Abstract
The presence of a narrow shape and size distribution for magnetite crystals within magnetotactic organisms suggests strongly that there are species-specific mechanisms that control the process of biomineralization. In order to explore the extent of this control, cultures of Aquaspirillum magnetotacticum in the exponential growth phase were exposed to increasing magnetic pulses with the aim of separating cell populations on the basis of their magnetic coercivities. Isothermal remanent magnetization and anhysteretic remanent magnetization studies were performed with freeze-dried magnetic cells after the remagnetization treatment. Subpopulations of A. magnetotacticum that showed an increase in coercivity correlated with the intensity of the magnetic pulses were isolated. After successive subcultures of the remaining north-seeking cells, a maximum bulk coercivity (Hbmax) of 40 mT was obtained after treatment with a 55-mT pulse. Although we obtained A. magnetotacticum variants displaying higher coercivities than the wild-type strain, changes in crystal size or shape of the magnetite crystals were below reliable detection limits with transmission electron microscopy. Attempts to shift the coercivity towards higher values caused it to decrease, a change which was accompanied by an increase in magnetostatic interactions of the magnetosome chains as well as an increase in the cell population displaying an abnormal distribution of the magnetosome chains. Ultrastructural analyses of cells and magnetosomes revealed the appearance of cystlike bodies which occasionally contained magnetosomes. The increase in cystlike cells and abnormal magnetosome chains when higher magnetic pulses were used suggested that magnetosomes were collapsing because of stronger interparticle magnetostatic forces.
Full text
PDF

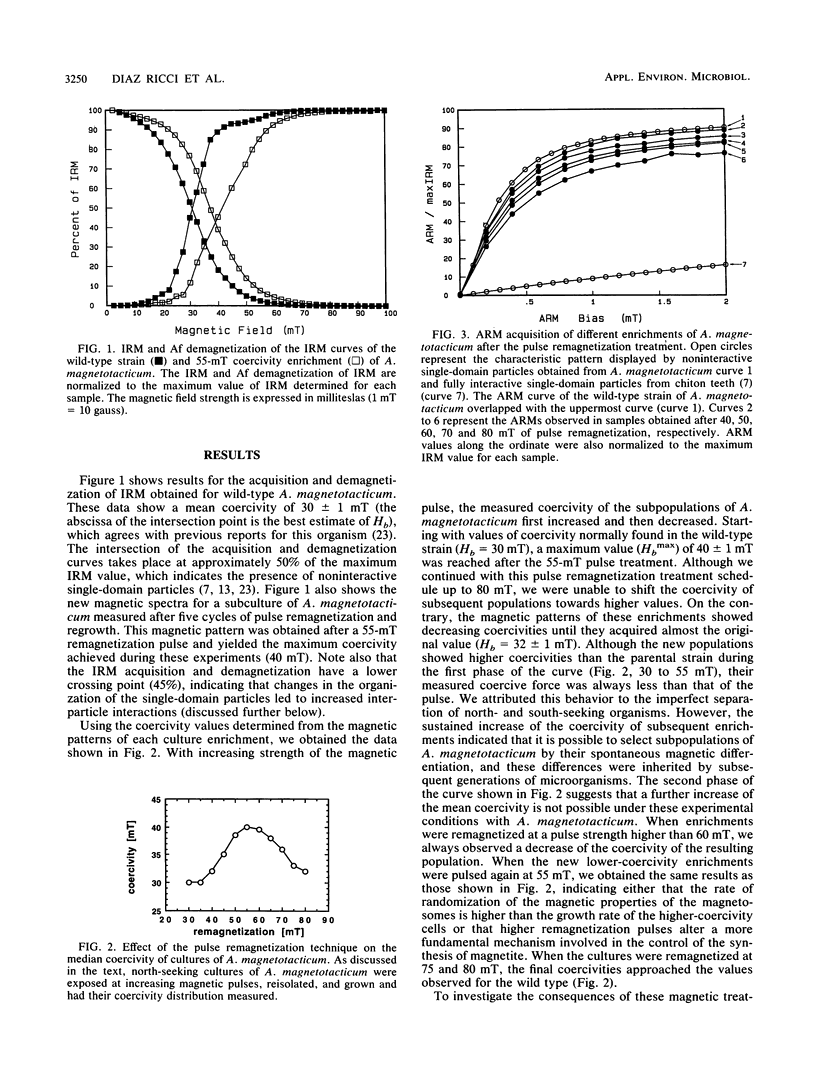
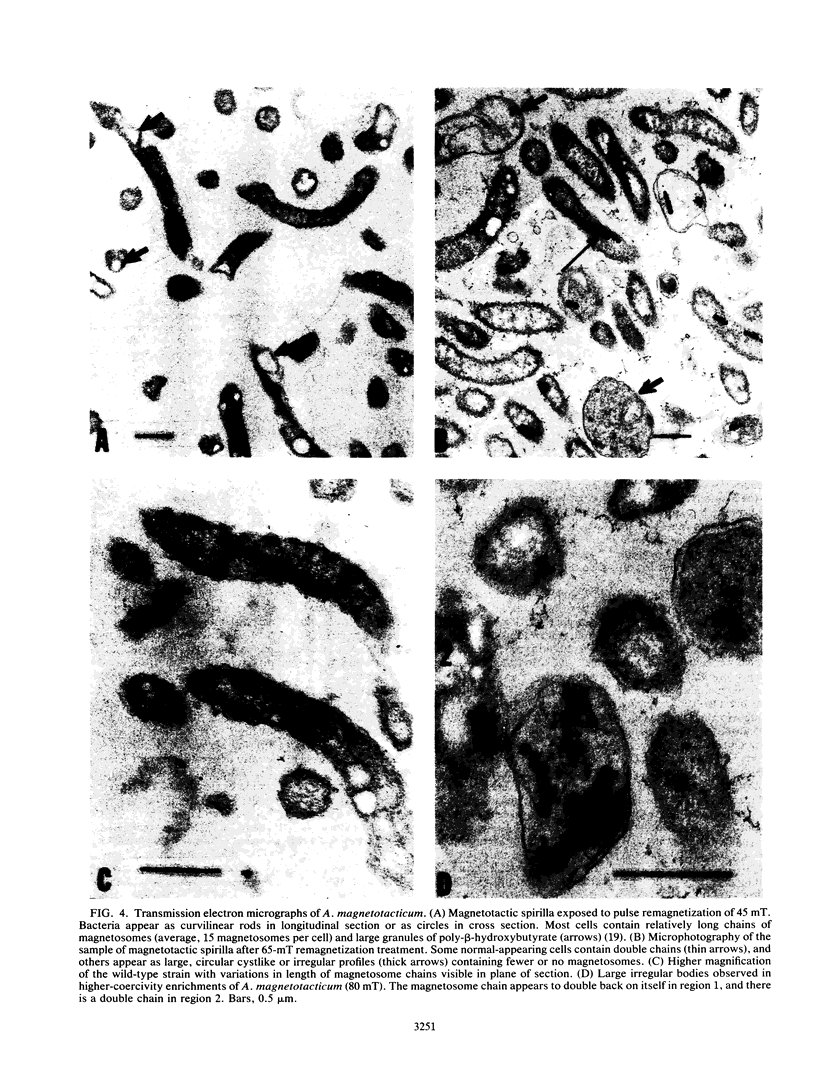
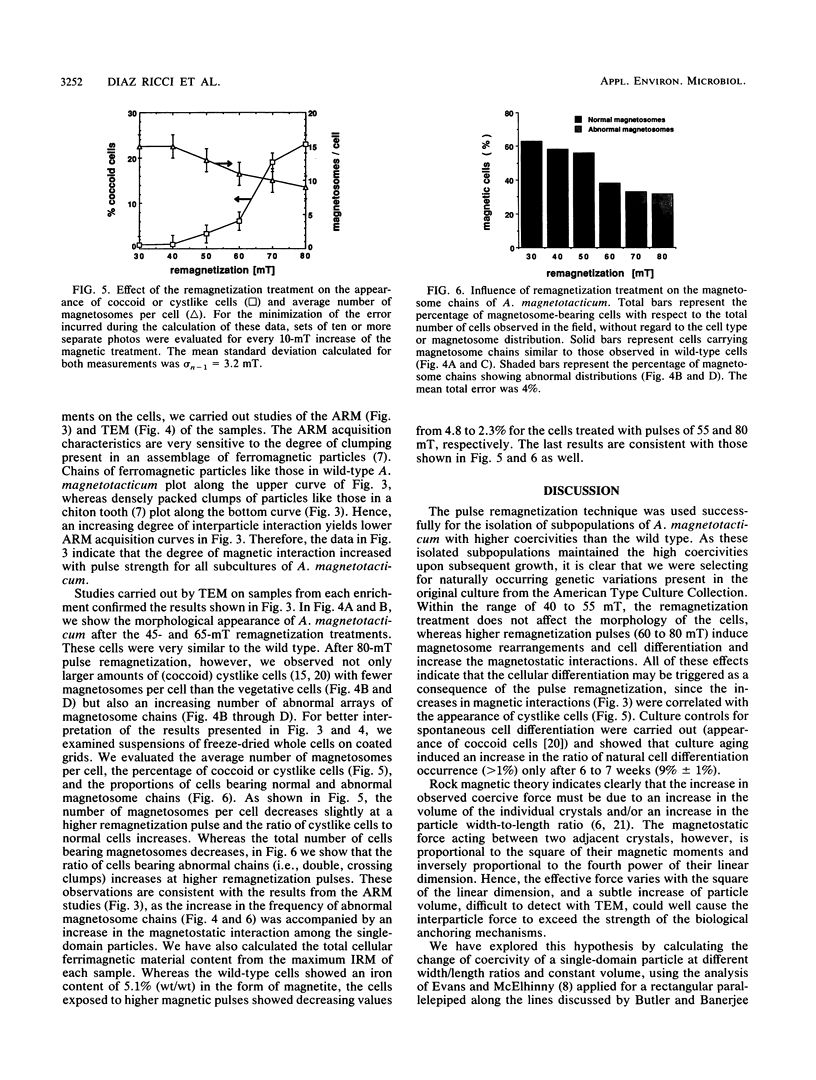

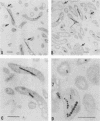
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakemore R. P. Magnetotactic bacteria. Annu Rev Microbiol. 1982;36:217–238. doi: 10.1146/annurev.mi.36.100182.001245. [DOI] [PubMed] [Google Scholar]
- Blakemore R. P., Maratea D., Wolfe R. S. Isolation and pure culture of a freshwater magnetic spirillum in chemically defined medium. J Bacteriol. 1979 Nov;140(2):720–729. doi: 10.1128/jb.140.2.720-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore R. Magnetotactic bacteria. Science. 1975 Oct 24;190(4212):377–379. doi: 10.1126/science.170679. [DOI] [PubMed] [Google Scholar]
- Krieg N. R. Biology of the chemoheterotrophic spirilla. Bacteriol Rev. 1976 Mar;40(1):55–115. doi: 10.1128/br.40.1.55-115.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]