Abstract
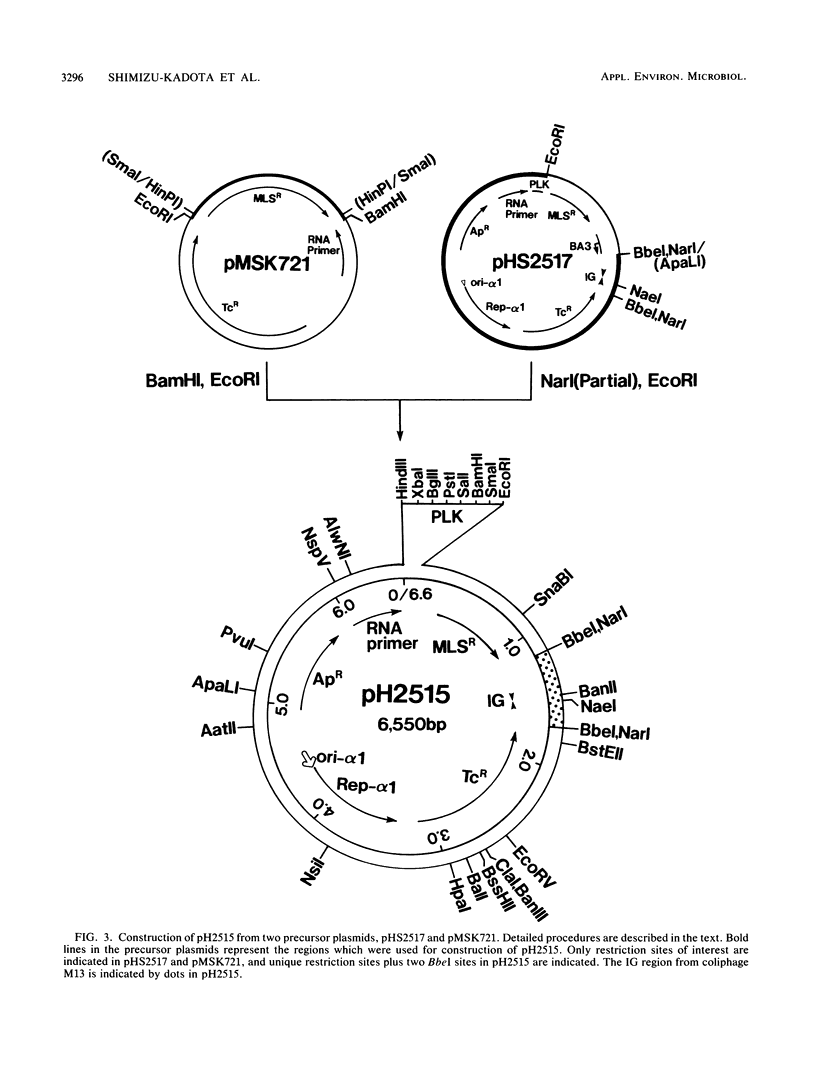
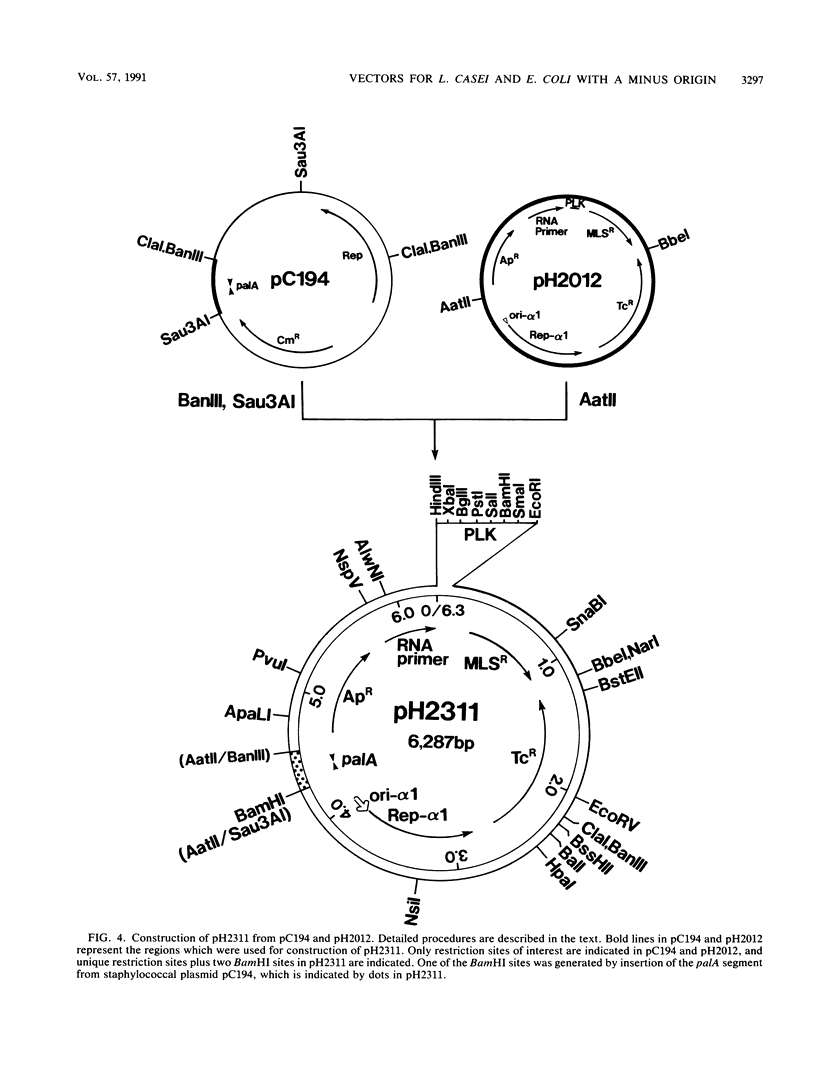
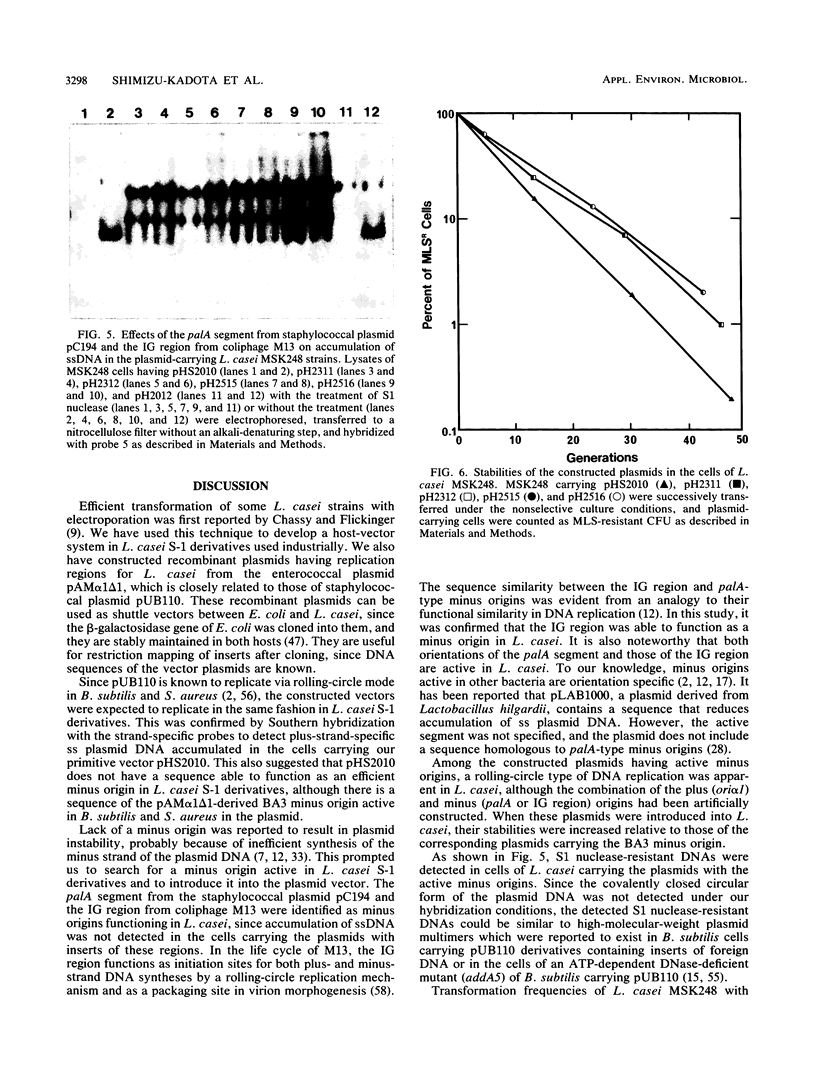
Recombinant plasmids which can be used as shuttle vectors between Escherichia coli and the industrially used strains of Lactobacillus casei were constructed. They have replication regions closely related to those of pUB110 and are likely to replicate by a rolling-circle mechanism via a plus-strand-specific DNA intermediate in L. casei. Both orientations of palA from the staphylococcal plasmid pC194 and those of the intergenic region from coliphage M13 are identified as active minus origins in L. casei, in contrast to the pAM alpha 1 delta 1-derived BA3 minus origin which does not function in L. casei. Stability of the plasmids increased in L. casei when one of these two active minus origins was inserted. All the DNA sequences of the constructed vectors were known.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates E. E., Gilbert H. J. Characterization of a cryptic plasmid from Lactobacillus plantarum. Gene. 1989 Dec 21;85(1):253–258. doi: 10.1016/0378-1119(89)90491-5. [DOI] [PubMed] [Google Scholar]
- Boe L., Gros M. F., te Riele H., Ehrlich S. D., Gruss A. Replication origins of single-stranded-DNA plasmid pUB110. J Bacteriol. 1989 Jun;171(6):3366–3372. doi: 10.1128/jb.171.6.3366-3372.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouia A., Bringel F., Frey L., Kammerer B., Belarbi A., Guyonvarch A., Hubert J. C. Structural organization of pLP1, a cryptic plasmid from Lactobacillus plantarum CCM 1904. Plasmid. 1989 Nov;22(3):185–192. doi: 10.1016/0147-619x(89)90001-2. [DOI] [PubMed] [Google Scholar]
- Brantl S., Behnke D., Alonso J. C. Molecular analysis of the replication region of the conjugative Streptococcus agalactiae plasmid pIP501 in Bacillus subtilis. Comparison with plasmids pAM beta 1 and pSM19035. Nucleic Acids Res. 1990 Aug 25;18(16):4783–4790. doi: 10.1093/nar/18.16.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm J., Salmond G., Minton N. Sequence of the adenine methylase gene of the Streptococcus faecalis plasmid pAM beta 1. Nucleic Acids Res. 1987 Apr 10;15(7):3177–3177. doi: 10.1093/nar/15.7.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringel F., Frey L., Hubert J. C. Characterization, cloning, curing, and distribution in lactic acid bacteria of pLP1, a plasmid from Lactobacillus plantarum CCM 1904 and its use in shuttle vector construction. Plasmid. 1989 Nov;22(3):193–202. doi: 10.1016/0147-619x(89)90002-4. [DOI] [PubMed] [Google Scholar]
