Abstract
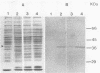
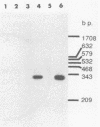
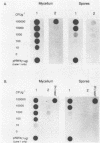
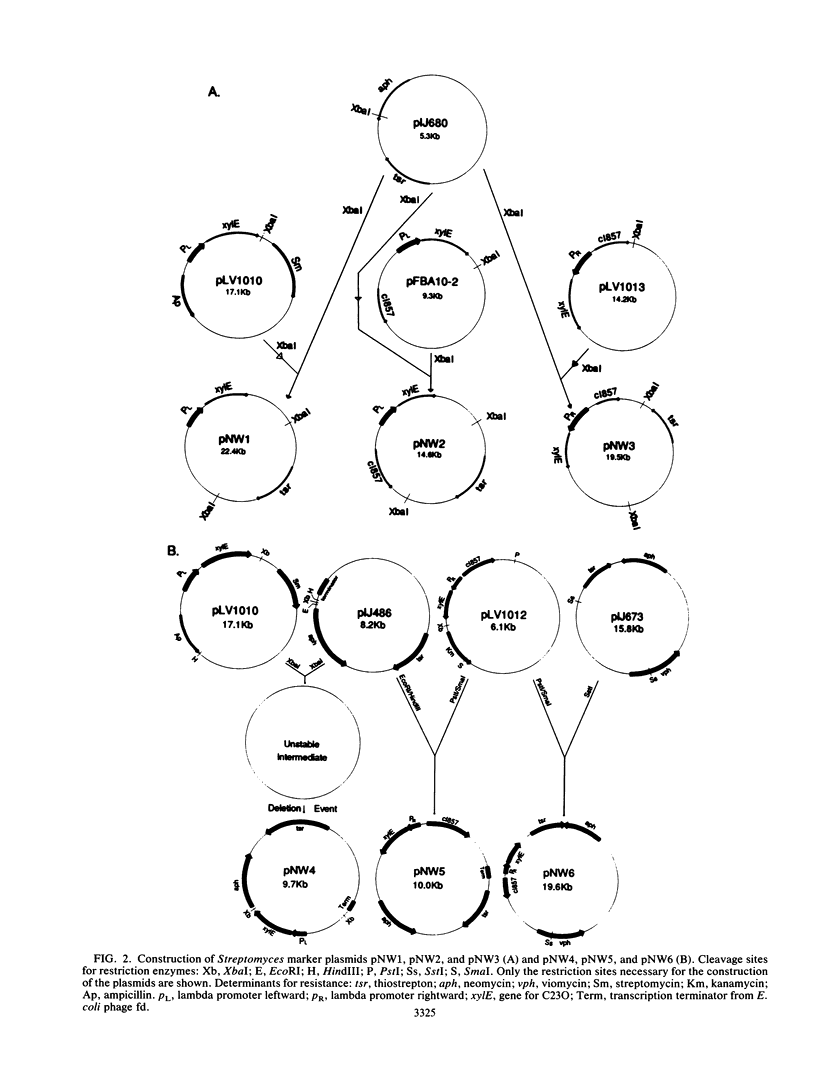
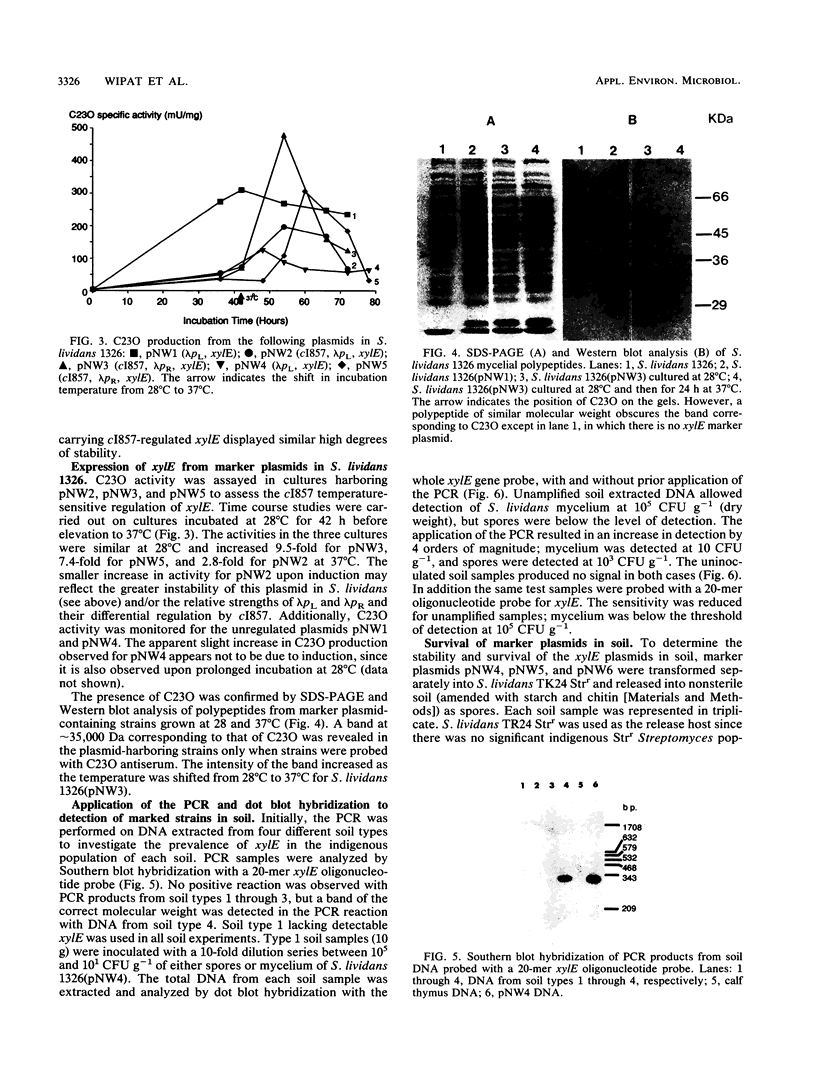
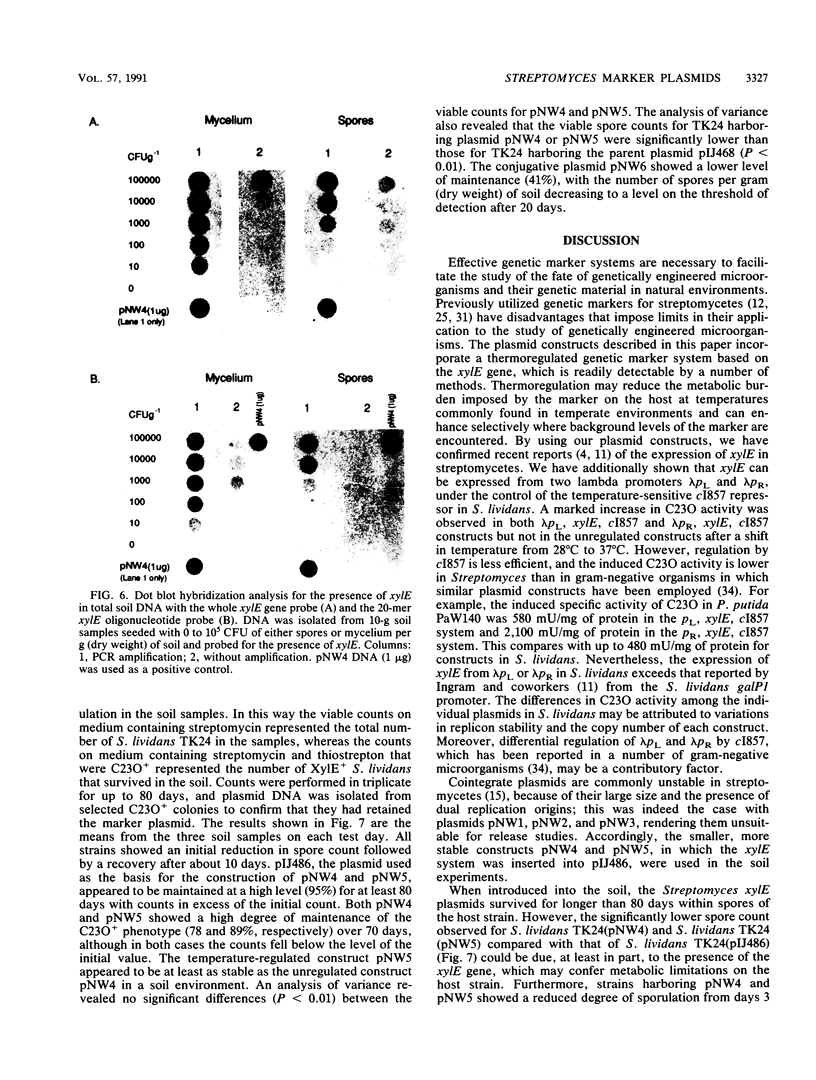
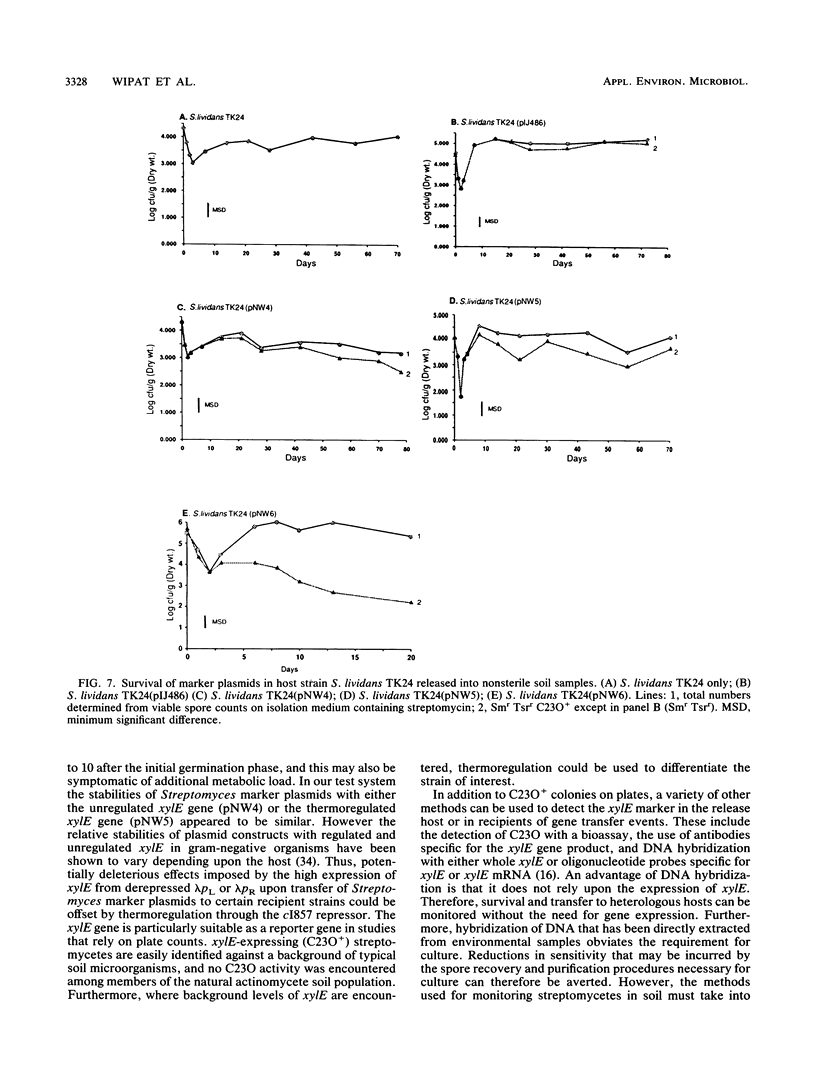
Plasmid constructs pNW1 through pNW6 containing a controllable xylE gene (for catechol 2,3-dioxygenase) were introduced into Streptomyces lividans strains to provide a selectable marker system. xylE functions in S. lividans under the control of bacteriophage lambda promoters lambda pL and lambda pR. Thermoregulated expression of xylE is provided through the lambda repressor cI857. Catechol 2,3-dioxygenase activity was increased 2.8-fold from plasmid construct pNW2 (lambda pL, xylE, cI857) and 9.5- and 7.4-fold from constructs pNW3 (lambda pR, xylE, cI857) and pNW5 (lambda pR, xylE, cI857), respectively, when the temperature was shifted from 28 degrees C to 37 degrees C. The