Abstract
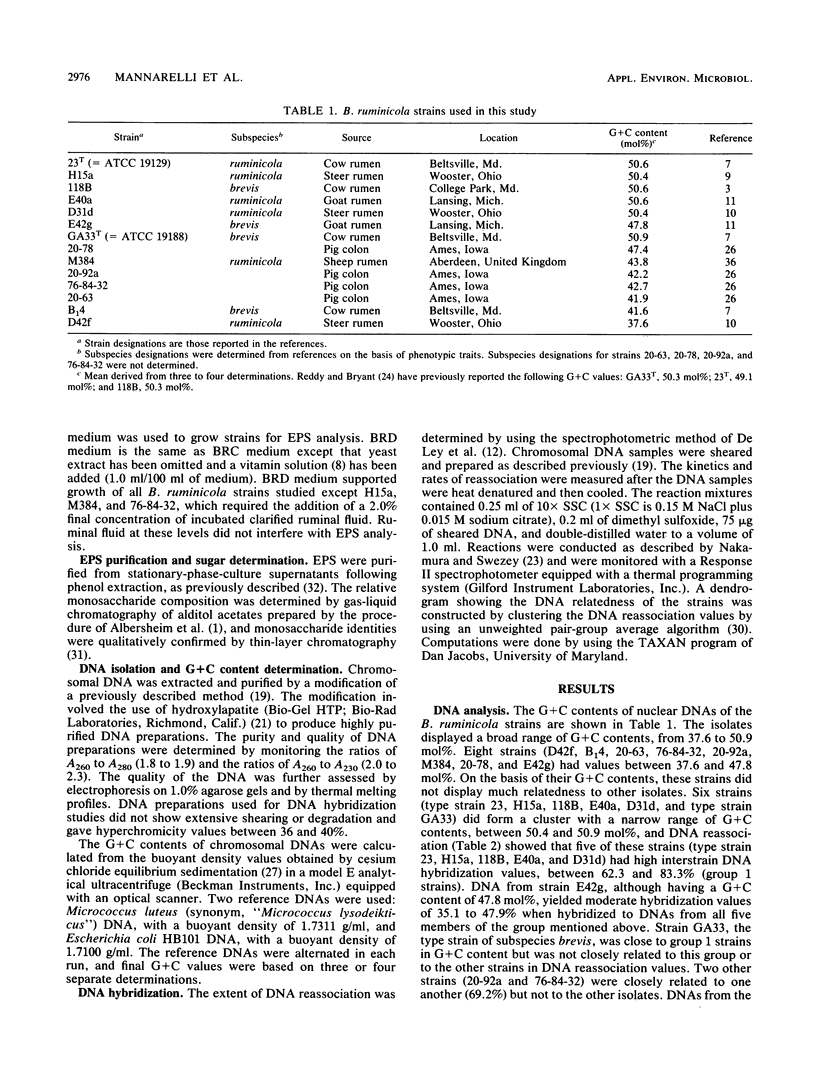
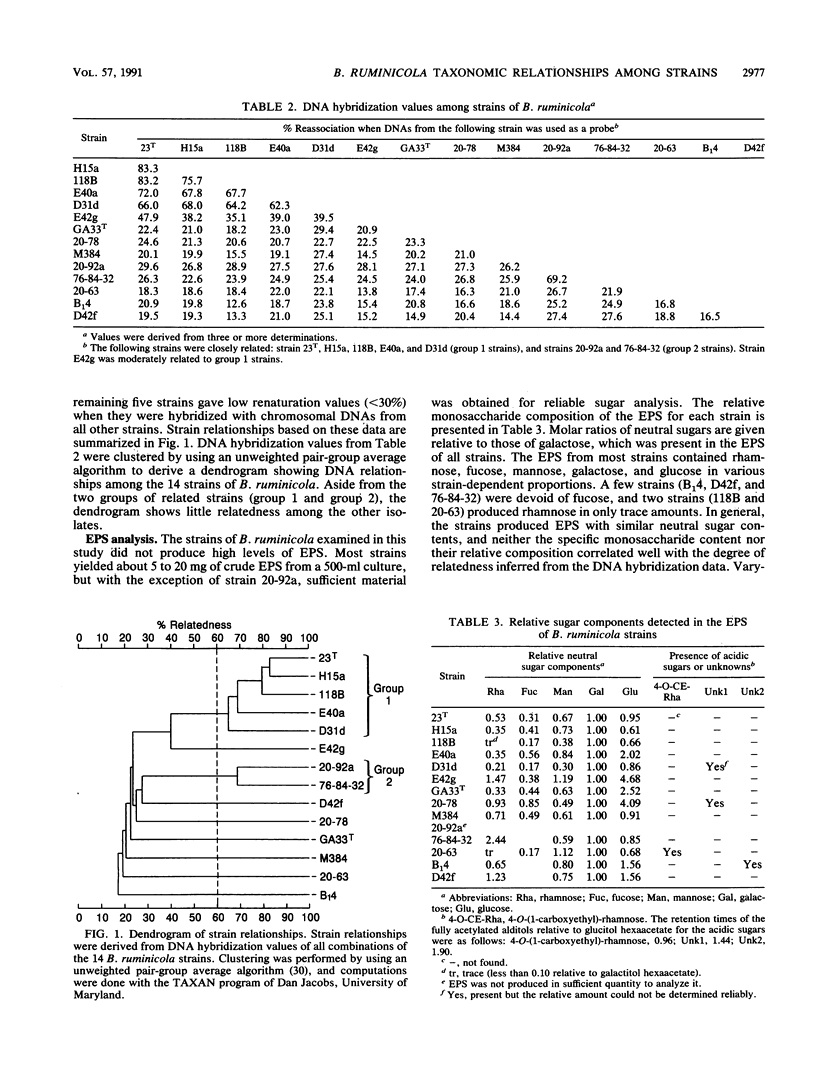
DNA and extracellular polysaccharide (EPS) analyses were performed on 14 strains of Bacteroides ruminicola. The guanine-plus-cytosine (G+C) base contents, determined from the buoyant densities of chromosomal DNAs, showed a broad range of values, from 37.6 to 50.9 mol%. DNA hybridization showed generally low DNA relatedness among the strains. Seven strains formed two groups of closely related bacteria consisting of five (group 1) and two (group 2) strains, and another strain, E42g, showed moderate relatedness to group 1 strains. However, the remaining six strains were not related to any of the other strains. DNA reassociation indicates that the strains constitute a genetically diverse group representing as many as nine separate species. EPS analysis showed that the strains produced EPS with rather uniform sugar compositions, which did not correlate with strain relationships determined by DNA analysis. Four strains had EPS with acidic sugars or unknown compounds. The EPS of strain 20-63 contained the unusual acidic sugar 4-O-(1-carboxyethyl)-rhamnose. This monosaccharide has been shown to occur in nature in only one other bacterial species.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann R. I., Binder B. J., Olson R. J., Chisholm S. W., Devereux R., Stahl D. A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990 Jun;56(6):1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N., BOUMA C., CHU H. Bacteroides ruminicola n. sp. and Succinimonas amylolytica; the new genus and species; species of succinic acid-producing anaerobic bacteria of the bovine rumen. J Bacteriol. 1958 Jul;76(1):15–23. doi: 10.1128/jb.76.1.15-23.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H. A., Bryant M. P., Doetsch R. N. A Study of Bacterial Species from the Rumen Which Produce Ammonia from Protein Hydrolyzate. Appl Microbiol. 1961 Mar;9(2):175–180. doi: 10.1128/am.9.2.175-180.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotta M. A., Hespell R. B. Proteolytic activity of the ruminal bacterium Butyrivibrio fibrisolvens. Appl Environ Microbiol. 1986 Jul;52(1):51–58. doi: 10.1128/aem.52.1.51-58.