- Bron S., Luxen E., Swart P. Instability of recombinant pUB110 plasmids in Bacillus subtilis: plasmid-encoded stability function and effects of DNA inserts. Plasmid. 1988 May;19(3):231–241. doi: 10.1016/0147-619x(88)90041-8. [DOI] [PubMed] [Google Scholar]
- Dao M. L., Ferretti J. J. Streptococcus-Escherichia coli shuttle vector pSA3 and its use in the cloning of streptococcal genes. Appl Environ Microbiol. 1985 Jan;49(1):115–119. doi: 10.1128/aem.49.1.115-119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFTHYMIOU C., HANSEN P. A. An antigenic analysis of Lactobacillus acidophilus. J Infect Dis. 1962 May-Jun;110:258–267. doi: 10.1093/infdis/110.3.258. [DOI] [PubMed] [Google Scholar]
- Gruss A. D., Ross H. F., Novick R. P. Functional analysis of a palindromic sequence required for normal replication of several staphylococcal plasmids. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2165–2169. doi: 10.1073/pnas.84.8.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss A., Ehrlich S. D. Insertion of foreign DNA into plasmids from gram-positive bacteria induces formation of high-molecular-weight plasmid multimers. J Bacteriol. 1988 Mar;170(3):1183–1190. doi: 10.1128/jb.170.3.1183-1190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss A., Ehrlich S. D. The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol Rev. 1989 Jun;53(2):231–241. doi: 10.1128/mr.53.2.231-241.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hashiba H., Takiguchi R., Ishii S., Aoyama K. Transformation of Lactobacillus helveticus subsp. jugurti with plasmid pLHR by electroporation. Agric Biol Chem. 1990 Jun;54(6):1537–1541. [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwa H., Tsuchida N. New shuttle vectors for Escherichia coli and Bacillus subtilis. I. Construction and characterization of plasmid pHY460 with twelve unique cloning sites. Gene. 1984 Dec;32(1-2):129–134. doi: 10.1016/0378-1119(84)90040-4. [DOI] [PubMed] [Google Scholar]
- Iwata M. Characterization of a pAM beta 1 deletion derivative isolated from Lactobacillus casei after conjugation. Biochimie. 1988 Apr;70(4):553–558. doi: 10.1016/0300-9084(88)90092-2. [DOI] [PubMed] [Google Scholar]
- Josson K., Soetaert P., Michiels F., Joos H., Mahillon J. Lactobacillus hilgardii plasmid pLAB1000 consists of two functional cassettes commonly found in other gram-positive organisms. J Bacteriol. 1990 Jun;172(6):3089–3099. doi: 10.1128/jb.172.6.3089-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I., Kobayashi S., Yokokura T., Mutai M. Antitumor activity of Lactobacillus casei in mice. Gan. 1981 Aug;72(4):517–523. [PubMed] [Google Scholar]
