Abstract
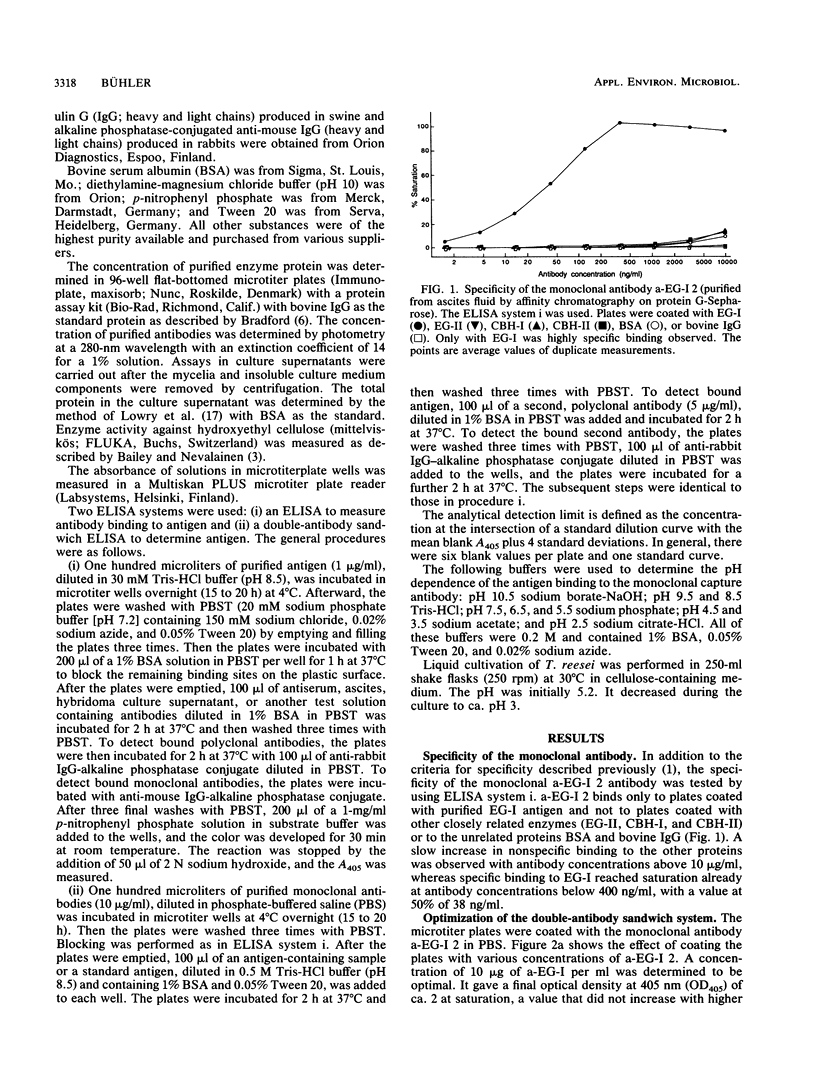
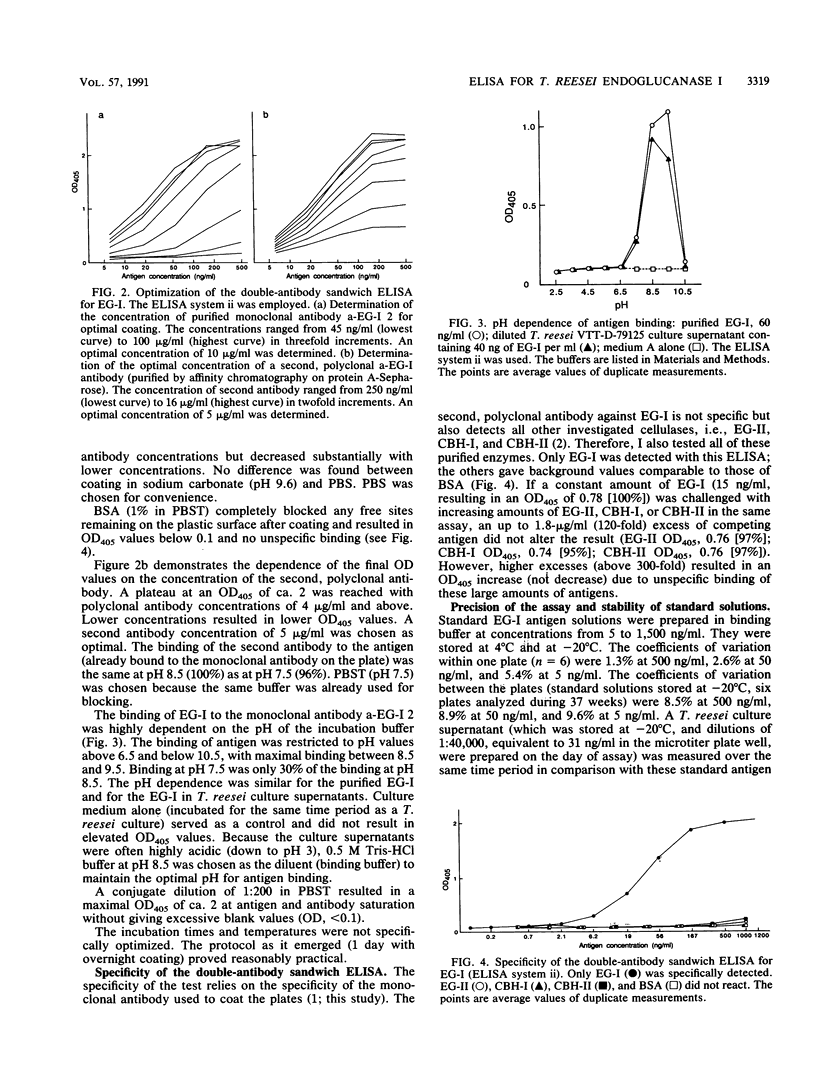
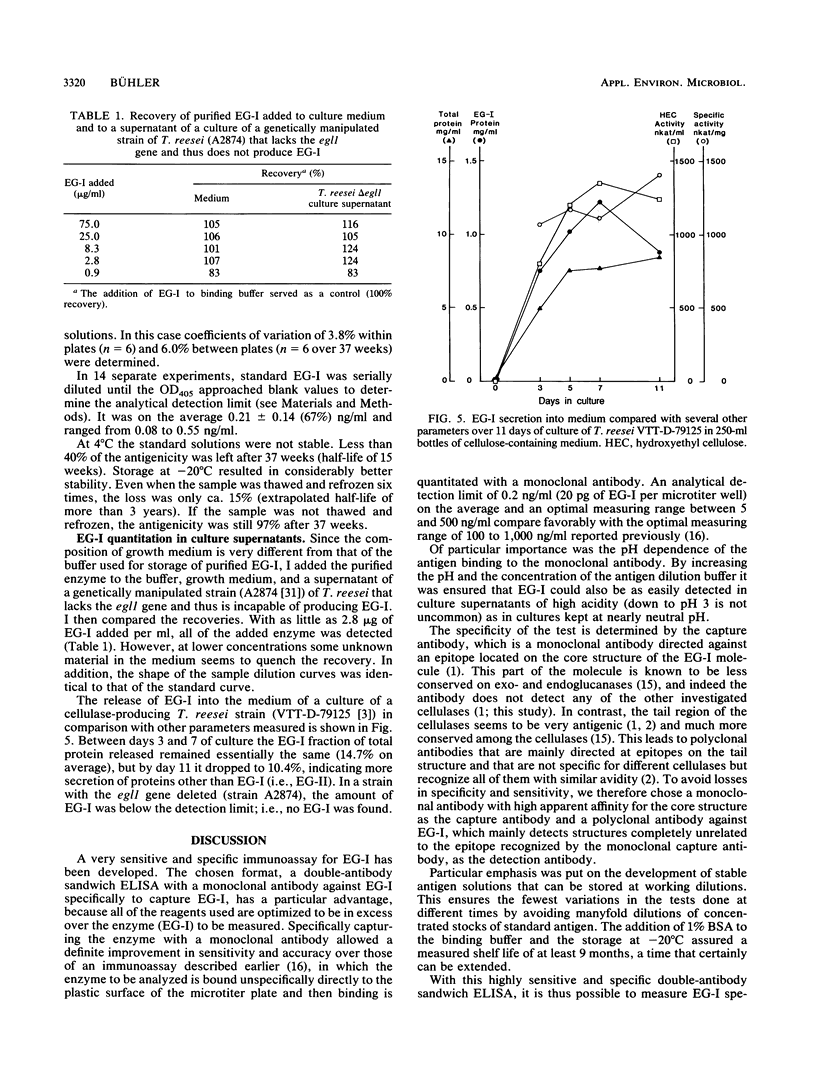
A sensitive and specific enzyme-liked immunosorbent assay for endoglucanase I (EG-I) has been developed. The monoclonal antibody a-EG-I 2, directed against an epitope on the core part of the enzyme, was used to capture the antigen in microtiter plate wells. A second, polyclonal antibody against the enzyme was then used to detect and quantitate the bound antigen. The test was specific for EG-I; neither endoglucanase II nor cellobiohydrolase I or II interfered. As little as 20 pg of EG-I protein could be detected. The coefficients of variation were 3.8% within plates and 6% between plates for a diluted Trichoderma reesei culture supernatant that contained 31 ng of EG-I per ml. Binding of the antigen to the monoclonal antibody was pH dependent and restricted to values between pH 6.5 and 10.5 with a maximum around pH 9. Standard solutions of EG-I were very stable at concentrations as low as 5 ng/ml when prepared in buffer that contained 1% bovine serum albumin and that was stored at -20 degrees C. After 37 weeks the antigenicity was still 97%. With this test it was possible to monitor the production of EG-I in a cellulase-producing strain of T. reesei and to demonstrate the apparent absence of the enzyme in a strain with the eglI gene deleted.