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ley J., Cattoir H., Reynaerts A. The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem. 1970 Jan;12(1):133–142. doi: 10.1111/j.1432-1033.1970.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Dehority B. A. Characterization of several bovine rumen bacteria isolated with a xylan medium. J Bacteriol. 1966 May;91(5):1724–1729. doi: 10.1128/jb.91.5.1724-1729.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A., Grubb J. A. Characterization of the predominant bacteria occurring in the rumen of goats (Capra hircus). Appl Environ Microbiol. 1977 May;33(5):1030–1036. doi: 10.1128/aem.33.5.1030-1036.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Pectin-fermenting bacteria isolated from the bovine rumen. J Bacteriol. 1969 Jul;99(1):189–196. doi: 10.1128/jb.99.1.189-196.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux R., He S. H., Doyle C. L., Orkland S., Stahl D. A., LeGall J., Whitman W. B. Diversity and origin of Desulfovibrio species: phylogenetic definition of a family. J Bacteriol. 1990 Jul;172(7):3609–3619. doi: 10.1128/jb.172.7.3609-3619.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden B. I. The isolation and identification of Bacteroides spp. from the normal human faecal flora. J Med Microbiol. 1980 Feb;13(1):69–78. doi: 10.1099/00222615-13-1-69. [DOI] [PubMed] [Google Scholar]
- Hespell R. B., Wolf R., Bothast R. J. Fermentation of xylans by Butyrivibrio fibrisolvens and other ruminal bacteria. Appl Environ Microbiol. 1987 Dec;53(12):2849–2853. doi: 10.1128/aem.53.12.2849-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdeman L. V., Good I. J., Moore W. E. Human fecal flora: variation in bacterial composition within individuals and a possible effect of emotional stress. Appl Environ Microbiol. 1976 Mar;31(3):359–375. doi: 10.1128/aem.31.3.359-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., FALKOW S., MANDEL M. NEW APPROACHES TO BACTERIAL TAXONOMY. Annu Rev Microbiol. 1963;17:329–372. doi: 10.1146/annurev.mi.17.100163.001553. [DOI] [PubMed] [Google Scholar]
- Markov G. G., Ivanov I. G. Hydroxyapatite column chromatography in procedures for isolation of purified DNA. Anal Biochem. 1974 Jun;59(2):555–563. doi: 10.1016/0003-2697(74)90309-1. [DOI] [PubMed] [Google Scholar]
