Abstract
Tyrosine hydroxylase (TyrH), the catalyst for the key regulatory step in catecholamine biosynthesis, is phosphorylated by cAMP-dependent protein kinase A (PKA) on a serine residue in a regulatory domain. In the case of the rat enzyme, phosphorylation of Ser40 by PKA is critical in regulating the enzyme activity; the effect of phosphorylation is to relieve the enzyme from inhibition by dopamine and dihydroxyphenylalanine (DOPA). There are four isoforms of human tyrosine hydroxylase (hTyrH), differing in the size of an insertion after Met30. The effects of phosphorylation by PKA on the binding of DOPA and dopamine have now been determined for all four human isoforms. There is an increase of about two-fold in the Kd value for DOPA for isoform 1 upon phosphorylation, from 4.4 to 7.4 μM; this effect decreases with the larger isoforms such that there is no effect of phosphorylation on the Kd value for isoform 4. Dopamine binds more much tightly, with Kd values less than 3 nM for all four unphosphorylated isoforms. Phosphorylation decreases the affinity for dopamine at least two orders of magnitude, resulting in Kd values of about 0.1 μM for the phosphorylated human enzymes, due primarily to increases in the rate constant for dissociation of dopamine. Dopamine binds about two-fold less tightly to the phosphorylated isoform 1 than to the other three isoforms. The results extend the regulatory model developed for the rat enzyme, in which the activity is regulated by the opposing effects of catecholamine binding and phosphorylation by PKA. The small effects on the relatively high Kd values for DOPA suggest that DOPA levels do not regulate the activity of hTyrH.
Keywords: DOPA, dopomine, phosphorylation, regulation, tyrosine hydroxylase
The biosynthesis of the catecholamine neurotransmitters begins with the hydroxylation of tyrosine to form dihydroxyphenylalanine (DOPA). This step, catalyzed by the enzyme tyrosine hydroxylase (TyrH), is generally accepted to be the rate-limiting step in the pathway (Fitzpatrick 1999). The TyrH reaction is a monooxygenation, in that molecular oxygen is the source of the atom of oxygen incorporated into DOPA (Daly et al. 1968), whereas tetrahydrobiopterin serves as the source of the two electrons required for reduction of the other atom of oxygen to the level of water. Activation of oxygen for the reaction involves an active site non-heme iron atom; for catalysis, the iron atom must be in the ferrous form (Fitzpatrick 1989). In the presence of oxygen the iron is readily oxidized to the ferric form; tetrahydropterins can reduce the iron back to the active ferrous state (Ramsey et al. 1996). The active site containing this iron atom is a deep cleft in a catalytic domain of about 300 amino acids that is homologous to the catalytic domains of the two other pterin-dependent aromatic amino acid hydroxylases, phenylalanine hydroxylase and tryptophan hydroxylase (Grenett et al. 1987; Goodwill et al. 1997).
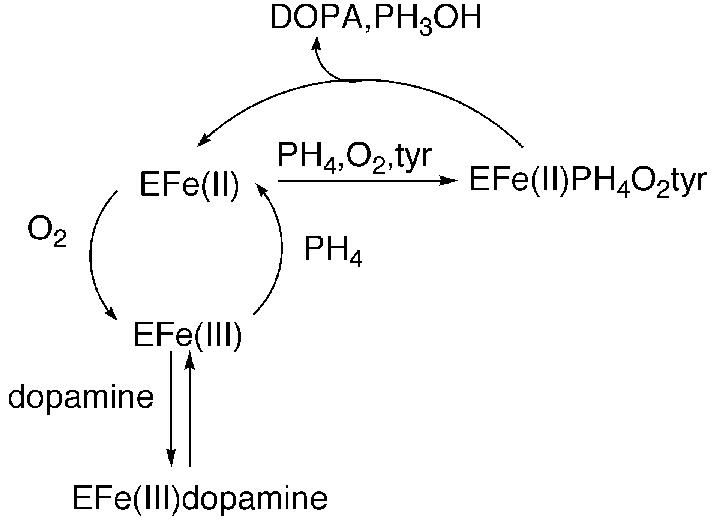
All three enzymes also contain N-terminal regulatory domains. The sequences of these regulatory domains differ among the three enzymes, consistent with their discrete regulatory properties. In the case of TyrH, short-term increases in the formation of DOPA in vivo correlate with increased phosphorylation of serine residues in this regulatory domain (Zigmond et al. 1989; Haycock 1990). In rat TyrH, the serine residues that exhibit altered phosphorylation when dopamine biosynthesis is stimulated are located at positions 19, 31, and 40; Ser8 has also been found to be phosphorylated in adrenal cells, but no physiologically relevant conditions that alter its level of phosphorylation have been described (Haycock 1990). A large number of studies have examined the effects of such phosphorylation on the catalytic activity of TyrH in vitro, utilizing protein kinases specific for selected sites (Zigmond et al. 1989; Kumer and Vrana 1996; Fitzpatrick 1999). cAMP-Dependent protein kinase A (PKA) was demonstrated early on to be specific for Ser40 (Campbell et al. 1986), and more recently the mitogen-activated protein (MAP) kinases extracellular signal-regulated kinase-1 (ERK1) and ERK2 have been demonstrated to phosphorylate Ser31 (Haycock et al. 1992; Royo et al. 2004). No kinase specific for Ser19 has been described to date, but several will phosphorylate both Ser19 and Ser40 (Campbell et al. 1986; Sutherland et al. 1993). Activation of TyrH upon phosphorylation by PKA has been demonstrated by a number of laboratories (Zigmond et al. 1989; Kumer and Vrana 1996; Fitzpatrick 1999). However, the effects on the enzyme activity are quite modest, changes of about two-fold in the Km value for tetrahydrobiopterin (Le Bourdellès et al. 1991; Daubner et al. 1992) and the Ki value for dopamine (Le Bourdellès et al. 1991), when purified recombinant enzyme is used. The degree of activation upon phosphorylation by a MAP kinase is smaller (Haycock et al. 1992; Sutherland et al. 1993), whereas phosphorylation of both Ser19 and Ser40 has a comparable effect to phosphorylation of Ser40 alone (Sutherland et al. 1993). In the case of rat TyrH, Ser40 phosphorylation results in much more dramatic effects on the affinity of the ferric enzyme for catecholamines due to modest increases in the rate constants for binding of catecholamines and much larger increases in the rate constants for dissociation (Ramsey and Fitzpatrick 1998, 2000). The greatest effects are seen upon binding of dopamine, norepinephrine, and epinephrine; the rate constants for dissociation of all three increase by three orders of magnitude when Ser40 is phosphorylated, whereas the dissociation rate constants for DOPA increase about 20-fold. These results are consistent with the model of Scheme 1 for the effects of Ser40 phosphorylation on rat TyrH (Daubner et al. 1992; Ramsey and Fitzpatrick 1998). The active ferrous form of the enzyme catalyzes the formation of DOPA from tyrosine. This form of the enzyme can be oxidized to the inactive ferric form; reduction by tetrahydrobiopterin restores activity. However, if a catecholamine accumulates in the cell, it can bind to the ferric enzyme, trapping it in the inactive complex. Consistent with such a model, when TyrH is isolated from rat or bovine cells, it is in the form of a complex containing ferric iron and bound catecholamines (Haavik et al. 1988; Andersson et al. 1992). The rate constants for dissociation of dopamine, norepinephrine, and epinephrine from the rat enzyme suggest that catecholamine binding to the ferric enzyme is effectively irreversible in the cell until Ser40 is phosphorylated (Ramsey and Fitzpatrick 2000).
