Abstract
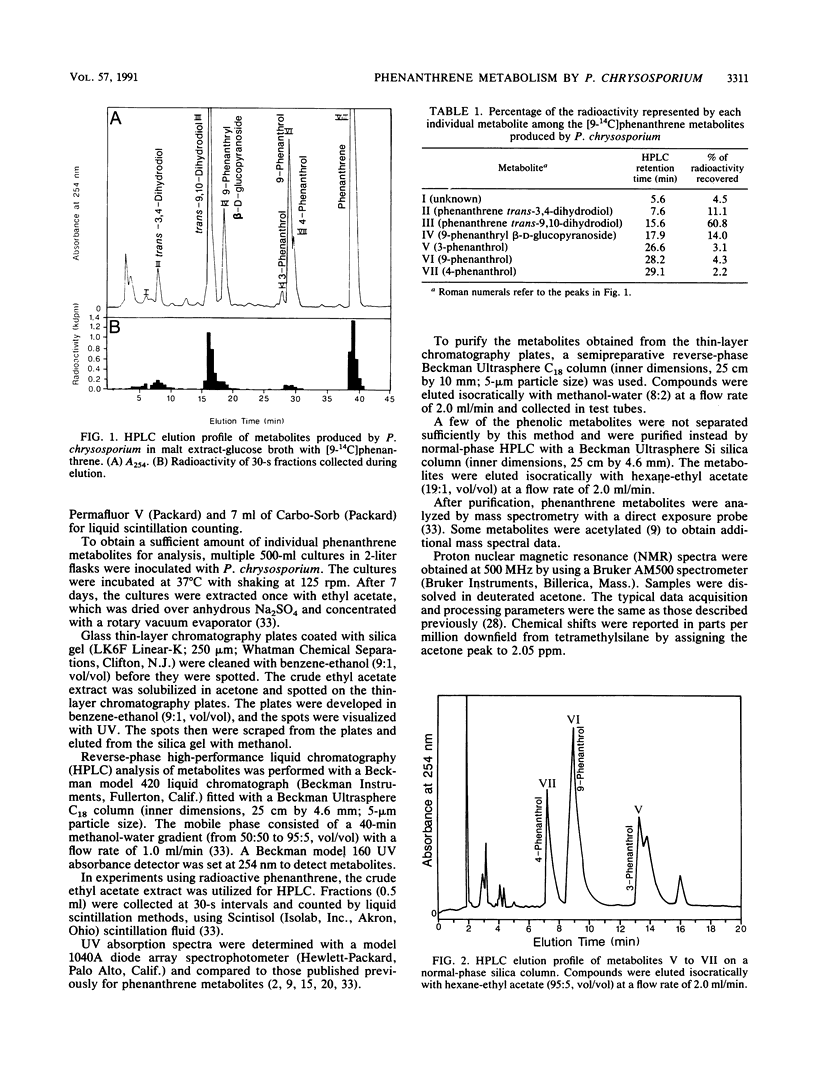
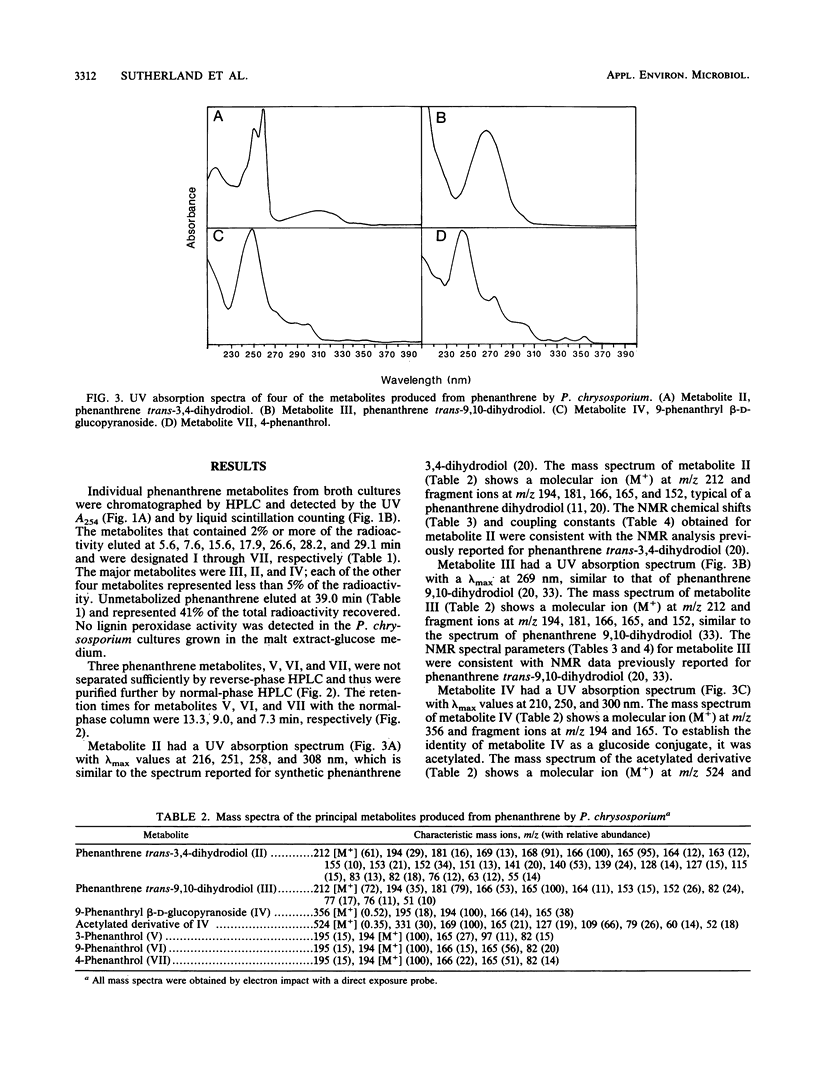
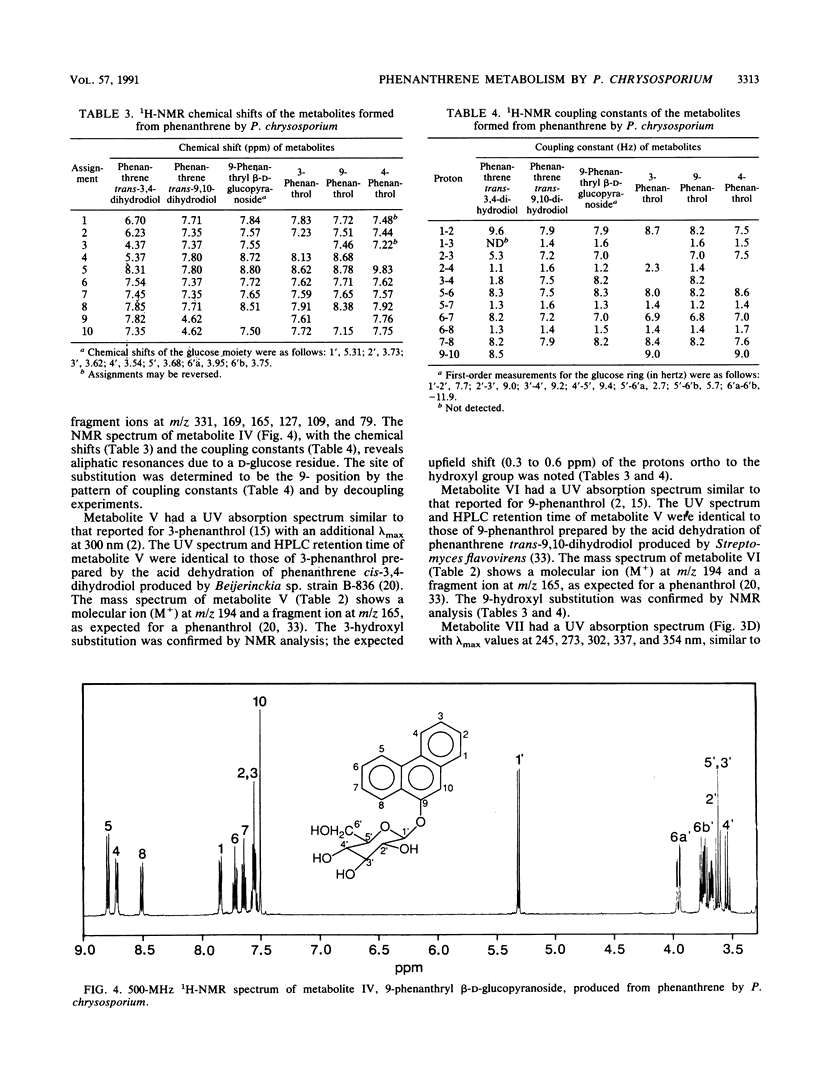
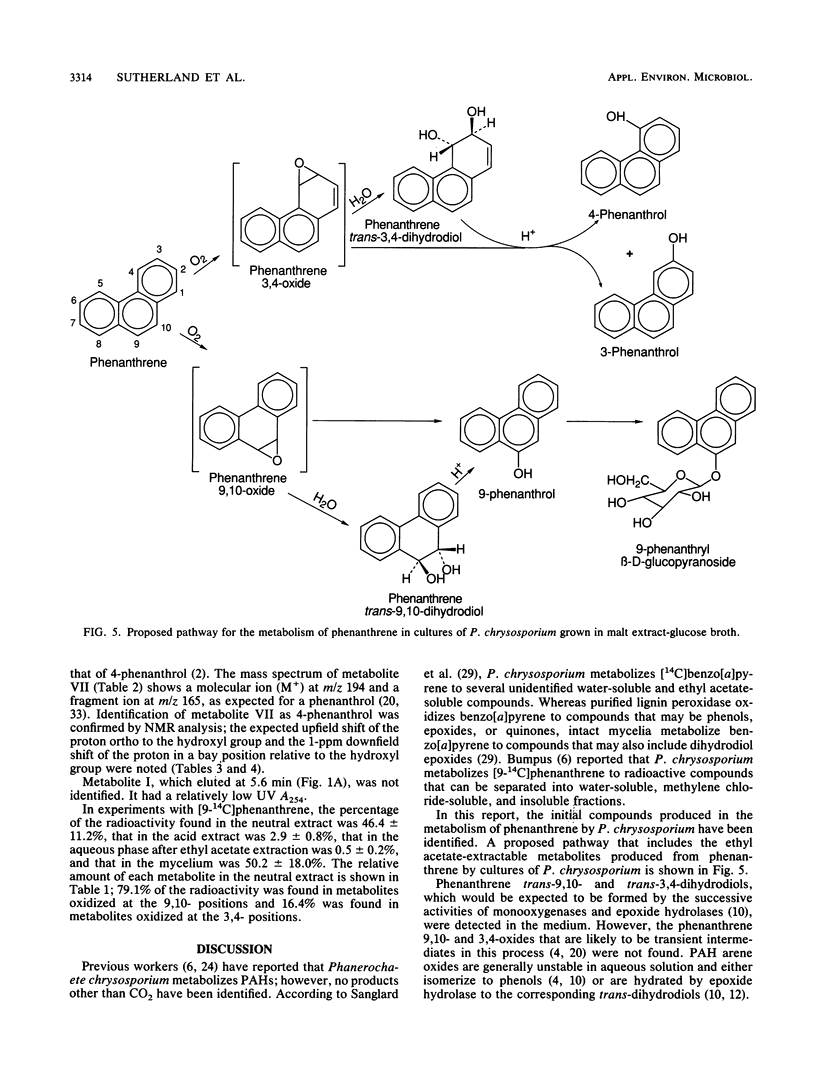
The white rot fungus Phanerochaete chrysosporium metabolized phenanthrene when it was grown for 7 days at 37 degrees C in a medium containing malt extract, D-glucose, D-maltose, yeast extract, and Tween 80. After cultures were grown with [9-14C]phenanthrene, radioactive metabolites were extracted from the medium with ethyl acetate, separated by high-performance liquid chromatography, and detected by liquid scintillation counting. Metabolites from cultures grown with unlabeled phenanthrene were identified as phenanthrene trans-9,10-dihydrodiol, phenanthrene trans-3,4-dihydrodiol, 9-phenanthrol, 3-phenanthrol, 4-phenanthrol, and the novel conjugate 9-phenanthryl beta-D-glucopyranoside. Identification of the compounds was based on their UV absorption, mass, and nuclear magnetic resonance spectra. Since lignin peroxidase was not detected in the culture medium, these results suggest the involvement of monooxygenase and epoxide hydrolase activity in the initial oxidation and hydration of phenanthrene by P. chrysosporium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black J. A., Birge W. J., Westerman A. G., Francis P. C. Comparative aquatic toxicology of aromatic hydrocarbons. Fundam Appl Toxicol. 1983 Sep-Oct;3(5):353–358. doi: 10.1016/s0272-0590(83)80004-9. [DOI] [PubMed] [Google Scholar]
