Abstract
Clinical guideline authors, health information technology (HIT) standards development organizations, and information system implementers all work to improve the processes of healthcare, but have long functioned independently towards realizing these goals. This has led to clinical standards of care that often poorly align with the functional and technical HIT standards developed to realize them. We describe the shortcomings and inefficiencies inherent in this current process and introduce two national initiatives that attempt to unite these communities. The mission of these two initiatives is to create examples of unambiguous, decidable, and executable clinical guidelines which both utilize and inform HIT terminology and logical expression standards. All of the products of this work aim to facilitate enterprise-wide guideline implementation and create a rising tide which lifts all ships.
Introduction
The ultimate promise of Health information technology (HIT) is a leap forward in quality of care: up-to-date health information aggregated from all of the patient’s providers; computerized order entry; and decision support systems that help realize the standards of care documented within clinical guidelines1. Foundational work within informatics research institutions has delivered early evidence of this promise2–5, but we now face the difficult prospect of scaling these successes throughout the larger healthcare enterprise6.
Unfortunately, developers of these systems face many obstacles. Given the documented challenges care providers have when attempting to interpret clinical guidelines7, 8, it makes sense that implementers often struggle to create effective systems incorporating this content9, 10. Additionally, mismatches in the level of abstraction, availability, and semantic meaning of pre-existing clinical data elements within systems either make the implementation of complex care pathways impossible, or require specific applications that gather these data prospectively11. These realities limit the scalability and complexity of HIT systems to support standards of care both in real time and retrospectively for audit and quality control. Those who demonstrate success often commit significant resources to systems that encode fairly basic guidelines which rely on limited data sets. We believe many of these issues could be remedied through direct integration of standard development efforts already concurrently occurring in both the medical informatics and clinical guideline development communities.
Background
Medical Informatics Community
Beginning in the early 1980’s, the medical informatics community began developing standards as frameworks that could allow system builders to approach HIT development projects consistently. For example, terminology standards such as LOINC12, SNOMED13, and RxNorm14 standardize the type and meaning of data encoded by information systems. Logical expression standards such as Arden Syntax15 provide a language to relate clinical knowledge with individual patient data so that information systems can facilitate health decision making processes and identify both missed opportunities and the potential for patient harm. At a higher level, guideline representation standards (GEM16) and execution formalisms (i.e., GLIF317 and SAGE18) are intended to represent the collection of terminologies and logical constructs that define a care pathway and a standard of care for a given topic area.
A survey of these standards illustrates varied levels of both completeness and use. The reasons for this are myriad. For one, standards developers have struggled to define a viable enrichment process. Considering the massive amount of content to encode, how do you define the project scope and what subject areas should be prioritized? How can the content of an evolving knowledge base be maintained? Additionally, those who create content with these standards frameworks are often not medical content experts and do so voluntarily to meet local needs. Because of this, the resulting standards often have a focus with limited utility to an enterprise with a more global mission and goals. It also implies that large “holes” in the content exist, many of which might have high relevance to clinical care. Given these realities, it’s not surprising to see slow uptake and integration of these standards into commercial HIT products.
Clinical Guideline Development Community
Much as the medical informatics community has created vocabulary and knowledge representation standards for health information systems, the medical community at large began developing clinical guidelines in earnest in the late 1980s to standardize the management of disease states. In synthesizing the consensus of current evidence-based management strategies, guideline developers created succinct documents that summarized standards of care. Over the last 20 years, clinical guidelines have become an essential product of most specialty organizations, with significant intellectual and financial resources allocated toward their development and upkeep19. However, much like medical informatics standards, the potential of clinical guidelines to provide an implementation framework for practice change is largely unrealized.
A well described obstacle that limits the usefulness of many guidelines is effective expression of the knowledge content itself. The critical knowledge components that differentiate guidelines from topic summaries and reviews are the recommendations. Ambiguity and vagueness in the wording of recommendations, incomplete decision logic pathways, and poor differentiation between evidence and opinion are common shortcomings of many guidelines20–23. As an indicator of the problem’s pervasiveness, instruments such as AGREE24 and GLIA25 have been developed specifically to assess the intrinsic quality and implementability of a clinical guideline. Additionally, a lack of transparency in both the quality of evidence and strength of recommendations makes prioritization and implementation decisions difficult. Probably most importantly, care providers face many external pressures, including time constraints, shrinking resource margins, and a growing evidence base which hinders their ability to assimilate new guidelines into routine workflows26. Therefore, the ultimate success of a guideline is dependent upon tools built to facilitate specific clinical guidance27.