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aho S., Paloheimo M. The conserved terminal region of Trichoderma reesei cellulases forms a strong antigenic epitope for polyclonal antibodies. Biochim Biophys Acta. 1990 Oct 23;1087(2):137–141. doi: 10.1016/0167-4781(90)90197-a. [DOI] [PubMed] [Google Scholar]
- Bhikhabhai R., Johansson G., Pettersson G. Isolation of cellulolytic enzymes from Trichoderma reesei QM 9414. J Appl Biochem. 1984 Oct-Dec;6(5-6):336–345. [PubMed] [Google Scholar]
- Bissett F. H. Analysis of cellulase proteins by high-performance liquid chromatography. J Chromatogr. 1979 Oct 31;178(2):515–523. doi: 10.1016/s0021-9673(00)92510-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Fägerstam L. G., Pettersson L. G. The cellulolytic complex of Trichoderma reesei QM 9414. An immunochemical approach. FEBS Lett. 1979 Feb 15;98(2):363–367. doi: 10.1016/0014-5793(79)80218-5. [DOI] [PubMed] [Google Scholar]
- Kolbe J., Kubicek C. P. Quantification and identification of the main components of the Trichoderma cellulase complex with monoclonal antibodies using an enzyme-linked immunosorbent assay (ELISA). Appl Microbiol Biotechnol. 1990 Oct;34(1):26–30. doi: 10.1007/BF00170918. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandels M. Applications of cellulases. Biochem Soc Trans. 1985 Apr;13(2):414–416. doi: 10.1042/bst0130414. [DOI] [PubMed] [Google Scholar]
