin-containing monomers onto high performance fibers by a continuous process. J Appl Polym Sci. 2003;88:1032–1039. [Google Scholar]
- 31.Worley SD, Williams DE. Halamine water disinfectants. CRC Crit Rev Environ Control. 1988;18:133–175. [Google Scholar]
- 32.Sun G, Chen TY, Worley SD. A novel biocidal styrenetriazinedione polymer. Polymer. 1996;37:3753–3756. [Google Scholar]
- 33.Sun YY, Sun G. Synthesis, characterization, and antibacterial activities of novel N-halamine polymer beads prepared by suspension copolymerization. Macromolecules. 2002;35:8909–8912. [Google Scholar]
- 34.Chen ZB, Sun YY. N-chloro-hindered amines as multifunctional polymer additives. Macromolecules. 2005;38:8116–8119. [Google Scholar]
- 35.Makal U, Wood L, Ohman DE, Wynne KJ. Polyurethane biocidal polymeric surface modifier. Biomaterials. 2006;27:1316–1326. doi: 10.1016/j.biomaterials.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 36.Mora EC, Kohl HH, Wheatley WB, Worley SD, Faison JH, Burkett HD, Boder N. Properties of a new chloramines disinfectant and detoxicant. Poultry Science. 1982;61:1968–1971. doi: 10.3382/ps.0611968. [DOI] [PubMed] [Google Scholar]
- 37.Burkett HD, Faison JH, Kohl HH, Wheatley WB, Worley SD, Bodor N. A novel chloramines compound for water disinfection. Water Resources Bulletin. 1981;17:874–879. [Google Scholar]
- 38.Worley SD, Burkett HD, Price JF. The tendency of a new water disinfectant to produce toxic trihalomethanes. Water Resources Bulletin. 1984;20:369–371. [Google Scholar]
- 39.Chen ZB, Sun YY. Antimicrobial polymers containing melamine derivatives. II. Biocidal polymers derived from 2-vinyl-4,6-diamino-1,3,5-triazine. J Polym Sci Part A; Polym Chem. 2005;43:4089–4098. [Google Scholar]
- 40.Chen ZB, Sun YY. N-Halamine-based antimicrobial additives for polymers: Preparation, characterization, and antimicrobial activity. Ind Eng Chem Res. 2006;45:2634–2640. doi: 10.1021/ie060088a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo J, Chen ZB, Sun YY. Controlling biofilm formation with an N-halamine-based polymeric additive. J Biomed Mater Res. 2006;77A:823–831. doi: 10.1002/jbm.a.30689. [DOI] [PubMed] [Google Scholar]
- 42.Dychdala GR. Chlorine and chlorine compounds. In: Block SS, editor. Disinfection, sterilization and preservation. 3rd edition Lea & Febiger; Philadelphia: 1983. pp. 157–182. [Google Scholar]
- 43.Kim SH, Bartholomew DG, Allen LB. Imidazo[1,2-a]-s-triazine nucleosides. Synthesis and antiviral activity of the N-bridgehead guanine, guanosine, and guanosine monophosphate analogues of imidazo[1,2-a]-s-triazine. J Med Chem. 1978;21:883–889. doi: 10.1021/jm00207a009. [DOI] [PubMed] [Google Scholar]
- 44.Sun YY, Chen ZB, Braun M. Preparation, and physical and antimicrobial properties of a cellulose-supported chloromelamine derivative. Ind Eng Chem Res. 2005;44:7916–7920. [Google Scholar]
- 45.Richmond JY, McKinney RW. Biosafaty in Microbiological and Biomedical Laboratories. 4th ed. U.S. Government Printing Office; Washington, DC: 1999. [Google Scholar]
- 46.Tiller JC, Liao CJ, Lewis K, Klibanov AM. Designing surfaces that kill bacteria on contact. Proc Natl Acad Sci U.S.A. 2001;98:5981–5985. doi: 10.1073/pnas.111143098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tiller JC, Lee SB, Lewis K, Klibanov AM. Polymer surfaces derivatized with poly(vinyl-N-hexylpyridinium) kill airborne and water borne bacteria. Biotech and Bioeng. 2002;79:465–471. doi: 10.1002/bit.10299. [DOI] [PubMed] [Google Scholar]
