Abstract
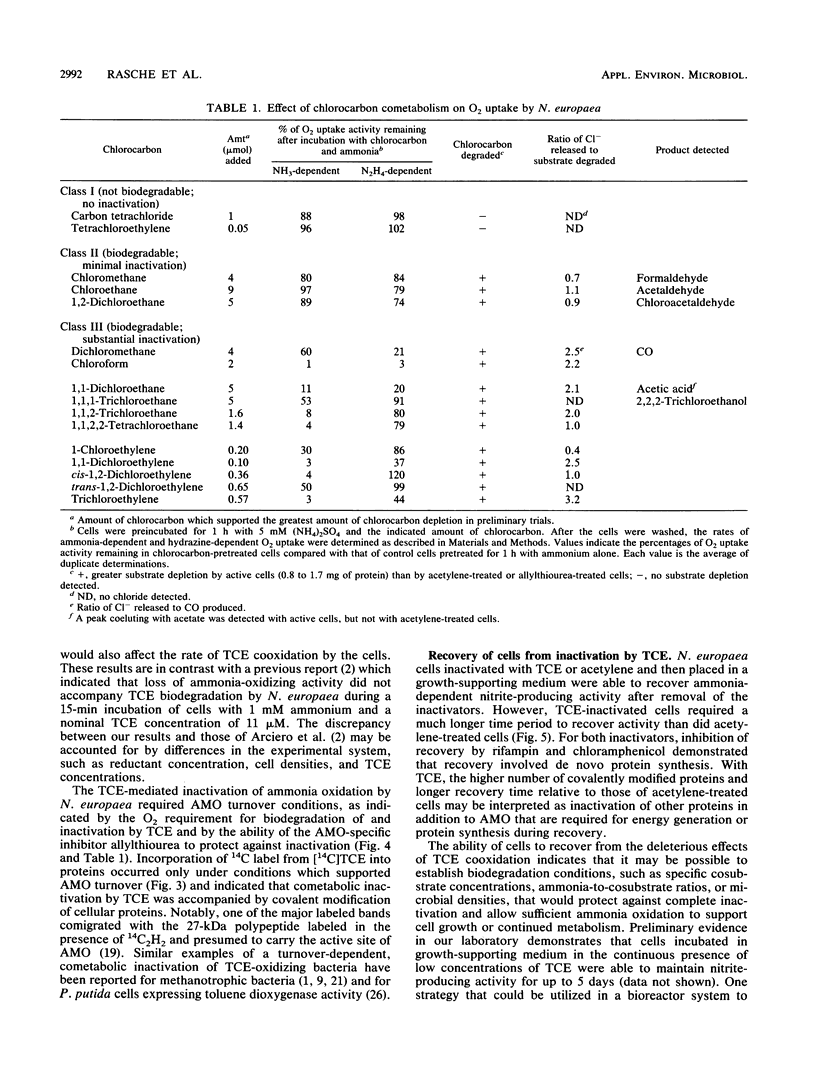
The soil nitrifying bacterium Nitrosomonas europaea is capable of degrading trichloroethylene (TCE) and other halogenated hydrocarbons. TCE cometabolism by N. europaea resulted in an irreversible loss of TCE biodegradative capacity, ammonia-oxidizing activity, and ammonia-dependent O2 uptake by the cells. Inactivation was not observed in the presence of allylthiourea, a specific inhibitor of the enzyme ammonia monooxygenase, or under anaerobic conditions, indicating that the TCE-mediated inactivation required ammonia monooxygenase activity. When N. europaea cells were incubated with [14C]TCE under conditions which allowed turnover of ammonia monooxygenase, a number of cellular proteins were covalently labeled with 14C. Treatment of cells with allylthiourea or acetylene prior to incubation with [14C]TCE prevented incorporation of 14C into proteins. The ammonia-oxidizing activity of cells inactivated in the presence of TCE could be recovered through a process requiring de novo protein synthesis. In addition to TCE, a series of chlorinated methanes, ethanes, and other ethylenes were screened as substrates for ammonia monooxygenase and for their ability to inactivate the ammonia-oxidizing system of N. europaea. The chlorocarbons could be divided into three classes depending on their biodegradability and inactivating potential: (i) compounds which were not biodegradable by N. europaea and which had no toxic effect on the cells; (ii) compounds which were cooxidized by N. europaea and had little or no toxic effect on the cells; and (iii) compounds which were cooxidized and produced a turnover-dependent inactivation of ammonia oxidation by N. europaea.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Cohen L., McCarty P. L. Effects of toxicity, aeration, and reductant supply on trichloroethylene transformation by a mixed methanotrophic culture. Appl Environ Microbiol. 1991 Jan;57(1):228–235. doi: 10.1128/aem.57.1.228-235.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciero D., Vannelli T., Logan M., Hooper A. B. Degradation of trichloroethylene by the ammonia-oxidizing bacterium Nitrosomonas europaea. Biochem Biophys Res Commun. 1989 Mar 15;159(2):640–643. doi: 10.1016/0006-291x(89)90042-9. [DOI] [PubMed] [Google Scholar]
- Bonam D., Murrell S. A., Ludden P. W. Carbon monoxide dehydrogenase from Rhodospirillum rubrum. J Bacteriol. 1984 Aug;159(2):693–699. doi: 10.1128/jb.159.2.693-699.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox B. G., Borneman J. G., Wackett L. P., Lipscomb J. D. Haloalkene oxidation by the soluble methane monooxygenase from Methylosinus trichosporium OB3b: mechanistic and environmental implications. Biochemistry. 1990 Jul 10;29(27):6419–6427. doi: 10.1021/bi00479a013. [DOI] [PubMed] [Google Scholar]
