Abstract
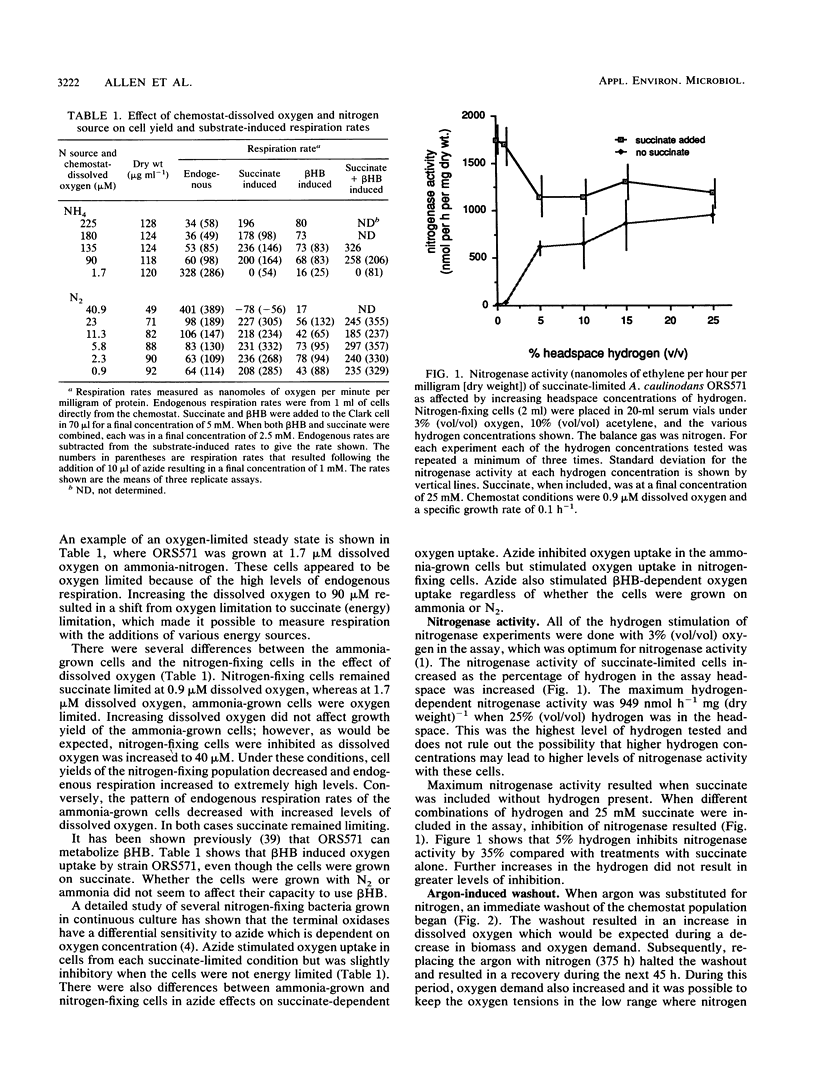
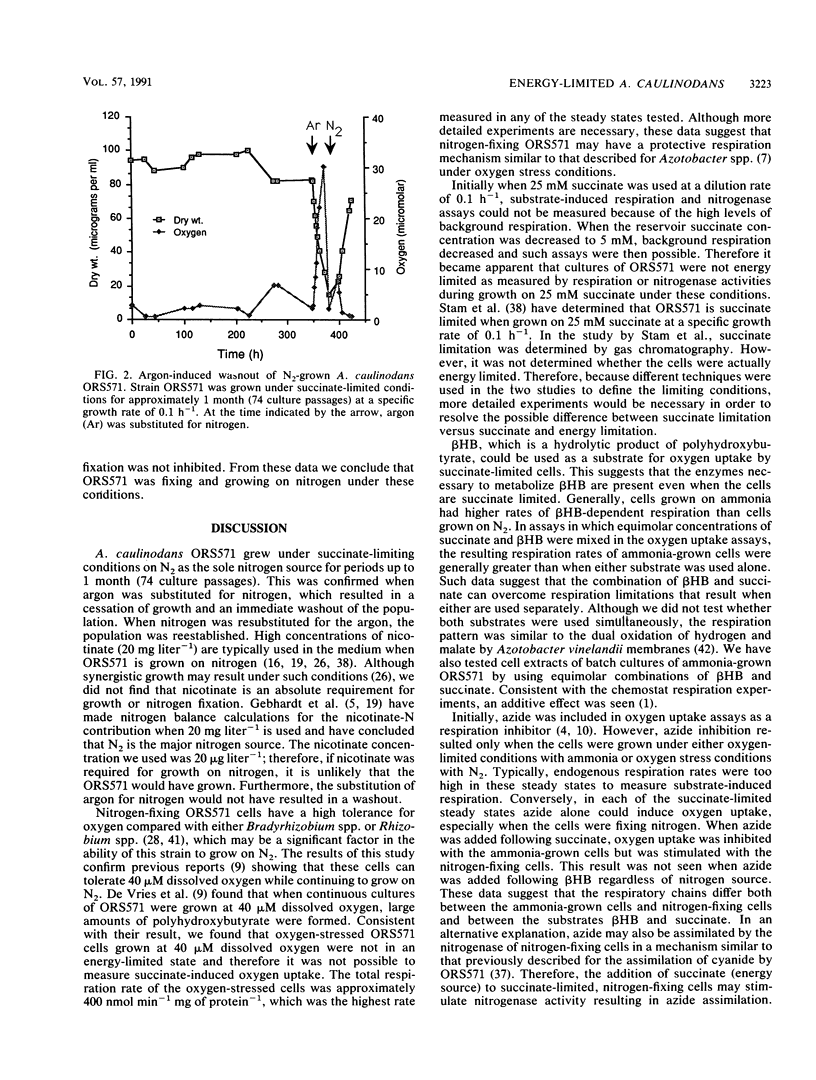
Succinate-limited continuous cultures of an Azorhizobium caulinodans strain were grown on ammonia or nitrogen gas as a nitrogen source. Ammonia-grown cells became oxygen limited at 1.7 μM dissolved oxygen, whereas nitrogen-fixing cells remained succinate limited even at dissolved oxygen concentrations as low as 0.9 μM. Nitrogen-fixing cells tolerated dissolved oxygen concentrations as high as 41 μM. Succinate-dependent oxygen uptake rates of cells from the different steady states ranged from 178 to 236 nmol min−1 mg of protein−1 and were not affected by varying chemostat-dissolved oxygen concentration or nitrogen source. When equimolar concentrations of succinate and β-hydroxybutyrate were combined, oxygen uptake rates were greater than when either substrate was used alone. Azide could also used alone as a respiratory substrate regardless of nitrogen source; however, when azide was added following succinate additions, oxygen uptake was inhibited in ammonia-grown cells and stimulated in nitrogen-fixing cells. Use of 25 mM succinate in the chemostat resevoir at a dilution rate of 0.1 h−1 resulted in high levels of background respiration and nitrogenase activity, indicating that the cells were not energy limited. Lowering the reservoir succinate to 5 mM imposed energy limitation. Maximum succinate-dependent nitrogenase activity was 1,741 nmol of C2H4h−1 mg (dry weight)−1, and maximum hydrogen-dependent nitrogenase activity was 949 nmol of C2H4 h−1 mg (dry weight)−1. However, when concentration of 5% (vol/vol) hydrogen or greater were combined with succinate, nitrogenase activity decreased by 35% in comparison to when succinate was used alone. Substitution of argon for nitrogen in the chemostat inflow gas resulted in “washout,” proving that ORS571 can grow on N2 and that there was not a nitrogen source in the medium that could substitute.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. C., Elkan G. H. Growth, Respiration, and Polypeptide Patterns of Bradyrhizobium sp. (Arachis) Strain 3G4b20 from Succinate- or Oxygen-Limited Continuous Cultures. Appl Environ Microbiol. 