Working Collaborations: A Strategic Approach
The medical informatics and guideline authoring communities typically function independently of each other. This limits the utility of the healthcare standards they create. We believe that these communities should mutually work toward alleviating many of today’s challenges by informing each other’s work. For example, informaticians focused upon implementation can likely help guideline authors to word actions in less ambiguous ways and point out incomplete branches within care algorithms. Guideline authors in return can guide standards development activities by defining the granularity of necessary vocabularies, providing the content expertise necessary to build standardized, logical expression rules, and creating an important starting place for content enrichment. They can also guide system builders toward functional specifications will have the greatest impact on a health topic’s outcomes. Finally, the guideline development process provides the informatics community with a framework for continuous HIT standard revision and maintenance. As clinical guidelines are created, updated, and revised, the HIT standards underlying them could adapt in response.
Until recently, a missing ingredient towards realizing these economies was direct interaction between these communities during both the process of guideline development and the HIT standard enrichment process. Two national pilot projects initiated in 2005 aim to create models of these working relationships through completion of specific, concrete projects. These initiatives, while similar in their mission, focus on creating different portions of products which should be developed as part of the larger knowledge engineering and implementation process. We fully intend these initiatives to serve as practical examples of how these collaborations can feasibly occur throughout the larger healthcare arena.
Partnership for Policy Implementation
The Partnership for Policy Implementation (PPI), initiated in June 2005 by the American Academy of Pediatrics (AAP), aims to create fundamental paradigm shifts in how policy statements, technical reports, and clinical guidelines are both written and ultimately integrated into care. Given the well recognized lack of integration of these standards of care into practice, the AAP has taken a proactive approach to incorporating content created by its members into tools such as health information systems. Towards that end, the aim of the PPI is to create guidelines for child health professionals that are highly decidable and actionable from their outset. This type of guideline will ultimately serve as substrate for many HIT standards development activities. It is the hope that statements written within this framework will also provide more straight-forward guidance for all pediatric health care providers, even those who continue to use paper records.
A process has been established to realize these goals. Members of the PPI team (one pediatric-trained medical informatician and one quality improvement expert per statement) consult directly with guideline authors during the authorship of either a new or a revision to a preexisting statement. During the two year pilot period, the explicit goal is to produce 6–8 published examples of guidelines written within this framework, which will both serve as substrate for HIT standards development and illustrate mechanisms to alleviate the many common shortcomings of such standard-of care statements.
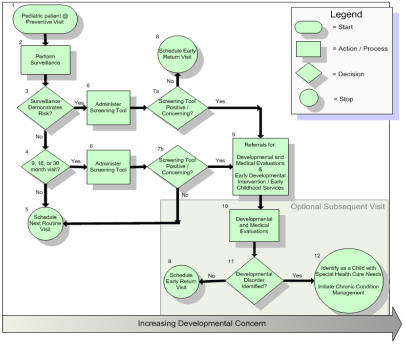
A recently completed guideline for developmental surveillance and screening of children up to 5 years of age illustrates some of the products that will emerge from the PPI process. For example, figure 1 demonstrates ways in which the informatics team increases the executability of recommendations by assisting in iterative revisions of the guideline text. In the early draft, authors asked pediatricians to assess social and demographic risk factors for developmental surveillance, which include “low maternal age”. However, as with many such ambiguous statements, implementers could build decision support logic which scans patient record data at many different age cut-points. Adding specificity curtails this behavior and thus speeds up system development processes. Additionally, the PPI provides a variety of different frameworks for discussion, such as clinical algorithms (figure 2). Within the developmental screening guideline, the medical informatician created an algorithm up front to capture the spirit of a previous edition of the guideline. Having this algorithm early in the process allowed authors to visualize their intended guidance and quickly see where content should be added or changed. All subsequent text within the final guideline focuses on explaining shapes within the algorithm, including clear definitions of actions and decision points. These various approaches were universally lauded by the guideline development committee as helpful adjuncts to the process.
Figure 1.
An example of concept disambiguation. Medical Informaticians within the PPI initiative work with guideline authors to increase specificity of recommendations and clinical guidance by pointing out ambiguities (i.e., low maternal education) and referencing evidence when feasible.
Figure 2. 2006 Developmental Screening and Surveillance Algorithm created within the American Academy of Pediatrics’ PPI initiative.
A medical informatician created this algorithm based on content contained within the initial 2001 statement along with iterative feedback from pediatric developmental experts. It serves as the cornerstone of the revised statement, which helped the guideline development team focus on creating explicit definitions of concepts such as developmental surveillance and screening. Each shape within this algorithm is fully described within the context of the document. The informatician also helped the guideline team develop a grid of validated developmental screening instruments with information which will allow future system developers to make implementation choices based upon the varied workflows of different clinic types.