Scheme 1.
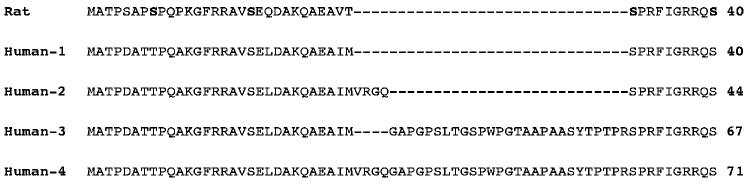
In both rats and humans there is a single gene for TyrH (Brown et al. 1987; O'Malley et al. 1987). However, in humans four different TyrH isoforms are synthesized from this single gene by differential splicing (Grima et al. 1987; Kaneda et al. 1987; O'Malley et al. 1987). The differences among the isoforms are limited to their regulatory domains (Fig. 1). Isoform 1 (hTyrH1) most closely resembles rat TyrH. Isoform 2 contains an additional four amino acids after Met30 of isoform 1, isoform 3 contains an insertion of 27 amino acids at the same point, and isoform 4 contains both insertions, giving 31 additional residues. These differences in the sequences of the regulatory domains of the four human isoforms raise obvious questions regarding the contribution of the additional sequences to the regulatory and catalytic properties of the isoforms. All four isoforms are present in the human brain, although not at identical levels, and all four can be found in the same cell (Lewis et al. 1993). Characterization of recombinant forms of all four human isoforms has shown that their steady state kinetic parameters are not dramatically different (Haavik et al. 1991; Le Bourdellès et al. 1991; Boularand et al. 1995), although hTyrH1 is reported to have a slightly higher Km for tetrahydrobiopterin (Le Bourdellès et al. 1991). The effects of phosphorylation by different kinases on steady state kinetic parameters are reported to be comparable for all four isoforms (Sutherland et al. 1993), although there is one report that hTyrH3 is not activated significantly upon phosphorylation by PKA (Alterio et al. 1998). However, as noted above the primary effect of phosphorylation on the rat enzyme is not on the steady state kinetic parameters but on the binding of inhibitory catecholamines. The goals of the experiments described here were to determine if the mechanism of Scheme 1 was equally applicable to human and rat TyrH and whether the different human isoforms differ in the effect of phosphorylation of Ser40 on the binding of DOPA and dopamine.
Fig. 1.
N-Terminal amino acid sequences of human tyrosine hydroxylase isoforms and rat tyrosine hydroxylase. The remaining sequences are identical among the human isoforms. The phosphorylated residues in the rat enzyme are in bold.
Experimental procedures
Materials
L-Tyrosine was purchased from Sigma Chemical Corp. (St Louis, MO, USA). 6-Methyltetrahydropterin (6MPH4) was purchased from B. Schircks Laboratories (Jona, Switzerland). All other reagents were of the highest purity commercially available. The catalytic subunit of beef heart PKA was purifed by the method of Flockhart and Corbin (Flockhart et al 1984).
Construction of vectors for expression of human tyrosine hydroxylase isoforms in Escherichia coli
Plasmid pHTh-63, which contains the cDNA for hTyrH2, was a gift from Dr Karen O'Malley, Washington University (St. Louis, MO, USA). PCR was used to add an NcoI site at the 5′ end of the coding region and a BamHI site at the 3′ end, allowing insertion into the pET-23d expression vector (Novagen, Madison, WI, USA). Plasmids containing hTyrH2 cDNA were identified by restriction enzyme digestion and confirmed by sequencing; the plasmid selected for further use was named p23dHTH2. To obtain the other human isoforms, modifications of the QuikChange method (Stratagene) were used to delete or insert DNA coding for multiple amino acids. To obtain p23dHTH4, the plasmid for expression of hTyrH4, two separate PCR reactions, each containing only one of the oligonucleotides in Table 1, were run for five cycles. The two reactions were then combined for 12 additional PCR cycles. To obtain the plasmids for expression of hTyrH1 and hTyrH3, p23dHTH1 and p23dHTH3, respectively, the oligonucleotides in Table 1 were used to delete 12 base pairs after the codon for Met30, using p23dHTH2 and p23dHTH4 as templates. To avoid formation of dimers between the primers, only one oligonucleotide primer was present for the first PCR cycle, and the other was then added for the remaining 17 cycles. The desired plasmids were identified by restriction digestion and confirmed by sequencing.
Table 1.
Oligonucleotides used for construction of expression plasmids for human tyrosine hydroxylase isoforms
| Modification | Product | Plus strand oligonucleotide | Minus strand oligonucleotide |
|---|---|---|---|
| Deletion of 12 base pairs from p23dHTH2 |
p23dHTH1 | 5′-GGCAGAGGCCATCATGTCC CCGCGGTTCATTGGG-3′ |
5′-CCCAATGAACCGCGGGGAC ATGATGGCCTCTGCC-3′ |
| Insertion of 81 base pairs into p23dHTH2 |
p23dHTH4 | 5′-CCGTGGCCTGGAACTGCAG CCCCAGCTGCATCCTACACCC CCACCCCAAGGTCCCCGCGG TTCATTGGGCG-3′ |
5′-GCAGTTCCAGGCCACGGAG AGCCTGTGAGGCTGGGCCCCG GGGCGCCCTGCCCTTTACCAT GATGGCCTCTGC-3′ |
| Deletion of 12 base pairs from p23dHTH4 |
p23dHTH3 | 5′-GCAGGCAGAGGCCATCATG GGCGCCCCGGGGCCCAGCCTCACAG-3′ |
5′-GCTGGGCCCCGGGGCGCCC ATGATGGCCTCTGCCTGCTTGGC-3′ |
Purification
Expression and purification of the human enzymes was performed using modifications of methods developed for the rat enzyme (Daubner et al. 1992). Escherichia coli BL21 Star™ (DE3) cells transformed with p23dHTH1, p23dHTH2, p23dHTH3 or p23dHTH4 were grown in Luria Bertani medium containing 100 μ/mL carbenicillin at 25°C. When the absorbance at 600 nm reached 0.5, expression was induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside. After 6 h, cells were harvested by centrifugation and resuspended in 75 μM diethylenetriaminepentaacetic acid (DTPA), 10% glycerol, 1 μM pepstatin, 1 μM leupeptin, 100 μg/mL phenylmethylsulfonylfluoride, 25 μg/mL lysozyme, 50 mM HEPES, pH 7.0. The cells were lysed by sonication at 4°C and centrifuged at 28 000 × g for 30 min. Streptomycin sulfate was added to the supernatant to a final concentration of 1% to allow precipitation of nucleic acids at 28 000 × g for 20 min. The resulting supernatant was made 45% saturated in ammonium sulfate, stirred for 20 min and centrifuged for another 20 min. The resulting pellet was resuspended in 75 μM DTPA, 10% glycerol, 50 mM HEPES, pH 7.0, and dialyzed against the same buffer. After centrifugation to remove precipitated protein, the sample was loaded onto a Hitrap-Heparin column; this was eluted with a linear gradient of 0–0.8 M KC1 in 75 μM DTPA, 10% glycerol, 50 mM HEPES, pH 7.0. Fractions containing tyrosine hydroxylase were pooled and precipitated by making the solution 65% saturated in ammonium sulfate. The pellet was resuspended to give a concentration of ~300 μM TyrH in 100 mM KC1, 10% glycerol, 1 μM pepstatin, 1 μM leupeptin, 50 mM HEPES, pH 7.0. To this, ferrous ammonium sulfate was added to give a 20% molar excess of iron over TyrH active sites; excess iron was removed by dialysis (Ramsey and Fitzpatrick 2000). All protein purification steps were carried out at 4°C. Purified enzymes were stored at –80°C. Typical yields were 150–200 mg of pure protein from 6 L of culture.