- Bumpus J. A. Biodegradation of polycyclic hydrocarbons by Phanerochaete chrysosporium. Appl Environ Microbiol. 1989 Jan;55(1):154–158. doi: 10.1128/aem.55.1.154-158.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus J. A., Tien M., Wright D., Aust S. D. Oxidation of persistent environmental pollutants by a white rot fungus. Science. 1985 Jun 21;228(4706):1434–1436. doi: 10.1126/science.3925550. [DOI] [PubMed] [Google Scholar]
- Bücker M., Glatt H. R., Platt K. L., Avnir D., Ittah Y., Blum J., Oesch F. Mutagenicity of phenanthrene and phenanthrene K-region derivatives. Mutat Res. 1979 Apr;66(4):337–348. doi: 10.1016/0165-1218(79)90044-2. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Campbell W. L., Freeman J. P., Evans F. E. Identification of a novel metabolite in phenanthrene metabolism by the fungus Cunninghamella elegans. Appl Environ Microbiol. 1989 Sep;55(9):2275–2279. doi: 10.1128/aem.55.9.2275-2279.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E., Yang S. K. Stereoselective metabolism of anthracene and phenanthrene by the fungus Cunninghamella elegans. Appl Environ Microbiol. 1984 Jan;47(1):119–124. doi: 10.1128/aem.47.1.119-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturapit S., Holder G. M. Studies on the hepatic microsomal metabolism of (14C) phenanthrene. Biochem Pharmacol. 1978;27(14):1865–1871. doi: 10.1016/0006-2952(78)90034-5. [DOI] [PubMed] [Google Scholar]
- Ferris J. P., MacDonald L. H., Patrie M. A., Martin M. A. Aryl hydrocarbon hydroxylase activity in the fungus Cunninghamella bainieri: evidence for the presence of cytochrome P-450. Arch Biochem Biophys. 1976 Aug;175(2):443–452. doi: 10.1016/0003-9861(76)90532-4. [DOI] [PubMed] [Google Scholar]
- Haemmerli S. D., Leisola M. S., Sanglard D., Fiechter A. Oxidation of benzo(a)pyrene by extracellular ligninases of Phanerochaete chrysosporium. Veratryl alcohol and stability of ligninase. J Biol Chem. 1986 May 25;261(15):6900–6903. [PubMed] [Google Scholar]
- Hammel K. E., Kalyanaraman B., Kirk T. K. Oxidation of polycyclic aromatic hydrocarbons and dibenzo[p]-dioxins by Phanerochaete chrysosporium ligninase. J Biol Chem. 1986 Dec 25;261(36):16948–16952. [PubMed] [Google Scholar]
- Jerina D. M., Selander H., Yagi H., Wells M. C., Davey J. F., Mahadevan V., Gibson D. T. Dihydrodiols from anthracene and phenanthrene. J Am Chem Soc. 1976 Sep 15;98(19):5988–5996. doi: 10.1021/ja00435a035. [DOI] [PubMed] [Google Scholar]