1990 Apr;56(4):1025–1032. doi: 10.1128/aem.56.4.1025-1032.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedmar E. J., Phillips D. A. A transmissible plant shoot factor promotes uptake hydrogenase activity in Rhizobium symbionts. Plant Physiol. 1984 Jul;75(3):629–633. doi: 10.1104/pp.75.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L. The role of O2-limitation in control of nitrogenase in continuous cultures of Rhizobrium sp. Biochem Biophys Res Commun. 1976 Nov 22;73(2):524–531. doi: 10.1016/0006-291x(76)90738-5. [DOI] [PubMed] [Google Scholar]
- Bethlenfalvay G. J., Phillips D. A. Effect of Light Intensity on Efficiency of Carbon Dioxide and Nitrogen Reduction in Pisum sativum L. Plant Physiol. 1977 Dec;60(6):868–871. doi: 10.1104/pp.60.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton H., Postgate J. R. Effect of oxygen on growth of Azotobacter chroococcum in batch and continuous cultures. J Gen Microbiol. 1968 Dec;54(3):463–473. doi: 10.1099/00221287-54-3-463. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Nitrogenase--hydrogenase interrelationships in Rhizobia. Biochimie. 1978;60(3):233–236. doi: 10.1016/s0300-9084(78)80819-0. [DOI] [PubMed] [Google Scholar]
- Dreyfus B. L., Elmerich C., Dommergues Y. R. Free-living Rhizobium strain able to grow on n(2) as the sole nitrogen source. Appl Environ Microbiol. 1983 Feb;45(2):711–713. doi: 10.1128/aem.45.2.711-713.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D. L. Acetylene reduction by pure cultures of Rhizobia. J Bacteriol. 1975 Sep;123(3):1265–1268. doi: 10.1128/jb.123.3.1265-1268.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepo J. E., Hickok R. E., Cantrell M. A., Russell S. A., Evans H. J. Revertible hydrogen uptake-deficient mutants of Rhizobium japonicum. J Bacteriol. 1981 May;146(2):614–620. doi: 10.1128/jb.146.2.614-620.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. T., Shanmugam K. T. Regulation of hydrogen utilisation in Rhizobium japonicum by cyclic AMP. Biochim Biophys Acta. 1979 May 16;584(3):479–492. doi: 10.1016/0304-4165(79)90121-1. [DOI] [PubMed] [Google Scholar]