Assays
The iron contents of the purified enzymes were determined by atomic absorption spectrophotometry as previously described for the rat enzyme (Ramsey et al. 1996). Concentrations of the purified proteins were determined using an A2801% value of 10.4 (Haavik et al. 1988). A colorimetric end point assay for DOPA was used to measure tyrosine hydroxylase activity (Fitzpatrick 1991). Assays were carried out in 100 μg/mL catalase, 1 mM dithiothreitol, 10 μM ferrous ammonium sulfate, at 32°C. Standard conditions include 0.5 μM enzyme, 400 μM 6MPH4 and 200 μM tyrosine in 50 mM HEPES, pH 7.0. For determination of the Km value for tyrosine, assays contained 400 μM 6MPH4 and 10–360 μM tyrosine. For determination of the Km value for 6MPH4, assays contained 200 μM tyrosine and 10–360 μM 6MPH4. 6-Methyltetrahydropterin was used as substrate because the substrate inhibition seen with tyrosine in this case is much less than when tetrahydrobiopterin is used, allowing intrinsic kinetic parameters to be more readily measured (Fitzpatrick 1991). To obtain the kinetic parameters in Table 2, the kinetic data were fit to the Michaelis–Menten equation using the program KaleidaGraph (Synergy Software, Reading, PA, USA).
Table 2.
Steady state kinetic parameters of human tyrosine hydroxylase isoformsa
| Enzyme | Ktyr (μM)b | K6MPH4 (μM)c | Vmax (min−1) |
|---|---|---|---|
| hTyrH1 | 77 ± 10 | 116 ± 10 | 135 ± 7 |
| hTyrH2 | 66 ± 5 | 75 ± 6 | 100 ± 2 |
| hTyrH3 | 79 ± 9 | 70 ± 9 | 119 ± 5 |
| hTyrH4 | 73 ± 6 | 63 ± 5 | 108 ± 3 |
Conditions: 100 μg/mL catalase, 1 mM dithiothreitol, 10 μM ferrous ammonium sulfate, 50 mM HEPES, pH 7.0, 32°C.
400 μM 6MPH4.
200 μM tyrosine.
Phosphorylation of tyrosine hydroxylase
The method for phosphorylation of human tyrosine hydroxylase was based on that developed for the rat enzyme (Ramsey and Fitzpatrick 1998). Each TyrH isoform (20 μM) was incubated with 60 μM ATP, 6 mM MgCl2, and 100 nM PKA catalytic subunit in 100 mM KCl, 10% glycerol, 50 mM HEPES, pH 7.0, at 4°C. After 2 h, additional ATP, MgCl2 and PKA were added to yield final concentrations of 125 μM, 12.5 mM, and 200 nM, respectively; the reaction was continued for 6 h. The phosphorylated enzyme was isolated on a MonoQ column using a gradient of 0–500 mM NaCl in the same buffer. The phosphorylation status was confirmed by incubating a sample of phosphorylated enzyme eluted from the MonoQ column with 15 mM MgCl2 , 88 nM PKA, 560 μM [γ-32P]ATP (1.97 × 105 cpm/nmol) in 10% glycerol, 50 mM HEPES, pH 7.0, at room temperature. Aliquots were removed at 5 min intervals for 15 min and spotted onto phosphocellulose filter paper; these were washed twice with 75 mM phosphoric acid and once with acetone. The amount of radioactivity remaining on the filters was determined by scintillation counting. In all cases the previously phosphorylated enzymes incorporated less than 0.02 nmol phosphate/nmol TyrH; unphosphorylated enzyme incorporated a stoichiometric amount of phosphate under these conditions.
Catecholamine binding
Catecholamine binding studies were performed by using previously described procedures (Ramsey and Fitzpatrick 1998). To determine the rate constants for dissociation of DOPA and dopamine, 40 μM unphosphorylated or phosphorylated TyrH was incubated with 60 μM DOPA or dopamine in 100 mM KCl, 10% glycerol, 50 mM HEPES, pH 7.0, at 10°C. Formation of the enzyme–catecholamine complex was monitored by observing the increase in absorbance at 690 nm (Haavik et al. 1991). After formation of the catecholamine complex was complete, 2,3-dihydroxynaphthalene (DHN) was added to give a final concentration of 1 mM; the increase in absorbance at 550 nm due to formation of the enzyme–DHN complex was then monitored. The dissociation rate constant was calculated by fitting the absorbance versus time to equation 1.
| (1) |
To determine association rate constants, 125 μM to 2 mM DOPA or dopamine was mixed with 15 μM (final concentrations) tyrosine hydroxylase in an Applied Photophysics SX-18MV stopped flow spectrophotometer. The increase in absorbance at 690 nm was monitored and the resulting data fit to equation 1.
Results
Characterization of human tyrosine hydroxylase isoforms
Plasmids were constructed for expression of all four human TyrH isoforms in E. coli by starting with the cDNA for hTyrH2 . All four proteins expressed well and could be purified using the protocol developed for the rat enzyme. The specific activities of the purified hTyrH isoforms were comparable: 1.8 μmol/(min mg) (hTyrH1), 1.2 μmol/(min mg) (hTyrH2), 1.3 μmol/(min mg) (hTyrH3) and 1.5 μmol/(min mg) (hTyrH4). After incubation with a slight excess of ferrous ammonium sulfate and removal of excess iron, each isoform contained one atom of iron per monomer. The steady state kinetic parameters with tyrosine and 6MPH4 as substrates were measured for each isoform and are summarized in Table 2. The Vmax and Ktyr values are essentially the same among all four isoforms. The Km values for 6MPH4 for isoforms 2–4 are similar, whereas that for hTyrH1 is slightly larger.