Future strategic planning within this initiative will focus on how to best integrate PPI methodology as a standard into all future AAP policy writing processes. Work in 2006 will focus on the development of guidebooks to illustrate proper guideline authoring approaches. These guidebooks will encapsulate lessons learned in the pilot phase of the PPI, and will facilitate the training of new pediatric informaticians who will contribute to the PPI effort. Additionally, the PPI will establish mentorships between these informaticians and other Academy members to scale these practices into the larger pediatric authorship community. The team is also developing methods to assess the project’s impact on resultant guidelines. This includes providing funding mechanisms for pilot implementations of revised guidelines and using the GLIA25 instrument to compare revised guidelines to those written prior to PPI involvement. As the AAP continues to explore new ways to expand involvement within the health information technology landscape, it is hoped that through the redesign of AAP policy, a new foundation will be laid that will provide a road map for future initiatives.
National Child Health Data Standards Workgroup
Also initiated in 2005, the National Child Health Data Standards Workgroup (NCHDSWG) convened experts from a number of pediatric stakeholder groups to evaluate the state of HIT standards and their potential to realize quality care for children. One of the initial tasks of this workgroup assembled a technical expert panel (TEP) to develop and study an example of a disease state which utilized HIT standards. Asthma was chosen given it’s pervasiveness in multiple care settings (outpatient clinics, hospitals, emergency rooms) and relevance to a large set of care providers. Given the clinical relevance and high impact of the National Heart, Lung, and Blood Institute’s (NHLBI) asthma guideline, the TEP has embarked on a collaborative effort with this group to produce companion references for an upcoming guideline revision.
The TEP is comprised of a broad cross sampling of experts experienced in issues related to HIT implementation. Among this group are representatives from the medical informatics community, quality improvement experts, asthma content experts, and health services researchers. Whereas the PPI initiative described previously places emphasis on fostering good guideline development techniques, members of the TEP have all agreed to focus more upon the actual identification and/or creation of specific content within vocabulary and logical expression standards. Given the previously described shortcomings of clinical guidelines, the TEP initially has worked with the NHLBI to identify ambiguities and vagueness within drafts of the guideline revision. Focus however will soon toward development of a few specific companion guideline products. The first is a terminology glossary that clearly defines all decision variables with written definitions, much like a dictionary. However, these definitions will additionally include mappings (synonymies) to standardized vocabularies wherever pertinent. To fill in content gaps, missing concepts will be submitted through relationships established between the TEP and standards development organizations.
Once the TEP defines this asthma “lingua franca,” effort will turn towards development of two other supplemental products: sample decision support rules and quality measures that rely upon the decision variables defined in the concept glossary. The goal of these activities is to demonstrate the feasibility and potential utility of creating higher level standards-based end user products. The group will release all of these products into the public domain, and will encourage pilot implementations using these products.
Conclusion
The American Academy of Pediatrics’ PPI initiative and National Child Health Data Standards Workgroup’s TEP represent long overdue attempts to practically merge the efforts of two constituencies equally dedicated to improving the process and quality of healthcare. The work products of these two initiatives will tangibly demonstrate the potential benefits (and challenges) of providing a framework that allows guideline authors and medical informaticians to work alongside each other. We believe that these initiatives are important building blocks toward national HIT standard development that truly impacts clinical care, given the strong focus on quality improvement efforts. We hope that these initiatives will additionally illustrate approaches that other medical specialty groups will emulate in the future.
References
- 1.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 2.McDonald CJ. Protocol-based computer reminders, the quality of care and the non-perfectability of man. N Engl J Med. 1976;295(24):1351–1355. doi: 10.1056/NEJM197612092952405. [DOI] [PubMed] [Google Scholar]
- 3.Rind DM, Safran C, Phillips RS, Wang Q, Calkins DR, Delbanco TL, et al. Effect of computer-based alerts on the treatment and outcomes of hospitalized patients. Arch Intern Med. 1994;154(13):1511–1517. [PubMed] [Google Scholar]
