Abstract
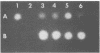
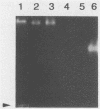
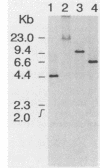
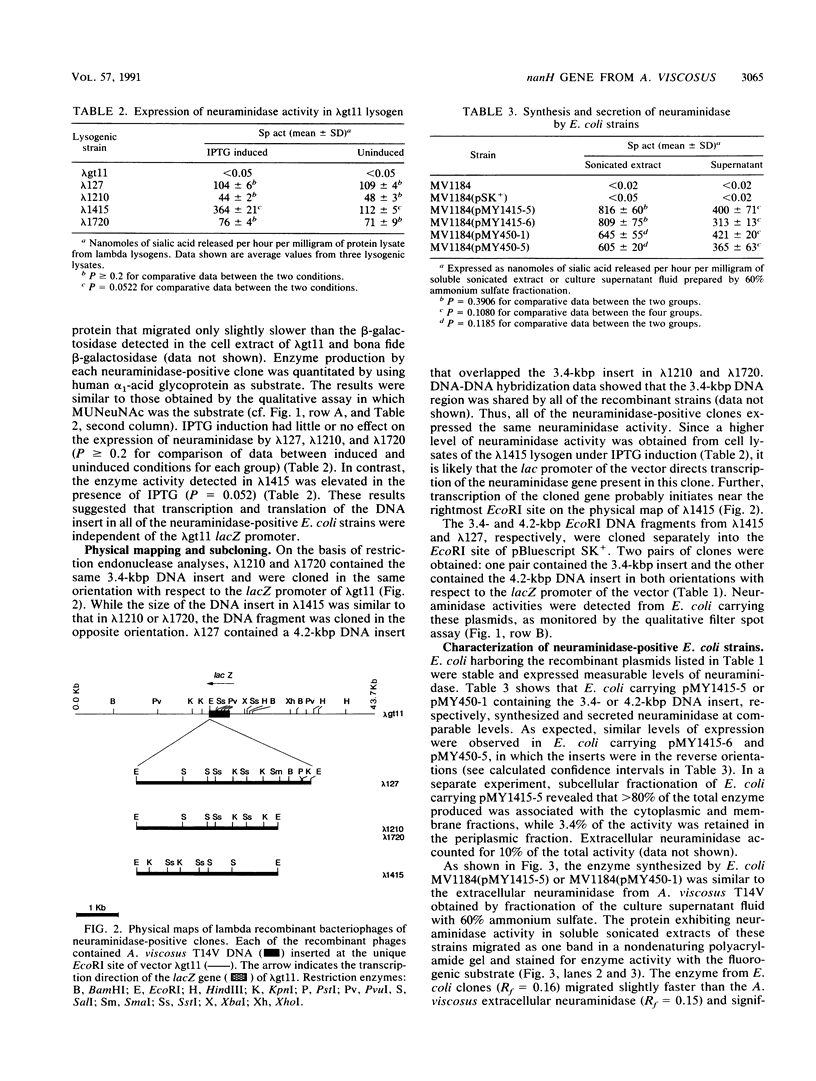
A genomic library of Actinomyces viscosus T14V DNA in lambda gt11 was screened for expression of neuraminidase activities. Four recombinant clones were detected that gave blue fluorescence upon incubation with a fluorogenic substrate, 2'-(4-methylumbelliferyl)-alpha-D-N-acetylneuraminic acid. Of these, two were identical, and all of the neuraminidase-positive clones shared a common 3.4-kbp DNA region. Expression of the enzyme activities in Escherichia coli carrying the cloned DNA was independent of the lacZ promoter of the vector. Maxicell analysis revealed that the 3.4-kbp DNA insert directed synthesis of a protein with an apparent molecular mass of 100,000 Da. The protein from cell extracts of E. coli clones migrated as a single band that stained for enzyme activity after electrophoresis in a nondissociating polyacrylamide gel. Moreover, human erythrocytes incubated previously with cell lysates from neuraminidase-positive E. coli were hemagglutinated by Actinomyces spp. The enzyme expressed by E. coli was active on substrates containing alpha-2,3 and alpha-2,6 ketosidic linked sialyl residues. Similar substrate specificities were obtained for both the extracellular and cell-associated neuraminidases from A. viscosus T14V. The 3.4-kbp insert hybridized to DNA fragments in a Southern blot containing A. viscosus T14V chromosomal DNA that had been digested with various restriction endonucleases. Data from hybridization studies show that A. viscosus T14V contains a single copy of the neuraminidase gene.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Beighton D., Whiley R. A. Sialidase activity of the "Streptococcus milleri group" and other viridans group streptococci. J Clin Microbiol. 1990 Jun;28(6):1431–1433. doi: 10.1128/jcm.28.6.1431-1433.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. J., Cisar J. O., Sandberg A. L. A 160-kilodalton epithelial cell surface glycoprotein recognized by plant lectins that inhibit the adherence of Actinomyces naeslundii. Infect Immun. 1986 Jun;52(3):840–845. doi: 10.1128/iai.52.3.840-845.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. J., Cisar J. O., Vatter A. E., Sandberg A. L. Lectin-dependent attachment of Actinomyces naeslundii to receptors on epithelial cells. Infect Immun. 1984 Nov;46(2):459–464. doi: 10.1128/iai.46.2.459-464.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy J. T., Jourdian G. W., Roseman S. The sialic acids. VI. Purification and properties of sialidase from Clostridium perfringens. J Biol Chem. 1965 Sep;240(9):3501–3506. [PubMed] [Google Scholar]
- Cisar J. O., Barsumian E. L., Curl S. H., Vatter A. E., Sandberg A. L., Siraganian R. P. Detection and localization of a lectin on Actinomyces viscosus T14V by monoclonal antibodies. J Immunol. 1981 Oct;127(4):1318–1322. [PubMed] [Google Scholar]