Catecholamine binding
Previous analyses of rat TyrH have shown that phosphorylation has a large effect on the binding of catecholamines to the ferric form of the enzyme (Ramsey and Fitzpatrick 1998). The rate constants for dissociation and association of DOPA and dopamine were determined for all four human isoforms by monitoring the formation of the broad charge-transfer absorbance band centered at 690 nm due to the complex. In the case of complex formation, the rate constants were determined by mixing the enzyme with various concentrations of DOPA or dopamine (data not shown). The observed rate constants for binding derived from these analyses show a linear dependence on the concentration of DOPA or dopamine, as illustrated in Fig. 2 for DOPA binding to unphosphorylated hTyrH1 and hTyrH4. This behavior is consistent with a simple bimolecular binding reaction. The second order rate constants for binding of DOPA or dopamine to each of the isoforms could be determined from the slopes of plots such as those in Fig. 2. The resulting values are summarized in Tables 3 and 4. The values decrease slightly as one proceeds from isoform 1–4, with dopamine binding exhibiting slightly higher association rate constants overall than DOPA.
Fig. 2.
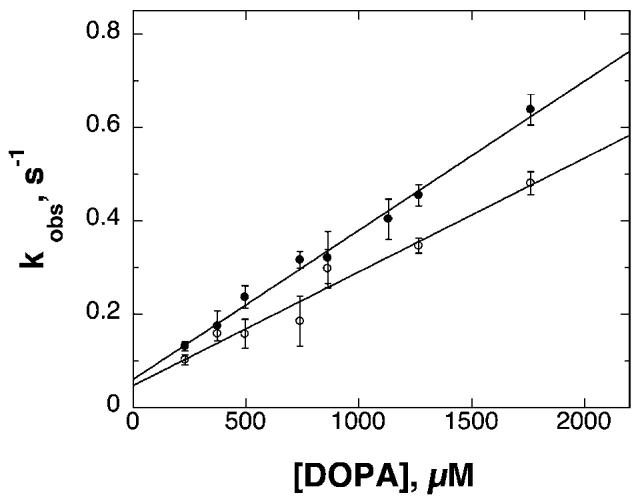
Concentration dependence of DOPA binding to hTyrH1 (filled circles) and hTyrH4 (open circles). The conditions were as described for Table 2.
Table 3.
Kinetic parameters for DOPA binding to human tyrosine hydroxylase isoforms
| Unphosphorylated enzyme |
Phosphorylated enzyme |
|||||
|---|---|---|---|---|---|---|
| Isoform | kona (mM−1 min−1) | koffb (min−1) | Kdc (μM) | kona (mM−1 min−1) | koffb (min−1) | Kdc (μM) |
| hTyrH1 | 18.6 ± 0.6 | 0.082 ± 0.001 | 4.45 ± 0.16 | 61.2 ± 2.4 | 0.453 ± 0.012 | 7.41 ± 0.36 |
| hTyrH2 | 17.4 ± 1.2 | 0.078 ± 0.001 | 4.52 ± 0.32 | 51.6 ± 3.0 | 0.334 ± 0.005 | 6.48 ± 0.40 |
| hTyrH3 | 10.8 ± 0.6 | 0.034 ± 0.001 | 3.17 ± 0.21 | 40.2 ± 1.8 | 0.235 ± 0.015 | 5.87 ± 0.47 |
| hTyrH4 | 14.4 ± 1.2 | 0.060 ± 0.001 | 4.21 ± 0.36 | 35.4 ± 1.2 | 0.142 ± 0.002 | 4.02 ± 0.16 |
| Rat TyrHd | 39.0 ± 3.6 | 0.102 ± 0.006 | 2.70 ± 0.29 | 66.0 ± 4.8 | 2.76 ± 0.54 | 41.7 ± 13.9 |
Conditions: 10% glycerol, 100 mM KCl, 50 mM HEPES, pH 7, 10°C.
Conditions: 1 mM DHN, 60 μm DOPA, 10% glycerol, 100 mM KCl, 0.2 mM DTPA, 50 mM HEPES, pH 7, 10°C.
Calculated from the corresponding kon and koff values.
From reference (McCulloch et al. 2001), determined at 15°C.
Table 4.
Dopamine binding to human tyrosine hydroxylase isoforms
| Unphosphorylated enzyme |
Phosphorylated enzyme |
|||||
|---|---|---|---|---|---|---|
| Isoform | kona (mM−1 min−1) | koffb (min−1) | Kdc (nM) | kona (mM−1 min−1) | koffb (min−1) | Kdc (μM) |
| hTyrH1 | 66.6 ± 3.0 | < 0.00015 | < 2.2 | 172 ± 7 | 0.024 ± 0.002 | 143 ± 15 |
| hTyrH2 | 43.2 ± 2.4 | < 0.00015 | < 3.5 | 198 ± 7 | 0.016 ± 0.001 | 82 ± 4 |
| hTyrH3 | 48.6 ± 2.4 | < 0.00015 | < 3.1 | 148 ± 7 | 0.012 ± 0.001 | 85 ± 9 |
| hTyrH4 | 55.2 ± 1.8 | < 0.00015 | < 2.7 | 145 ± 4 | 0.011 ± 0.001 | 78 ± 4 |
| rat TyrHc | 312 ± 18 | 0.000041 ± 0.000004 | 0.13 ± 0.02 | 474 ± 2 | 0.10 ± 0.002 | 208 ± 20 |
Conditions: 10% glycerol, 100 mM KCl, 50 mM HEPES, pH 7, 10°C.
Conditions: 1 mM DHN, 60 μm dopamine, 10% glycerol, 100 mM KCl, 0.2 mM DTPA, 50 mM HEPES, pH 7, 10°C.
From reference (McCulloch et al. 2001); determined at 15°C.
Although the rate constants for dissociation of catecholamines from TyrH can in principle be determined from the y-intercepts of plots such as those in Fig. 2, the precision of the resulting values is low, especially in the case of dopamine. Consequently, the dissociation rate constants were measured directly. This was done by using DHN to displace the bound DOPA or dopamine, taking advantage of the spectral differences between the complexes with DHN, which exhibit maximal absorbance at 550 nm, and those with catecholamines, with maximal absorbance at 690 nm. Each hTyrH isoform (30 μM) was incubated with 60 μM DOPA or dopamine until binding was complete, as indicated by no further spectral changes. DHN was then added to give a final concentration of 1 mM; this concentration is sufficiently high that all of the catecholamine is displaced by the end of the reaction, and the binding of DHN is much more rapid than the dissociation of the catecholamine (Ramsey and Fitzpatrick 1998). Under these conditions, the rate constant for the formation of the DHN complex is equal to the rate constant for dissociation of the bound catecholamine. The spectral changes that occur when this is done with DOPA and the unphosphorylated enzymes are illustrated in Fig. 3a for hTyrH4. DHN could similarly displace DOPA from the other isoforms. The first order rate constants for dissociation of DOPA from the unphosphorylated isoforms determined in this fashion are summarized in Table 3.