- 4.Dexter PR, Perkins S, Overhage JM, Maharry K, Kohler RB, McDonald CJ. A Computerized Reminder System to Increase the Use of Preventive Care for Hospitalized Patients. N Engl J Med. 2001;345(13):965–970. doi: 10.1056/NEJMsa010181. [DOI] [PubMed] [Google Scholar]
- 5.Dexter PR, Perkins SM, Maharry KS, Jones K, McDonald CJ. Inpatient Computer-Based Standing Orders vs Physician Reminders to Increase Influenza and Pneumococcal Vaccination Rates: A Randomized Trial. JAMA. 2004;292(19):2366–2371. doi: 10.1001/jama.292.19.2366. [DOI] [PubMed] [Google Scholar]
- 6.Southon FCG, Sauer C, Dampney CNG. Information Technology in Complex Health Services: Organizational Impediments to Successful Technology Transfer and Diffusion. J Am Med Inform Assoc. 1997;4(2):112–124. doi: 10.1136/jamia.1997.0040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christakis DA, Rivara FP. Pediatricians' Awareness of and Attitudes About Four Clinical Practice Guidelines. Pediatrics. 1998;101(5):825–830. doi: 10.1542/peds.101.5.825. [DOI] [PubMed] [Google Scholar]
- 8.Hardern RD, Hampshaw S. What do accident and emergency medical staff think of practice guidelines? Eur J Emerg Med. 1997;4(2):68–71. doi: 10.1097/00063110-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Tierney WM, Overhage JM, Takesue BY, Harris LE, Murray MD, Vargo DL, et al. Computerizing guidelines to improve care and patient outcomes: the example of heart failure. J Am Med Inform Assoc. 1995;2(5):316–22. doi: 10.1136/jamia.1995.96073834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz DA. Barriers between guidelines and improved patient care: an analysis of AHCPR's Unstable Angina Clinical Practice Guideline. Agency for Health Care Policy and Research. Health Serv Res. 1999;34(1 Pt 2):377–89. [PMC free article] [PubMed] [Google Scholar]
- 11.Anand V, Biondich PG, Liu G, Rosenman M, Downs SM. Child Health Improvement through Computer Automation: the CHICA system. Medinfo. 2004;11(Pt 1):187–91. [PubMed] [Google Scholar]
- 12.McDonald CJ, Huff SM, Suico JG, Hill G, Leavelle D, Aller R, et al. LOINC, a universal standard for identifying laboratory observations: a 5-year update. Clinical Chemistry. 2003;49(4):624–33. doi: 10.1373/49.4.624. [DOI] [PubMed] [Google Scholar]
- 13.Herbert I, Hawking M. Bringing SNOMED-CT into use within primary care. Informatics in Primary Care. 2005;13(1):61–4. doi: 10.14236/jhi.v13i1.581. [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Wei M, Moore R, Ganesan V, Nelson S. RxNorm: prescription for electronic drug information exchange. IT Professional. 2005;7(5):17–23. [Google Scholar]
- 15.Hripcsak G, Ludemann P, Pryor TA, Wigertz OB, Clayton PD. Rationale for the Arden Syntax. Comput Biomed Res. 1994;27(4):291–324. doi: 10.1006/cbmr.1994.1023. [DOI] [PubMed] [Google Scholar]
- 16.Shiffman RN, Michel G, Essaihi A, Thornquist E. Bridging the guideline implementation gap: a systematic, document-centered approach to guideline implementation. J Am Med Inform Assoc. 2004;11(5):418–26. doi: 10.1197/jamia.M1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boxwala AA, Peleg M, Tu S, Ogunyemi O, Zeng QT, Wang D, et al. GLIF3: a representation format for sharable computer-interpretable clinical practice guidelines. J Biomed Inform. 2004;37(3):147–61. doi: 10.1016/j.jbi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Parker CG, Rocha RA, Campbell JR, Tu SW, Huff SM. Detailed clinical models for sharable, executable guidelines. Medinfo. 2004;11(Pt 1):145–8. [PubMed] [Google Scholar]
- 19.Institute of Medicine. Guidelines for Clinical Practice: From Development to Use. Washington, DC: National Academy Press; 1992. [Google Scholar]
- 20.Tierney WM, Overhage JM, McDonald CJ. Computerizing guidelines: factors for success. Proc AMIA Annu Fall Symp. 1996:459–62. [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald CJ, Overhage JM. Guidelines you can follow and can trust. An ideal and an example. Jama. 1994;271(11):872–3. [PubMed] [Google Scholar]
- 22.Greenes RA, Peleg M, Boxwala A, Tu S, Patel V, Shortliffe EH. Sharable computer-based clinical practice guidelines: rationale, obstacles, approaches, and prospects. Medinfo. 2001;10(Pt 1):201–5. [PubMed] [Google Scholar]
- 23.Shiffman RN, Shekelle P, Overhage JM, Slutsky J, Grimshaw J, Deshpande AM. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Ann Intern Med. 2003;139(6):493–8. doi: 10.7326/0003-4819-139-6-200309160-00013. [DOI] [PubMed] [Google Scholar]
- 24.Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12(1):18–23. doi: 10.1136/qhc.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiffman R, Dixon J, Brandt C, Essaihi A, Hsiao A, Michel G, et al. The GuideLine Implementability Appraisal (GLIA): development of an instrument to identify obstacles to guideline implementation. BMC Medical Informatics and Decision Making. 2005;5(1):23. doi: 10.1186/1472-6947-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud P-AC, et al. Why Don't Physicians Follow Clinical Practice Guidelines? A Framework for Improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 27.James BC. Making It Easy to Do It Right. N Engl J Med. 2001;345(13):991–993. doi: 10.1056/NEJM200109273451311. [DOI] [PubMed] [Google Scholar]