- Kok J., van der Vossen J. M., Venema G. Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl Environ Microbiol. 1984 Oct;48(4):726–731. doi: 10.1128/aem.48.4.726-731.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag I. E., Viret J. F., Alonso J. C. Replication and incompatibility properties of plasmid pUB110 in Bacillus subtilis. Mol Gen Genet. 1988 May;212(2):232–240. doi: 10.1007/BF00334690. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T., Yokokura T., Mutai M. Antitumor effect of intrapleural administration of Lactobacillus casei in mice. Cancer Immunol Immunother. 1988;26(3):209–214. doi: 10.1007/BF00199931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie T., Hoshino T., Tanaka T., Sueoka N. Correction. A revision of the nucleotide sequence and functional map of pUB110. Plasmid. 1987 Jan;17(1):83–85. doi: 10.1016/0147-619x(87)90015-1. [DOI] [PubMed] [Google Scholar]
- McKenzie T., Hoshino T., Tanaka T., Sueoka N. The nucleotide sequence of pUB110: some salient features in relation to replication and its regulation. Plasmid. 1986 Mar;15(2):93–103. doi: 10.1016/0147-619x(86)90046-6. [DOI] [PubMed] [Google Scholar]
- Natori Y., Kano Y., Imamoto F. Genetic transformation of Lactobacillus casei by electroporation. Biochimie. 1990 Apr;72(4):265–269. doi: 10.1016/0300-9084(90)90082-r. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Staphylococcal plasmids and their replication. Annu Rev Microbiol. 1989;43:537–565. doi: 10.1146/annurev.mi.43.100189.002541. [DOI] [PubMed] [Google Scholar]
- Perkins J. B., Youngman P. Streptococcus plasmid pAM alpha 1 is a composite of two separable replicons, one of which is closely related to Bacillus plasmid pBC16. J Bacteriol. 1983 Aug;155(2):607–615. doi: 10.1128/jb.155.2.607-615.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzin K. M., Shimizu-Kadota M. Identification of a new insertion element, similar to gram-negative IS26, on the lactose plasmid of Streptococcus lactis ML3. J Bacteriol. 1987 Dec;169(12):5481–5488. doi: 10.1128/jb.169.12.5481-5488.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Kadota M. Properties of Lactose Plasmid pLY101 in Lactobacillus casei. Appl Environ Microbiol. 1987 Dec;53(12):2987–2991. doi: 10.1128/aem.53.12.2987-2991.