Fig. 3.
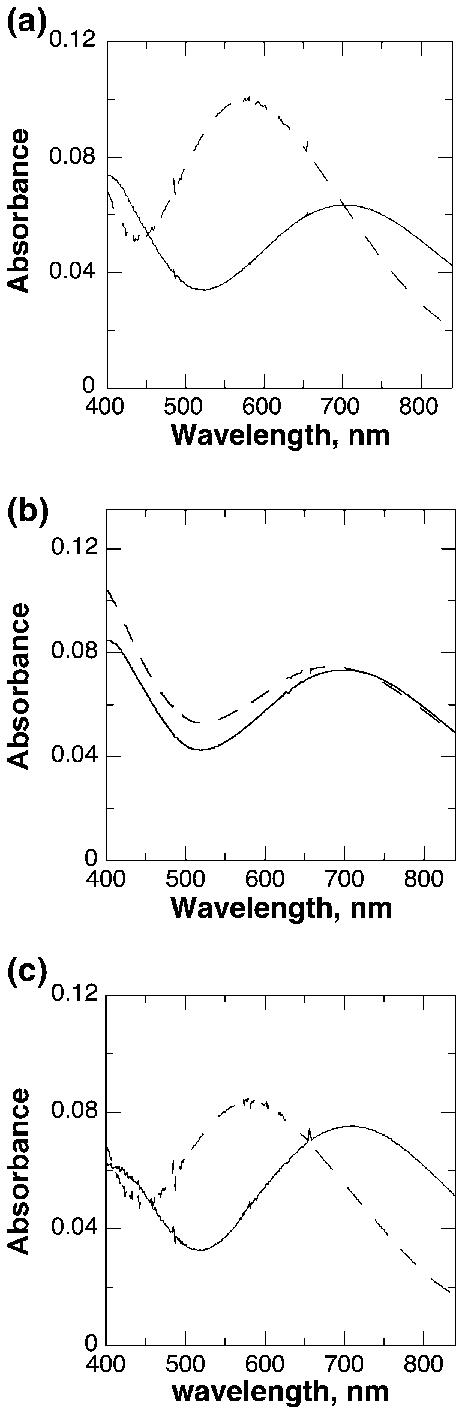
Spectral changes during displacement by DHN of DOPA (a) or dopamine (b and c) from unphosphorylated (a and b) and phosphorylated (c) hTyrH4. Enzyme (30 μM) was incubated with 60 μM L-DOPA or dopamine for 15 min in 10% glycerol, 100 mM KCl, 0.2 mM DTPA, 50 mM HEPES, pH 7, at 10°C, before the spectra indicated by solid lines were taken. DHN was then added to give a final concentration of 1 mM; the spectra indicated by dashed lines were taken after 2.8 h (a) or 12.5 h (b and c).
In contrast to the results with DOPA, with dopamine there was no evidence for significant displacement of dopamine by DHN from any of the unphosphorylated isoforms, even after 12 h. There was a slight increase in absorbance at 550 nm, but no accompanying decrease at long wavelength (Fig. 3b). At times longer than 12 h significant protein precipitation was seen. As a result it was not possible to determine the rate constant for dissociation of dopamine from any of the unphosphorylated isoforms. If one assumes that the absorbance change seen in Fig. 3(b) is completely due to replacement of dopamine by DHN and that the spectral changes are identical to those seen with the phosphorylated enzyme (see below), less than 10% of the dopamine is displaced by DHN after 12 h in the experiment illustrated in Fig. 3(b). This places an upper limit on the dissociation rate constant of 0.00015 min−1.
Effect of phosphorylation by protein kinase A on catecholamine binding to human TyrH
Each of the TyrH isoforms was phosphorylated with PKA, which is specific for the serine corresponding to Ser40 of hTyrH1 (Fig. 1), followed by anion exchange chromatography to remove components of the phosphorylation reaction. When [γ32P]ATP was used for the reaction, all four hTyrH isoforms were found to be phosphorylated to 0.9 ± 0.2 mol phosphate/mol enzyme. In addition, no further phosphate could be incorporated into the phosphorylated enzymes by further treatment with PKA and ATP, consistent with stoichiometric phosphorylation. Using the phosphorylated isoforms, the binding and dissociation rate constants for DOPA and dopamine were determined as described above for the unphosphorylated enzymes; the results are summarized in Tables 3 and 4 and illustrated in Fig. 4. In the case of DOPA binding, phosphorylation increases the dissociation rate constant by 2.5–5-fold and increases the association rate constant by 3–4-fold. The increase in the association rate constant is comparable for each isoform, although the absolute magnitude of the value decreases as the size of the regulatory domain increases from hTyrH1 to hTyrH4. As a result of the comparable increases in the association and dissociation rate constants, the change in the Kd value for DOPA varies from an increase of slightly less than two-fold for hTyrH1 to no effect for hTyrH4. Table 3 also shows the much larger increase seen with the rat enzyme (McCulloch et al. 2001).
Fig. 4.
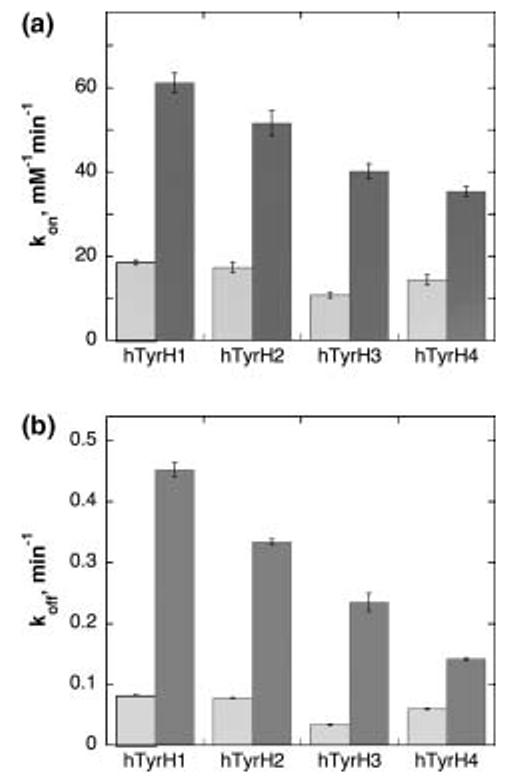
Effects of phosphorylation (solid bars) on the rate constants for association (a) and dissociation (b) of DOPA from TyrH isoforms. The conditions were as described for Fig. 3.
