Abstract
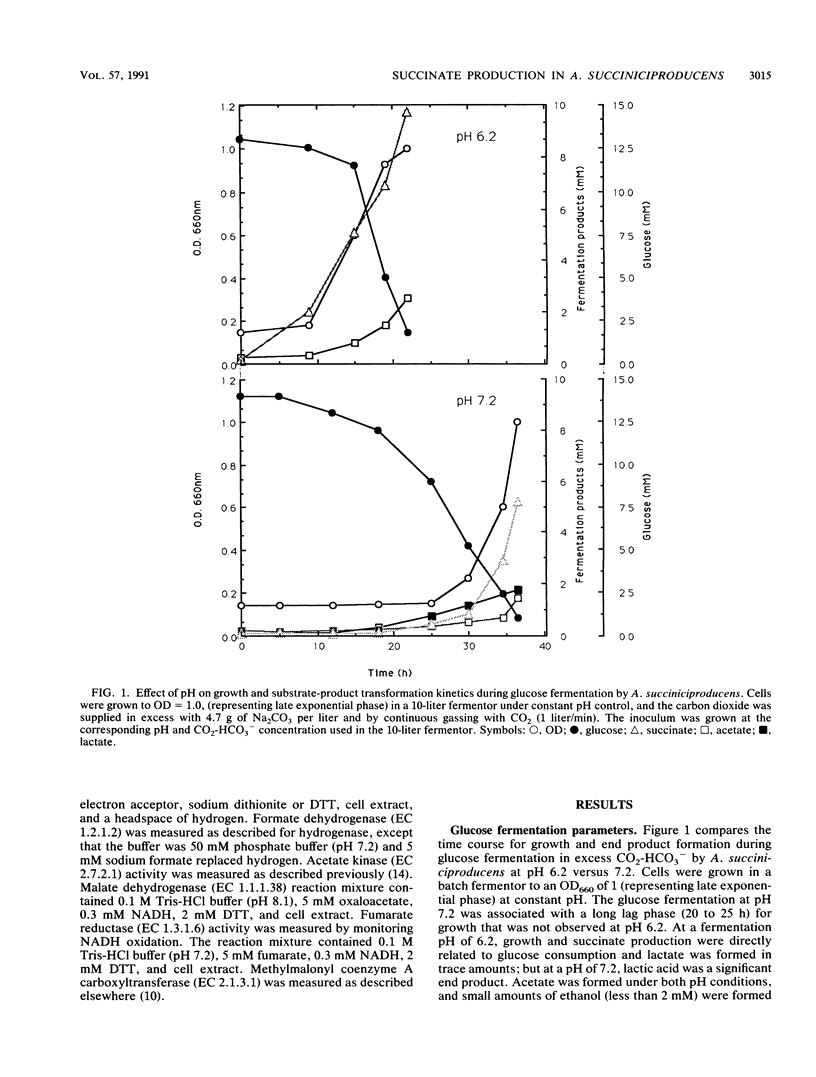
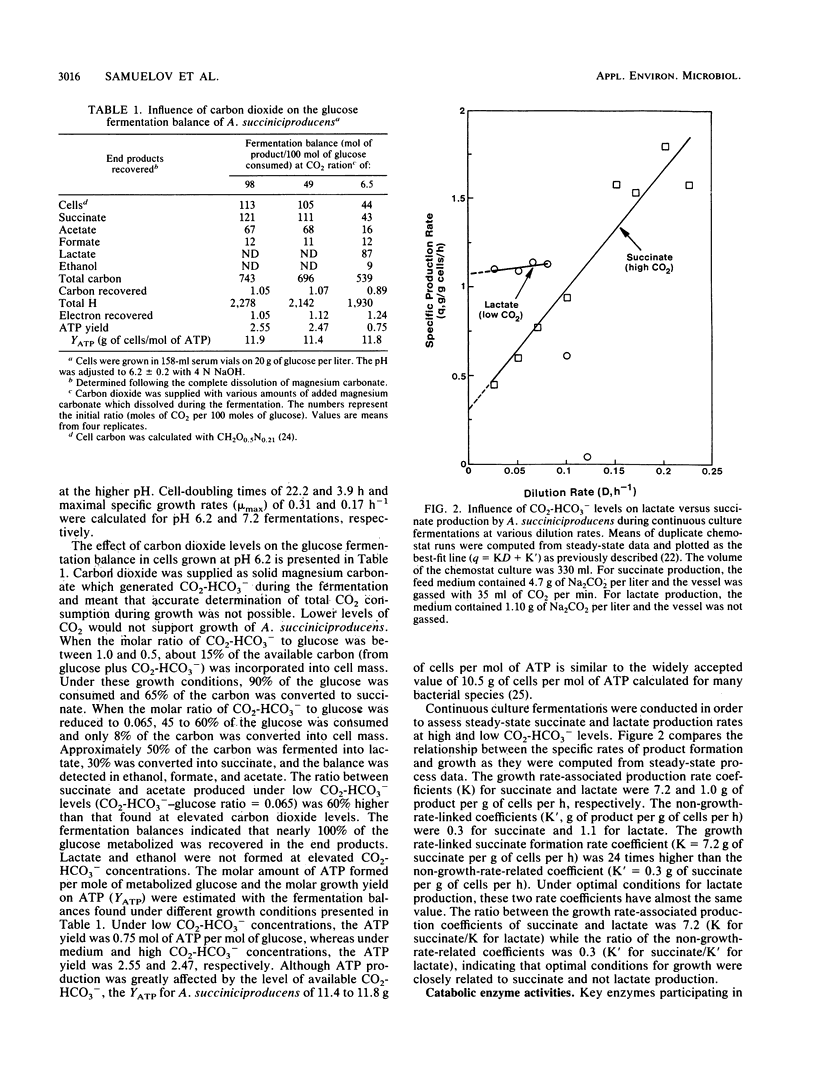
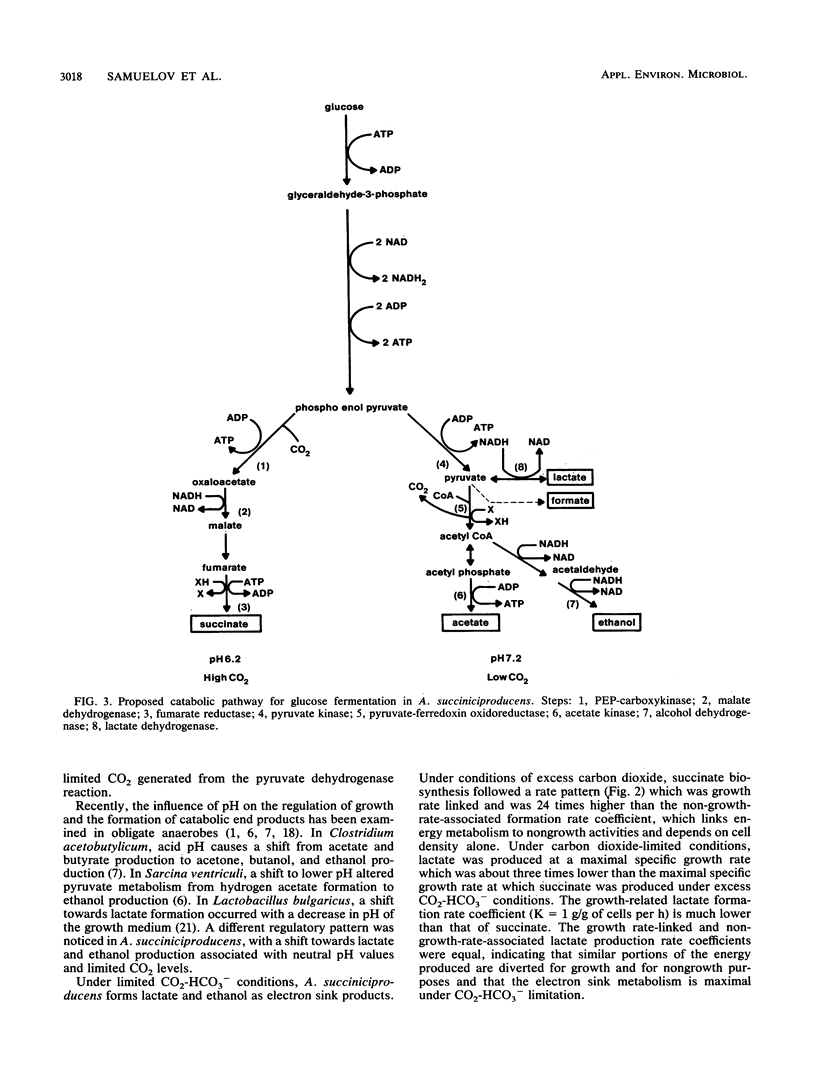
Growth and succinate versus lactate production from glucose by Anaerobiospirillum succiniciproducens was regulated by the level of available carbon dioxide and culture pH. At pH 7.2, the generation time was almost doubled and extensive amounts of lactate were formed in comparison with growth at pH 6.2. The succinate yield and the yield of ATP per mole of glucose were significantly enhanced under excess-CO2-HCO3− growth conditions and suggest that there exists a threshold level of CO2 for enhanced succinate production in A. succiniciproducens. Glucose was metabolized via the Embden-Meyerhof-Parnas route, and phosphoenolpyruvate carboxykinase levels increased while lactate dehydrogenase and alcohol dehydrogenase levels decreased under excess-CO2-HCO3− growth conditions. Kinetic analysis of succinate and lactate formation in continuous culture indicated that the growth rate-linked production rate coefficient (K) cells was much higher for succinate (7.2 versus 1.0 g/g of cells per h) while the non-growth-rate-related formation rate coefficient (K′) was higher for lactate (1.1 versus 0.3 g/g of cells per h). The data indicate that A. succiniciproducens, unlike other succinate-producing anaerobes which also form propionate, can grow rapidly and form high final yields of succinate at pH 6.2 and with excess CO2-HCO3− as a consequence of regulating electron sink metabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baronofsky J. J., Schreurs W. J., Kashket E. R. Uncoupling by Acetic Acid Limits Growth of and Acetogenesis by Clostridium thermoaceticum. Appl Environ Microbiol. 1984 Dec;48(6):1134–1139. doi: 10.1128/aem.48.6.1134-1139.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspari D., Macy J. M. The role of carbon dioxide in glucose metabolism of Bacteroides fragilis. Arch Microbiol. 1983 Aug;135(1):16–24. doi: 10.1007/BF00419476. [DOI] [PubMed] [Google Scholar]