- 48.Cen L, Neoh KG, Kang ET. Antibacterial activity of cloth functionalized with N-alkylated poly(4-vinylpyridine) J Biomed Mater Res. 2004;74A:70–80. doi: 10.1002/jbm.a.30125. [DOI] [PubMed] [Google Scholar]
- 49.Miyamoto T, Sato Y, Shibata T, Inagaki H. 13C nuclear magnetic resonance studies of cellulose acetate. J Polym Sci: Polym Chem Ed. 1984;22:2363–2370. [Google Scholar]
- 50.Doyle S, Pethrick RA, Harris RK, Lane JM, Packer KJ, Heatley F. 13C nuclear magnetic resonance studies of cellulose acetate in the solution and solid states. Polymer. 1986;27:19–24. [Google Scholar]
- 51.Goodlett VW, Dougherty JT, Patton HW. Characterization of cellulose acetates by nuclear magnetic resonance. J Polym Sci: Part A-1. 1971;9:155–160. [Google Scholar]
- 52.Buchanan CM, Edgar KJ, Hyatt JA, Wilson AK. Preparation of cellulose [1-13C] acetate and determination of monomer composition by NMR spectroscopy. Macromolecules. 1991;24:3050–3059. [Google Scholar]
- 53.Wu TK. Carbon-13 and proton nuclear magnetic resonance studies of cellulose nitrates. Macromolecules. 1980;13:74–79. [Google Scholar]
- 54.Helmer RD, Finch GR. Use of MS2 coliphage as a surrogate for enteric viruses in surface waters disinfected with ozone. Ozone: Science and Engineering. 1993;15:279–293. [Google Scholar]
- 55.Duran AE, Muniesa M, Mendez X, Valero F, Lucena F, Jofre J. Removal and inactivation of indicator bacteriophages in fresh waters. J App Microbiol. 2002;92:338–347. doi: 10.1046/j.1365-2672.2002.01536.x. [DOI] [PubMed] [Google Scholar]
- 56.Allwood PB, Malik YS, Hedberg CW, Goyal SM. Effect of temperature and sanitizers on survival of feline calicivirus, Escherichia coli, and F-specific coliphage MS2 on leafy salad vegetables. J Food Prot. 2004;67:1451–1456. doi: 10.4315/0362-028x-67.7.1451. [DOI] [PubMed] [Google Scholar]
- 57.Allwood PB, Malik YS, Hedgerg CW, Goyal SM. Survival of F-specific RNA coliphage, feline calicivirus, and Escherichia coli in water: a comparative study. App Environ Microbiol. 2003;69:5707–5710. doi: 10.1128/AEM.69.9.5707-5710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bloomfield SF, Arthur M. Mechanisms of inactivation and resistance of spores to chemical biocides. J Appl Bacteriol Symp Suppl. 1994;76:91S–104S. doi: 10.1111/j.1365-2672.1994.tb04361.x. [DOI] [PubMed] [Google Scholar]
- 59.Bloomfield SF, Megid R. Interaction of iodine with Bacillus subtilis spores and spore forms. J App Bacteriol. 1994;76:492–499. doi: 10.1111/j.1365-2672.1994.tb01107.x. [DOI] [PubMed] [Google Scholar]
- 60.Bayliss CE, Waites WM. The effect of hydrogen peroxide on spores of Clostridium bifermentans. J Gen Microbiol. 1976;96:401–407. doi: 10.1099/00221287-96-2-401. [DOI] [PubMed] [Google Scholar]
- 61.Sheth NK, Franson TR, Sohnle PG. Influence of bacterial adherence to intravascular catheters on in-vitro antibiotic susceptibility. Lancet. 1985;326:1266–1268. doi: 10.1016/s0140-6736(85)91552-1. [DOI] [PubMed] [Google Scholar]
- 62.An YH, Friedman RJ. Prevention of sepsis in total joint arthroplasty. J Hosp Infect. 1996;33:93–108. doi: 10.1016/s0195-6701(96)90094-8. [DOI] [PubMed] [Google Scholar]