In contrast to the very small effect of phosphorylation on the affinity of the human isoforms for DOPA, there is a large effect on the binding of dopamine (Table 4). As was the case with DOPA, phosphorylation increases the association rate constants for all four isoforms by 3–4-fold. Although no significant displacement of dopamine from the unphosphorylated enzyme could be seen upon incubation with DHN after 12 h, DHN was able to completely displace dopamine from the phosphorylated enzymes over the same time period (Fig. 3c). This reflects an increase of at least two orders of magnitude in the dissociation rate constants for dopamine upon phosphorylation. Again, the effect decreases as one progresses from hTyrH1 to hTyrH4. The Kd values for dopamine binding to all four phosphorylated human isoforms are comparable to that for the rat enzyme (McCulloch et al. 2001).
Discussion
The results presented here establish that phosphorylation of human TyrH decreases the affinity of the ferric enzyme for catecholamines and thereby extend the regulatory model developed for the rat enzyme. Still, the human and rat enzymes do show qualitative differences in their regulatory properties, and the four isoforms of human TyrH differ quantitatively in the effects of PKA phosphorylation.
The steady state kinetic parameters of the four human isoforms reported here do not differ substantially from one another, indicating that the different insertions after Met30 of hTyrH do not markedly affect catalysis. The Vmax values for the different isoforms are comparable and are similar to that of the rat enzyme (Fitzpatrick 1991), whereas hTyrH1 has a slightly higher Km value for 6MPH4 than the other three isoforms. A similar difference in Km values has been reported using tetrahydrobiopterin as substrate for the human isoforms (Le Bourdellès et al. 1991). However, when the latter pterin is used, there is substantial substrate inhibition (Le Bourdellès et al. 1991); this precludes measurement of actual Vmaxvalues with the physiological substrate, a problem not encountered with 6MPH4.
Phosphorylation by PKA of Ser40 in the regulatory domain of rat TyrH greatly decreases the affinity of that enzyme for catecholamines (Ramsey and Fitzpatrick 1998, 2000). The largest effect is on the affinities for dopamine, norepinephrine, and epinephrine,1 with a smaller effect on the affinity for the product of the TyrH reaction, DOPA. There is no structure available for the regulatory domain of TyrH from any source which would provide insight into the structural basis for this effect. However, there is evidence that phosphorylation alters the structure of the regulatory domain and the interaction between the regulatory and catalytic domains. Phosphorylation increases the sensitivity to trypsin of the residues surrounding Ser40 in the rat enzyme, whereas dopamine has the opposite effect (McCulloch and Fitzpatrick 1999), demonstrating that the conformation of this region is sensitive to the regulatory status of the enzyme. The effect of dopamine, which binds in the active site (Michaud-Soret et al. 1995; Erlandsen et al. 1998), on the conformation of the regulatory domain suggests that this region of the regulatory domain is close to the active site. The structure of the combined regulatory and catalytic domains of the related enzyme phenylalanine hydroxylase (Kobe et al. 1999), which shows that the N-terminus of the regulatory domain physically blocks the active site, supports such a model. The insertions found in hTyrH2, hTyrH3 and hTyrH4 are after Met30 and thus immediately precede the phosphorylation sensitive region, providing a link between the structural differences among the human isoforms and any differences in their response to phosphorylation.
Clearly, dopamine binds very tightly to all four human isoforms. In the case of the unphosphorylated enzyme, dopamine binding is effectively irreversible. For all four isoforms, phosphorylation results in an increase in the rate constants for dopamine dissociation of at least two orders of magnitude, with much smaller increases in the rate constants for association. Qualitatively this resembles the effects of phosphorylation of the rat enzyme, demonstrating that the regulatory model developed with rat TyrH (Scheme 1) also applies to the human isoforms. In addition, there are quantitative differences in the binding of dopamine to the different phosphorylated isoforms. There is a small decrease in the association rate constant as one progresses from hTyrH1 to hTyrH4 for both the unphosphorylated and phosphorylated enzymes. A more significant effect is seen on the dissociation rate constants for the phosphorylated enzymes, which decrease as the size of the insertion after Met30 increases. As a result of these two trends, phosphorylated hTyrH1 has a significantly lower affinity for dopamine than the other three isoforms. Whether the same trend is seen with the unphosphorylated enzyme cannot be determined from the present data, although the results with DOPA (vide infra) suggest that this is indeed the case. However, the data do establish that the effect of phosphorylation on dopamine binding is attenuated by the insertions.
The effects of phosphorylation on binding of DOPA to the human isoforms differ significantly from the effects on the binding of dopamine. The DOPA association and dissociation rate constants both decrease slightly as the size of the insertion increases from hTyrH1 to hTyrH4, resulting in the Kd values for DOPA binding to the unphosphorylated enzymes being essentially identical for all four isoforms and close to the value for the rat enzyme. However, phosphorylation by PKA has little to no effect on the affinity of human TyrH for DOPA. The association rate constants for all four isoforms increase about four-fold upon phosphorylation; this is greater than the increase seen with the rat enzyme (Ramsey and Fitzpatrick 1998), but comparable to the change in the association rate constants for dopamine binding to the human enzymes. The increases upon phosphorylation in the rate constants for DOPA dissociation from the human isoforms are much less than is seen for rat TyrH. As was the case for dopamine binding, the insertions attenuate the effects of phosphorylation, in that both the association and dissociation rate constants for DOPA show smaller increases upon phosphorylation as the size of the insertion increases. Because both rate constants increase by comparable amounts, the Kd values increase less than two-fold when hTyrH1 is phosphorylated. The effect of phosphorylation on the Kd value decreases as the size of the insertion increases, to the point where the value for hTyrH4shows no change upon phosphorylation. In contrast, upon phosphorylation of rat TyrH the Kd value for DOPA increases about 20-fold.
The lack of a substantial change in the affinity of hTyrH for DOPA upon phosphorylation suggests that inhibition of the human enzyme by DOPA is not important for regulation in vivo, whereas inhibition by dopamine is. This observation would be consistent with physiological levels of the two inhibitors. The concentration of DOPA in brain and adrenal tissues is quite low, well below μM, due to its rapid conversion to dopamine by DOPA decarboxylase (Carlsson et al. 1972; Kehr et al. 1972; Wagner et al. 1979; Buu et al. 1985). Thus, the Kd for DOPA for even unphosphorylated hTyrH is likely to be much higher than the physiological concentration. This suggests that even unphosphorylated TyrH is not significantly inhibited by DOPA in the cell. In contrast, the average concentration of dopamine in brain and adrenal tissue is 5–10 μM (Carlsson et al. 1972; Wagner et al. 1979; Buu et al. 1985); the concentration in the cytosol is undoubtedly much lower, since most of the dopamine is inside secretory vesicles (DeRobertis 1967; Serck-Hanssen and Helle 1972; Kirshner 1974). The much lower Kd value for dopamine for the phosphorylated isoforms is consistent with the much lower level of catecholamines in the cytosol. Moreover, when TyrH is isolated from bovine adrenal or rat pheochromocytoma cells, the enzyme has a combination of dopamine, epinephrine, and norepinephrine bound, but no DOPA (Haavik et al. 1988; Andersson et al. 1992). This lack of DOPA in enzyme isolated from physiological sources is consistent with it not being a physiologically important inhibitor.