- Cisar J. O., David V. A., Curl S. H., Vatter A. E. Exclusive presence of lactose-sensitive fimbriae on a typical strain (WVU45) of Actinomyces naeslundii. Infect Immun. 1984 Nov;46(2):453–458. doi: 10.1128/iai.46.2.453-458.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Corfield A. P., Veh R. W., Wember M., Michalski J. C., Schauer R. The release of N-acetyl- and N-glycolloyl-neuraminic acid from soluble complex carbohydrates and erythrocytes by bacterial, viral and mammalian sialidases. Biochem J. 1981 Aug 1;197(2):293–299. doi: 10.1042/bj1970293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A. H., Cisar J. O., Kolenbrander P. E., Gabriel O. Neuraminidase-dependent hamagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979 Nov;26(2):563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzeniek R., Scharmann W., Balke E. Neuraminidase and N-acetylneuraminate pyruvate-lyase of Pasteurella multocida. J Gen Microbiol. 1972 Sep;72(2):357–368. doi: 10.1099/00221287-72-2-357. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Fillery E. D., Chan K. H., Grove D. A. Sialidase-enhanced lectin-like mechanism for Actinomyces viscosus and Actinomyces naeslundii hemagglutination. Infect Immun. 1980 Feb;27(2):335–343. doi: 10.1128/iai.27.2.335-343.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flashner M., Wang P., Hurley J. B., Tanenbaum S. W. Properties of an inducible extracellular neuraminidase from an Arthrobacter isolate. J Bacteriol. 1977 Mar;129(3):1457–1465. doi: 10.1128/jb.129.3.1457-1465.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A. G., Brown R. Neuraminidase production by Bacteroidaceae. J Med Microbiol. 1981 Feb;14(1):63–76. doi: 10.1099/00222615-14-1-63. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. A. THE SENSITIVITY OF THE NEURAMINOSIDIC LINKAGE IN MUCOSUBSTANCES TOWARDS ACID AND TOWARDS NEURAMINIDASE. Biochem J. 1963 Nov;89:380–391. doi: 10.1042/bj0890380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimoto S., Aoyagi T., Takeuchi T., Umezawa H. Purification and characterization of Streptomyces sialidases. J Bacteriol. 1974 Aug;119(2):394–400. doi: 10.1128/jb.119.2.394-400.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moncla B. J., Braham P. Detection of sialidase (neuraminidase) activity in Actinomyces species by using 2'-(4-methylumbelliferyl)alpha-D-N-acetylneuraminic acid in a filter paper spot test. J Clin Microbiol. 1989 Jan;27(1):182–184. doi: 10.1128/jcm.27.1.182-184.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger A., Ratcliffe W. A. The acid and enzymic hydrolysis of O-acetylated sialic acid residues from rabbit Tamm-Horsfall glycoprotein. Biochem J. 1972 Sep;129(3):683–693. doi: 10.1042/bj1290683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoe G. I. The inducible neuraminidase (N-acyl-neuraminyl hydrolase EC 3.2.1.18) of Klebsiella aerogenes NCIB 9479. Pathol Microbiol (Basel) 1970;35(6):361–376. doi: 10.1159/000162248. [DOI] [PubMed] [Google Scholar]