- Reddy C. A., Bryant M. P. Deoxyribonucleic acid base composition of certain species of the genus Bacteroides. Can J Microbiol. 1977 Sep;23(9):1252–1256. doi: 10.1139/m77-187. [DOI] [PubMed] [Google Scholar]
- Robinson I. M., Allison M. J., Bucklin J. A. Characterization of the cecal bacteria of normal pigs. Appl Environ Microbiol. 1981 Apr;41(4):950–955. doi: 10.1128/aem.41.4.950-955.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson I. M., Whipp S. C., Bucklin J. A., Allison M. J. Characterization of predominant bacteria from the colons of normal and dysenteric pigs. Appl Environ Microbiol. 1984 Nov;48(5):964–969. doi: 10.1128/aem.48.5.964-969.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Seidler R. J., Knittel M. D., Brown C. Potential pathogens in the environment: cultural reactions and nucleic acid studies on Klebsiella pneumoniae from clinical and environmental sources. Appl Microbiol. 1975 Jun;29(6):819–825. doi: 10.1128/am.29.6.819-825.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah H. N., Collins D. M. Prevotella, a new genus to include Bacteroides melaninogenicus and related species formerly classified in the genus Bacteroides. Int J Syst Bacteriol. 1990 Apr;40(2):205–208. doi: 10.1099/00207713-40-2-205. [DOI] [PubMed] [Google Scholar]
- Slodki M. E., Wickerham L. J. Extracellular polysaccharides and classification of the genus Lipomyces. J Gen Microbiol. 1966 Mar;42(3):381–385. doi: 10.1099/00221287-42-3-381. [DOI] [PubMed] [Google Scholar]
- Stack R. J. Neutral sugar composition of extracellular polysaccharides produced by strains of Butyrivibrio fibrisolvens. Appl Environ Microbiol. 1988 Apr;54(4):878–883. doi: 10.1128/aem.54.4.878-883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack R. J., Weisleder D. 4-O-(1-carboxyethyl)-L-rhamnose, a second unique acidic sugar found in an extracellular polysaccharide from Butyrivibrio fibrisolvens strain 49. Biochem J. 1990 Jun 1;268(2):281–285. doi: 10.1042/bj2680281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Biosynthesis and composition of gram-negative bacterial extracellular and wall polysaccharides. Annu Rev Microbiol. 1985;39:243–270. doi: 10.1146/annurev.mi.39.100185.001331. [DOI] [PubMed] [Google Scholar]