- Ludwig R. A. Rhizobium sp. strain ORS571 grows synergistically on N2 and nicotinate as N sources. J Bacteriol. 1986 Jan;165(1):304–307. doi: 10.1128/jb.165.1.304-307.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Merberg D., O'Hara E. B., Maier R. J. Regulation of hydrogenase in Rhizobium japonicum: analysis of mutants altered in regulation by carbon substrates and oxygen. J Bacteriol. 1983 Dec;156(3):1236–1242. doi: 10.1128/jb.156.3.1236-1242.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Ortiz J. M., Burris R. H. Interactions among substrates and inhibitors of nitrogenase. J Bacteriol. 1975 Aug;123(2):537–545. doi: 10.1128/jb.123.2.537-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Evans H. J. Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1207–1211. doi: 10.1073/pnas.73.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Jennings N. T., Evans H. J. Hydrogen Reactions of Nodulated Leguminous Plants: II. Effects on Dry Matter Accumulation and Nitrogen Fixation. Plant Physiol. 1978 Mar;61(3):398–401. doi: 10.1104/pp.61.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson F. B., Burris R. H. A nitrogen pressure of 50 atmospheres does not prevent evolution of hydrogen by nitrogenase. Science. 1984 Jun 8;224(4653):1095–1097. doi: 10.1126/science.6585956. [DOI] [PubMed] [Google Scholar]
- Tjepkema J., Evans H. J. Nitrogen fixation by free-living Rhizobium in a defined liquid medium. Biochem Biophys Res Commun. 1975 Jul 22;65(2):625–628. doi: 10.1016/s0006-291x(75)80192-6. [DOI] [PubMed] [Google Scholar]
- de Vries W., Stam H., Duys J. G., Ligtenberg A. J., Simons L. H., Stouthamer A. H. The effect of the dissolved oxygen concentration and anabolic limitations on the behaviour of Rhizobium ORS571 in chemostat cultures. Antonie Van Leeuwenhoek. 1986;52(1):85–96. doi: 10.1007/BF00402690. [DOI] [PubMed] [Google Scholar]
- de Vries W., Stam H., Stouthamer A. H. Hydrogen oxidation and nitrogen fixation in rhizobia, with special attention focused on strain ORS 571. Antonie Van Leeuwenhoek. 1984;50(5-6):505–524. doi: 10.1007/BF02386223. [DOI] [PubMed] [Google Scholar]