- Dehority B. A. Carbon dioxide requirement of various species of rumen bacteria. J Bacteriol. 1971 Jan;105(1):70–76. doi: 10.1128/jb.105.1.70-76.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin S., Zeikus J. G. Physiological adaptations of anaerobic bacteria to low pH: metabolic control of proton motive force in Sarcina ventriculi. J Bacteriol. 1987 May;169(5):2150–2157. doi: 10.1128/jb.169.5.2150-2157.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopgood M. F., Walker D. J. Succinic acid production by rumen bacteria. I. Isolation and metabolism of Ruminococcus flavefaciens. Aust J Biol Sci. 1967 Feb;20(1):165–182. [PubMed] [Google Scholar]
- Howlett M. R., Mountfort D. O., Turner K. W., Roberton A. M. Metabolism and growth yields in Bacteroides ruminicola strain b14. Appl Environ Microbiol. 1976 Aug;32(2):274–283. doi: 10.1128/aem.32.2.274-283.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis B. D., Henderson C., Asmundson R. V. The role of carbonate in the metabolism of glucose by Butyrivibrio fibrisolvens. J Gen Microbiol. 1978 Apr;105(2):287–295. doi: 10.1099/00221287-105-2-287. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamed R., Zeikus J. G. Glucose fermentation pathway of Thermoanaerobium brockii. J Bacteriol. 1980 Mar;141(3):1251–1257. doi: 10.1128/jb.141.3.1251-1257.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd L., Kerby R., Zeikus J. G. Carbon monoxide metabolism of the methylotrophic acidogen Butyribacterium methylotrophicum. J Bacteriol. 1982 Jan;149(1):255–263. doi: 10.1128/jb.149.1.255-263.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel T. A., Macy J. M. Generation of a membrane potential by sodium-dependent succinate efflux in Selenomonas ruminantium. J Bacteriol. 