In conclusion, the data presented here show that the regulatory model developed for rat TyrH also applies to all four human isoforms. The human isoforms differ from the rat enzyme in the lack of an effect of phosphorylation on DOPA binding. In addition, the insertions in the regulatory domain that differentiate the four human isoforms do affect their regulatory properties.
Acknowledgements
We thank Dr Karen O'Malley for the generous gift of plasmid pHTh-63. This work was supported in part by NIH grant GM 47291 to PFF and a grant from the National Parkinson Foundation— Parkinson's Disease Foundation Joint Research Grant Program to SCD.
Abbreviations used
- DHN
2,3-dihydroxynaphthalene
- DOPA
dihydroxyphenylalanine
- DTPA
diethylenetriaminepentaacetic acid
- ERK
extracellular signal-regulated kinase
- hTyrH1, hTyrH2, hTyrH3, hTyrH4
isoforms 1–4 of human tyrosine hydroxylase
- MAP kinase
mitogen-activated protein kinase
- 6MPH4
6-methyltetrahydropterin
- PKA
cAMP-dependent protein kinase A
- TyrH
tyrosine hydroxylase
Footnotes
For rat TyrH, the affinities for dopamine, norepinephrine, and epinephrine are virtually identical, as is the effect of phosphorylation upon the affinities (Ramsey and Fitzpatrick 2000).
References
- Alterio J, Ravassard P, Haavik J, Le Caer J, Biguet N, Waksman G, Mallet J. Human tyrosine hydroxylase isoforms. Inhibition by excess tetrahydropterin and unusual behavior of isoform 3 after cAMP-dependent protein kinase phosphorylation. J. Biol. Chem. 1998;273:10196–10201. doi: 10.1074/jbc.273.17.10196. [DOI] [PubMed] [Google Scholar]
- Andersson KK, Vassort C, Brennan BA, Que L, Jr, Haavik J, Flatmark T, Gros F, Thibault J. Purification and characterization of the blue-green rat phaeochromocytoma (PC12) tyrosine hydroxylase with a dopamine-Fe (III) complex reversal of the endogenous feedback inhibition by phosphorylation of serine-40. Biochem. J. 1992;284:687–695. doi: 10.1042/bj2840687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boularand S, Darmon MC, Mallet J. The human tryptophan hydroxylase gene an unusual spicing complexity in the 5′-untranslated region. J. Biol. Chem. 1995;270:3748–3756. doi: 10.1074/jbc.270.8.3748. [DOI] [PubMed] [Google Scholar]
- Brown ER, Coker GT, III, O'Malley KL. Organization and evolution of the rat tyrosine hydroxylase gene. Biochemistry. 1987;26:5208–5212. doi: 10.1021/bi00390a046. [DOI] [PubMed] [Google Scholar]
- Buu NT, Duhaime J, Kuchel O. Effects of L-DOPA on the concentrations of free and sulfoconjugated catecholamines in plasma, cerebrospinal fluid, urine, and central and peripheral nervous system tissues of the rat. J. Neurochem. 1985;44:787–792. doi: 10.1111/j.1471-4159.1985.tb12884.x. [DOI] [PubMed] [Google Scholar]
- Campbell DG, Hardie DG, Vulliet PR. Identification of four phosphorylation sites in the N-terminal region of tyrosine hydroxylase. J. Biol. Chem. 1986;261:10489–10492. [PubMed] [Google Scholar]
- Carlsson A, Davis JN, Kehr W, Lindqvist M, Atack CV. Simultaneous measurement of tyrosine and tryptophan hydroxylase activities in brain in vivo using an inhibitor of the aromatic amino acid decarboxylase. Naunyn Schmiedeberg's Arch. Pharmacol. 1972;275:153–168. doi: 10.1007/BF00508904. [DOI] [PubMed] [Google Scholar]
- Daly J, Levitt M, Guroff G, Udenfriend S. Isotope studies on the mechanism of action of adrenal tyrosine hydroxylase. Arch. Biochem. Biophys. 1968;126:593–598. doi: 10.1016/0003-9861(68)90446-3. [DOI] [PubMed] [Google Scholar]
- Daubner SC, Lauriano C, Haycock JW, Fitzpatrick PF. Site-directed mutagenesis of Serine 40 of rat tyrosine hydroxylase. Effects of dopamine and cAMP-dependent phosphorylation on enzyme activity. J. Biol. Chem. 1992;267:12639–12646. [PubMed] [Google Scholar]
- DeRobertis E. Ultrastructure and cytochemistry of the synaptic region. Science. 1967;156:907–914. doi: 10.1126/science.156.3777.907. [DOI] [PubMed] [Google Scholar]
- Erlandsen H, Flatmark T, Stevens RC, Hough E. Crystallographic analysis of the human phenylalanine hydroxylase catalytic domain with bound catechol inhibitors at 2.0 Å resolution. Biochemistry. 1998;37:15638–15646. doi: 10.1021/bi9815290. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick PF. The metal requirement of rat tyrosine hydroxylase. Biochem. Biophys. Res. Commun. 1989;161:211–215. doi: 10.1016/0006-291x(89)91582-9. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick PF. The steady state kinetic mechanism of rat tyro-sine hydroxylase. Biochemistry. 1991;30:3658–3662. doi: 10.1021/bi00229a010. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick PF. The tetrahydropterin-dependent amino acid hydroxylases. Annu. Rev. Biochem. 1999;68:355–381. doi: 10.1146/annurev.biochem.68.1.355. [DOI] [PubMed] [Google Scholar]
- Flockhart DA, Corbin JD. In: Preparation of the Catalytic Subunit of Camp-Dependent Protein Kinase, in Brain Receptor Methodologies, Part A. Maranos PJ, Campbell IC, Cohen RM, editors. Academic Press; New York: 1984. pp. 209–215. [Google Scholar]
- Goodwill KE, Sabatier C, Marks C, Raag R, Fitzpatrick PF, Stevens RC. Crystal structure of tyrosine hydroxylase at 2.3 Å and its implications for inherited diseases. Nat. Struct. Biol. 1997;4:578–585. doi: 10.1038/nsb0797-578. [DOI] [PubMed] [Google Scholar]
- Grenett HE, Ledley FD, Reed LL, Woo SLC. Full-length cDNA for rabbit tryptophan hydroxylase: Functional domains and evolution of aromatic amino acid hydroxylases. Proc. Natl Acad. Sci. USA. 1987;84:5530–5534. doi: 10.1073/pnas.84.16.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B, Lamouroux A, Boni C, Julien J-F, Javoy-Agid F, Mallet J. A single human gene encoding multiple tyrosine hydroxylases with different predicted functional characteristics. Nature. 1987;326:707–711. doi: 10.1038/326707a0. [DOI] [PubMed] [Google Scholar]