- Pipe R. K., Moore M. N. Arylsulphatase activity associated with phenanthrene induced digestive cell deletion in the marine mussel Mytilus edulis. Histochem J. 1986 Oct;18(10):557–564. doi: 10.1007/BF01675197. [DOI] [PubMed] [Google Scholar]
- Pothuluri J. V., Freeman J. P., Evans F. E., Cerniglia C. E. Fungal transformation of fluoranthene. Appl Environ Microbiol. 1990 Oct;56(10):2974–2983. doi: 10.1128/aem.56.10.2974-2983.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino J. F., Tanabe L. L. Sublethal effects of phenanthrene, nicotine, and pinane on Daphnia pulex. Bull Environ Contam Toxicol. 1989 May;42(5):778–784. doi: 10.1007/BF01700403. [DOI] [PubMed] [Google Scholar]
- Shiaris M. P. Seasonal Biotransformation of Naphthalene, Phenanthrene, and Benzo[a]pyrene in Surficial Estuarine Sediments. Appl Environ Microbiol. 1989 Jun;55(6):1391–1399. doi: 10.1128/aem.55.6.1391-1399.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solbakken J. E., Palmork K. H. Metabolism of phenanthrene in various marine animals. Comp Biochem Physiol C. 1981;70(1):21–26. doi: 10.1016/0306-4492(81)90073-3. [DOI] [PubMed] [Google Scholar]
- Sutherland J. B., Freeman J. P., Selby A. L., Fu P. P., Miller D. W., Cerniglia C. E. Stereoselective formation of a K-region dihydrodiol from phenanthrene by Streptomyces flavovirens. Arch Microbiol. 1990;154(3):260–266. doi: 10.1007/BF00248965. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]