1990 Mar;172(3):1430–1435. doi: 10.1128/jb.172.3.1430-1435.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montville T. J., Parris N., Conway L. K. Influence of pH on organic acid production by Clostridium sporogenes in test tube and fermentor cultures. Appl Environ Microbiol. 1985 Apr;49(4):733–736. doi: 10.1128/aem.49.4.733-736.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., Clayton M. A. Control of Escherichia coli growth by CO2. J Bacteriol. 1978 Sep;135(3):1162–1164. doi: 10.1128/jb.135.3.1162-1164.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., Repaske A. C., Mayer R. D. Carbon dioxide control of lag period and growth of Streptococcus sanguis. J Bacteriol. 1974 Feb;117(2):652–659. doi: 10.1128/jb.117.2.652-659.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. K., Pack M. Y. Effect of environmental pH on fermentation balance of Lactobacillus bulgaricus. J Bacteriol. 1980 Oct;144(1):217–221. doi: 10.1128/jb.144.1.217-221.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L. F., Chapital D. C. Simultaneous determination of carbohydrates and products of carbohydrate metabolism in fermentation mixtures by HPLC. J Chromatogr Sci. 1987 Mar;25(3):112–117. doi: 10.1093/chromsci/25.3.112. [DOI] [PubMed] [Google Scholar]
- Scheifinger C. C., Wolin M. J. Propionate formation from cellulose and soluble sugars by combined cultures of Bacteroides succinogenes and Selenomonas ruminantium. Appl Microbiol. 1973 Nov;26(5):789–795. doi: 10.1128/am.26.5.789-795.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. Chemical and fuel production by anaerobic bacteria. Annu Rev Microbiol. 1980;34:423–464. doi: 10.1146/annurev.mi.34.100180.002231. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977 Nov;132(2):604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