- Haavik J, Andersson KK, Petersson L, Flatmark T. Soluble tyrosine hydroxylase (tyrosine 3-monooxygenase) from bovine adrenal medulla: Large-scale purification and physicochemical properties. Biochim. Biophys. Acta. 1988;953:142–156. doi: 10.1016/0167-4838(88)90019-2. [DOI] [PubMed] [Google Scholar]
- Haavik J, Le Bourdelles B, Martinez A, Flatmark T, Mallet J. Recombinant human tyrosine hydroxylase isozymes. Reconstitution with iron and inhibitory effect of other metal ions. Eur. J. Biochem. 1991;19:371–378. doi: 10.1111/j.1432-1033.1991.tb16133.x. [DOI] [PubMed] [Google Scholar]
- Haycock JW. Phosphorylation of tyrosine hydroxylase in situ at serine 8, 19, 31, and 40. J. Biol. Chem. 1990;265:11682–11691. [PubMed] [Google Scholar]
- Haycock JW, Ahn NG, Cobb MH, Krebs EG. ERK1 and ERK2, two microtubule-associated protein 2 kinases, mediate the phosphorylation of tyrosine hydroxylase at serine-31 in situ. Proc. Natl Acad. Sci. USA. 1992;89:2365–2369. doi: 10.1073/pnas.89.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda N, Kobayashi K, Ichinose H, Kishi F, Nakazawa A, Kurosawa Y, Fujita K, Nagatsu T. Isolation of a novel cDNA clone for human tyrosine hydroxylase: Alternative RNA splicing produces four kinds of mRNA from a single gene. Biochem. Biophys. Res. Commun. 1987;147:971–975. doi: 10.1016/0006-291x(87)90742-x. [DOI] [PubMed] [Google Scholar]
- Kehr W, Carlsson A, Lindqvist M. A method for the determination of 3,4-dihydroxyphenylalanine (DOPA) in brain. Naunyn Schmiedeberg's Arch. Pharmacol. 1972;274:273–280. doi: 10.1007/BF00501936. [DOI] [PubMed] [Google Scholar]
- Kirshner N. Function and organization of chromaffin vesicle. Life Sci. 1974;14:1153–1167. doi: 10.1016/0024-3205(74)90424-x. [DOI] [PubMed] [Google Scholar]
- Kobe B, Jennings IG, House CM, Michell BJ, Goodwill KE, Santarsiero BD, Stevens RC, Cotton RGH, Kemp BE. Structural basis of intrasteric and allosteric controls of phenylalanine hydroxylase. Nat. Struct. Biol. 1999;6:442–448. doi: 10.1038/8247. [DOI] [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J. Neurochem. 1996;67:443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Le Bourdellès B, Horellou P, Le Caer J-P, Denèfle P, Latta M, Haavik J, Guibert B, Mayaux J-F, Mallet J. Phosphorylation of human recombinant tyrosine hydroxylase isoforms 1 and 2: An additional phosphorylated residue in isoform 2, generated through alternative splicing. J. Biol. Chem. 1991;266:17124–17130. [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Haycock JW. Four isoforms of tyrosine hydroxylase are expressed in human brain. Neuroscience. 1993;54:477–492. doi: 10.1016/0306-4522(93)90267-j. [DOI] [PubMed] [Google Scholar]
- McCulloch RI, Fitzpatrick PF. Limited proteolysis of tyrosine hydroxylase identifies residues 33–50 as conformationally sensitive to phosphorylation state and dopamine binding. Arch. Biochem. Biophys. 1999;367:143–145. doi: 10.1006/abbi.1999.1259. [DOI] [PubMed] [Google Scholar]
- McCulloch RI, Daubner SC, Fitzpatrick PF. Effects of substitution at serine 40 of tyrosine hydroxylase on catecholamine binding. Biochemistry. 2001;40:7273–7278. doi: 10.1021/bi010546d. [DOI] [PubMed] [Google Scholar]
- Michaud-Soret I, Andersson KK, Que L, Jr, Haavik J. Resonance Raman studies of catecholate and phenolate complexes of recombinant human tyrosine hydroxylase. Biochemistry. 1995;34:5504–5510. doi: 10.1021/bi00016a022. [DOI] [PubMed] [Google Scholar]
- O'Malley KL, Anhalt MJ, Martin BM, Kelsoe JR, Winfield SL, Ginns EI. Isolation and characterization of the human tyrosine hydroxylase gene: Identification of 5′-alternative splice sites responsible for multiple mRNAs. Biochemistry. 1987;26:6910–6914. doi: 10.1021/bi00396a007. [DOI] [PubMed] [Google Scholar]
- Ramsey AJ, Fitzpatrick PF. Effects of phosphorylation of serine 40 of tyrosine hydroxylase on binding of catecholamines: Evidence for a novel regulatory mechanism. Biochemistry. 1998;37:8980–8986. doi: 10.1021/bi980582l. [DOI] [PubMed] [Google Scholar]
- Ramsey AJ, Fitzpatrick PF. Effects of phosphorylation on binding of catecholamines to tyrosine hydroxylase: specificity and thermodynamics. Biochemistry. 2000;39:773–778. doi: 10.1021/bi991901r. [DOI] [PubMed] [Google Scholar]
- Ramsey AJ, Hillas PJ, Fitzpatrick PF. Characterization of the active site iron in tyrosine hydroxylase: Redox states of the iron. J. Biol. Chem. 1996;271:24395–24400. doi: 10.1074/jbc.271.40.24395. [DOI] [PubMed] [Google Scholar]
- Royo M, Daubner SC, Fitzpatrick PF. Specificity of the MAP kinase ERK2 for phosphorylation of tyrosine hydroxylase. Arch. Biochem. Biophys. 2004;423:247–252. doi: 10.1016/j.abb.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Serck-Hanssen G, Helle KB. Biochemical and morphological characterization of chromaffin granules accumulating during in vitro secretion from perfused adrenal glands. Biochim. Biophys. Acta. 1972;273:199–207. doi: 10.1016/0304-4165(72)90207-3. [DOI] [PubMed] [Google Scholar]
- Sutherland C, Alterio J, Campbell DG, Le Bourdelles B, Mallet J, Haavik J, Cohen P. Phosphorylation and activation of human tyrosine hydroxylase in vitro by mitogen-activated protein (MAP) kinase and MAP-kinase-activated kinases 1 and 2. Eur. J. Biochem. 1993;217:715–722. doi: 10.1111/j.1432-1033.1993.tb18297.x. [DOI] [PubMed] [Google Scholar]
- Wagner J, Palfreyman M, Zraika M. Determination of dopa, dopamine, dopac, epinephrine, norepinephrine, α-monofluoromethyldopa and α-difluoromethyldopa in various tissues of mice and rats using reversed-phase ion-pair liquid chromatography with electrochemical detection. J. Chromatogr. 1979;164:41–54. doi: 10.1016/s0378-4347(00)81570-4. [DOI] [PubMed] [Google Scholar]
- Zigmond RE, Schwarzschild MA, Rittenhouse AR. Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Ann. Rev. Neurosci. 1989;12:415–461. doi: 10.1146/annurev.ne.12.030189.002215. [DOI] [PubMed] [Google Scholar]