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Kadota M., Sakurai T., Tsuchida N. Prophage Origin of a Virulent Phage Appearing on Fermentations of Lactobacillus casei S-1. Appl Environ Microbiol. 1983 Feb;45(2):669–674. doi: 10.1128/aem.45.2.669-674.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaugen M. The complete nucleotide sequence of a small cryptic plasmid from Lactobacillus plantarum. Plasmid. 1989 Sep;22(2):175–179. doi: 10.1016/0147-619x(89)90028-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Swinfield T. J., Oultram J. D., Thompson D. E., Brehm J. K., Minton N. P. Physical characterisation of the replication region of the Streptococcus faecalis plasmid pAM beta 1. Gene. 1990 Mar 1;87(1):79–90. [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Viret J. F., Alonso J. C. A DNA sequence outside the pUB110 minimal replicon is required for normal replication in Bacillus subtilis. Nucleic Acids Res. 1988 May 25;16(10):4389–4406. doi: 10.1093/nar/16.10.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viret J. F., Alonso J. C. Generation of linear multigenome-length plasmid molecules in Bacillus subtilis. Nucleic Acids Res. 1987 Aug 25;15(16):6349–6367. doi: 10.1093/nar/15.16.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zinder N. D., Horiuchi K. Multiregulatory element of filamentous bacteriophages. Microbiol Rev. 1985 Jun;49(2):101–106. doi: 10.1128/mr.49.2.101-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Solar G. H., Puyet A., Espinosa M. Initiation signals for the conversion of single stranded to double stranded DNA forms in the streptococcal plasmid pLS1. Nucleic Acids Res. 1987 Jul 24;15(14):5561–5580. doi: 10.1093/nar/15.14.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Riele H., Michel B., Ehrlich S. D. Single-stranded plasmid DNA in Bacillus subtilis and Staphylococcus aureus. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2541–2545. doi: 10.1073/pnas.83.8.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wezenbeek P. M., Hulsebos T. J., Schoenmakers J. G. Nucleotide sequence of the filamentous bacteriophage M13 DNA genome: comparison with phage fd. Gene. 1980 Oct;11(1-2):129–148. doi: 10.1016/0378-1119(80)90093-1. [DOI] [PubMed] [Google Scholar]
- van der Vossen J. M., Kok J., Venema G. Construction of cloning, promoter-screening, and terminator-screening shuttle vectors for Bacillus subtilis and Streptococcus lactis. Appl Environ Microbiol. 1985 Aug;50(2):540–542. doi: 10.1128/aem.50.2.540-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]