- 63.Gray ED, Verstegen M, Peters G, Regelmann W. Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response. Lancet. 1984;323:365–367. doi: 10.1016/s0140-6736(84)90413-6. [DOI] [PubMed] [Google Scholar]
- 64.Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ. Bacterial biofilms in nature and disease. Ann Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 65.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 66.Costerton JW, Stewart P, Greenberg EP. Bacterial biofilms: A common cause of persistant infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 67.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruseska I, Robbins J, Costerton JW, Lashen ES. Biocide testing against corrosion-causing oil-filed bacteria helps control plugging. Oil Gas J. 1982;80:253–254,256. 261-262,264. [Google Scholar]
- 69.Costerton JW. The formation of biocide-resistant biofilms in industrial, natural and medical systems. Dev Ind Microbiol. 1984;25:363–372. [Google Scholar]
- 70. http://grants.nih.gov/grants/guide/pa-files/PA-03-047.html.
- 71.Zobell CE. The effect of solid surfaces upon bacterial activity. J Bacteriol. 1943;46:39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.An YH, Friedman RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res. 1998;43:338–348. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 73.Bodor N, Kaminski JJ, Worley SD, Colton RJ, Lee TH, Rabalais JW. Photoelectron spectra, hydrolytic stability, and antimicrobial activity of N-chlorinated piperidines. J Pharm Sci. 1974;63:1387–1391. doi: 10.1002/jps.2600630911. [DOI] [PubMed] [Google Scholar]
- 74.Kaminski JJ, Bodor N, Higuchi T. N-halo derivatives III: stabilization of nitrogen-chlorine bond in N-chloramino acid derivatives. J Pharm Sci. 1976;65:553–557. doi: 10.1002/jps.2600650418. [DOI] [PubMed] [Google Scholar]
- 75.Dychdala GR. In: Disinfection, Sterilization and Preservation. 3rd edition Block SS, editor. Lea & Febiger; Philadelphia: 1983. pp. 157–182. [Google Scholar]
- 76.Williams DE, Swango LJ, Wilt GR, Worley SD. Effect of organic N-halamines on selected membrane functions in intact Staphylococcus aureus cells. Appl Environ Microbio. 1991;57:1121–1127. doi: 10.1128/aem.57.4.1121-1127.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bloomfield SF, Arthur M. Effect of chlorine-releasing agents on Bacillus subtilis vegetative cells and spores. Lett Appl Microbiol. 1989;8:101–104. [Google Scholar]
- 79.Williams DE, Elder ED, Worley SD. Is free chlorine necessary for disinfection? App Environ Microbiol. 1988;54:2583–2585. doi: 10.1128/aem.54.10.2583-2585.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wyatt LR, Waites WM. The effect of chlorine on spores of Clostridium bifermentans, Bacillus subtilis and Bacillus cereus. J Gen Microbiol. 1975;89:337–344. doi: 10.1099/00221287-89-2-337. [DOI] [PubMed] [Google Scholar]
- 81.Foegeding PM, Busta FF. Proposed mechanism for sensitization by hypochlorite treatment of Clostridium botulinum spores. Appl Environ Microbiol. 1983;45:1374–1379. doi: 10.1128/aem.45.4.1374-1379.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bloomfield SF, Arthur M. Interaction of Bacillus subtilis spores with sodium hypochlorite, sodium dichloroisocyanurate and chloramine-T. J Appl Bacteriol. 1992;72:166–172. doi: 10.1111/j.1365-2672.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]