- Perlitsh M. J., Glickman I. Salivary neuraminidase. I. The presence of neuraminidase in human saliva. J Periodontol. 1966 Sep-Oct;37(5):368–373. doi: 10.1902/jop.1966.37.5.368. [DOI] [PubMed] [Google Scholar]
- Potier M., Mameli L., Bélisle M., Dallaire L., Melançon S. B. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-D-N-acetylneuraminate) substrate. Anal Biochem. 1979 Apr 15;94(2):287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- Rogers R., Newbrun E., Tatevossian A. Neuraminidase activity in human dental plaque fluid. Arch Oral Biol. 1979;24(9):703–705. doi: 10.1016/0003-9969(79)90121-3. [DOI] [PubMed] [Google Scholar]
- Russo T. A., Thompson J. S., Godoy V. G., Malamy M. H. Cloning and expression of the Bacteroides fragilis TAL2480 neuraminidase gene, nanH, in Escherichia coli. J Bacteriol. 1990 May;172(5):2594–2600. doi: 10.1128/jb.172.5.2594-2600.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg A. L., Mudrick L. L., Cisar J. O., Brennan M. J., Mergenhagen S. E., Vatter A. E. Type 2 fimbrial lectin-mediated phagocytosis of oral Actinomyces spp. by polymorphonuclear leukocytes. Infect Immun. 1986 Nov;54(2):472–476. doi: 10.1128/iai.54.2.472-476.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Elledge S., Sweetser D., Young R. A., Davis R. W. Lambda gt 11: gene isolation with antibody probes and other applications. Methods Enzymol. 1987;154:107–128. doi: 10.1016/0076-6879(87)54073-3. [DOI] [PubMed] [Google Scholar]
- Teufel M., Roggentin P., Schauer R. Properties of sialidase isolated from Actinomyces viscosus DSM 43798. Biol Chem Hoppe Seyler. 1989 May;370(5):435–443. doi: 10.1515/bchm3.1989.370.1.435. [DOI] [PubMed] [Google Scholar]
- Tuyau J. E., Sims W. Occurrence of haemophili in dental plaque and their association with neuraminidase activity. J Dent Res. 1975 Jul-Aug;54(4):737–739. doi: 10.1177/00220345750540040701. [DOI] [PubMed] [Google Scholar]
- Uchida Y., Tsukada Y., Sugimori T. Enzymatic properties of neuraminidases from Arthrobacter ureafaciens. J Biochem. 1979 Nov;86(5):1573–1585. doi: 10.1093/oxfordjournals.jbchem.a132675. [DOI] [PubMed] [Google Scholar]
- Vimr E. R., Lawrisuk L., Galen J., Kaper J. B. Cloning and expression of the Vibrio cholerae neuraminidase gene nanH in Escherichia coli. J Bacteriol. 1988 Apr;170(4):1495–1504. doi: 10.1128/jb.170.4.1495-1504.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung M. K., Chassy B. M., Cisar J. O. Cloning and expression of a type 1 fimbrial subunit of Actinomyces viscosus T14V. J Bacteriol. 1987 Apr;169(4):1678–1683. doi: 10.1128/jb.169.4.1678-1683.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]