- Mischak H., Hofer F., Messner R., Weissinger E., Hayn M., Tomme P., Esterbauer H., Küchler E., Claeyssens M., Kubicek C. P. Monoclonal antibodies against different domains of cellobiohydrolase I and II from Trichoderma reesei. Biochim Biophys Acta. 1989 Jan 27;990(1):1–7. doi: 10.1016/s0304-4165(89)80003-0. [DOI] [PubMed] [Google Scholar]
- Nieves R. A., Himmel M. E., Todd R. J., Ellis R. P. Cross-reactive and specific monoclonal antibodies against cellobiohydrolases I and II and endoglucanases I and II of Trichoderma reesei. Appl Environ Microbiol. 1990 Apr;56(4):1103–1108. doi: 10.1128/aem.56.4.1103-1108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummi M., Niku-Paavola M. L., Enari T. M., Raunio V. Immunoelectrophoretic detection of cellulases. FEBS Lett. 1980 May 5;113(2):164–166. doi: 10.1016/0014-5793(80)80583-7. [DOI] [PubMed] [Google Scholar]
- Riske F. J., Eveleigh D. E., Macmillan J. D. Double-antibody sandwich enzyme-linked immunosorbent assay for cellobiohydrolase I. Appl Environ Microbiol. 1990 Nov;56(11):3261–3265. doi: 10.1128/aem.56.11.3261-3265.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker S. P., Brown R. D., Jr Characterization of endo-1,4-beta-D-glucanases purified from Trichoderma viride. Biochim Biophys Acta. 1978 Mar 14;523(1):147–161. doi: 10.1016/0005-2744(78)90017-7. [DOI] [PubMed] [Google Scholar]