- HOFMAN T., LEES H. The biochemistry of the nitrifying organisms. IV. The respiration and intermediary metabolism of Nitrosomonas. Biochem J. 1953 Jul;54(4):579–583. doi: 10.1042/bj0540579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper A. B., Terry K. R. Specific inhibitors of ammonia oxidation in Nitrosomonas. J Bacteriol. 1973 Aug;115(2):480–485. doi: 10.1128/jb.115.2.480-485.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Arp D. J. The small-scale production of [U-14C]acetylene from Ba14CO3: application to labeling of ammonia monooxygenase in autotrophic nitrifying bacteria. Anal Biochem. 1990 Nov 1;190(2):348–353. doi: 10.1016/0003-2697(90)90206-o. [DOI] [PubMed] [Google Scholar]
- Hyman M. R., Murton I. B., Arp D. J. Interaction of Ammonia Monooxygenase from Nitrosomonas europaea with Alkanes, Alkenes, and Alkynes. Appl Environ Microbiol. 1988 Dec;54(12):3187–3190. doi: 10.1128/aem.54.12.3187-3190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Wood P. M. Methane oxidation by Nitrosomonas europaea. Biochem J. 1983 Apr 15;212(1):31–37. doi: 10.1042/bj2120031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Wood P. M. Suicidal inactivation and labelling of ammonia mono-oxygenase by acetylene. Biochem J. 1985 May 1;227(3):719–725. doi: 10.1042/bj2270719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenhuis R., Oedzes J. Y., van der Waarde J. J., Janssen D. B. Kinetics of chlorinated hydrocarbon degradation by Methylosinus trichosporium OB3b and toxicity of trichloroethylene. Appl Environ Microbiol. 1991 Jan;57(1):7–14. doi: 10.1128/aem.57.1.7-14.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenhuis R., Vink R. L., Janssen D. B., Witholt B. Degradation of chlorinated aliphatic hydrocarbons by Methylosinus trichosporium OB3b expressing soluble methane monooxygenase. Appl Environ Microbiol. 1989 Nov;55(11):2819–2826. doi: 10.1128/aem.55.11.2819-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasche M. E., Hicks R. E., Hyman M. R., Arp D. J. Oxidation of monohalogenated ethanes and n-chlorinated alkanes by whole cells of Nitrosomonas europaea. J Bacteriol. 1990 Sep;172(9):5368–5373. doi: 10.1128/jb.172.9.5368-5373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasche Madeline E., Hyman Michael R., Arp Daniel J. Biodegradation of Halogenated Hydrocarbon Fumigants by Nitrifying Bacteria. Appl Environ Microbiol. 1990 Aug;56(8):2568–2571. doi: 10.1128/aem.56.8.2568-2571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannelli T., Logan M., Arciero D. M., Hooper A. B. Degradation of halogenated aliphatic compounds by the ammonia- oxidizing bacterium Nitrosomonas europaea. Appl Environ Microbiol. 1990 Apr;56(4):1169–1171. doi: 10.1128/aem.56.4.1169-1171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L. P., Householder S. R. Toxicity of Trichloroethylene to Pseudomonas putida F1 Is Mediated by Toluene Dioxygenase. Appl Environ Microbiol. 1989 Oct;55(10):2723–2725. doi: 10.1128/aem.55.10.2723-2725.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]