stability of the constructs varied from 4.7% for pNW2 to 99.4% for pNW4 (lambda pL, xylE) over two rounds of sporulation. Marked S. lividans strains released into soil systems retained the XylE phenotype for more than 80 days, depending on the marker plasmid, when examined by a selective plating method. Furthermore, S. lividans harboring plasmid pNW5 was detectable by nucleic acid hybridization at less than 10 CFU g-1 (dry weight) of soil as mycelium and 10(3) CFU g-1 (dry weight) of soil as spores with the xylE marker DNA extracted from soil and amplified by using the polymerase chain reaction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bej A. K., Steffan R. J., DiCesare J., Haff L., Atlas R. M. Detection of coliform bacteria in water by polymerase chain reaction and gene probes. Appl Environ Microbiol. 1990 Feb;56(2):307–314. doi: 10.1128/aem.56.2.307-314.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clayton T. M., Bibb M. J. Streptomyces promoter-probe plasmids that utilise the xylE gene of Pseudomonas putida. Nucleic Acids Res. 1990 Feb 25;18(4):1077–1077. doi: 10.1093/nar/18.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Hazen T. C., Jiménez L. Enumeration and identification of bacteria from environmental samples using nucleic acid probes. Microbiol Sci. 1988 Nov;5(11):340–343. [PubMed] [Google Scholar]
- Holben William E., Jansson Janet K., Chelm Barry K., Tiedje James M. DNA Probe Method for the Detection of Specific Microorganisms in the Soil Bacterial Community. Appl Environ Microbiol. 1988 Mar;54(3):703–711. doi: 10.1128/aem.54.3.703-711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram C., Brawner M., Youngman P., Westpheling J. xylE functions as an efficient reporter gene in Streptomyces spp.: use for the study of galP1, a catabolite-controlled promoter. J Bacteriol. 1989 Dec;171(12):6617–6624. doi: 10.1128/jb.171.12.6617-6624.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. A., Chater K. F. The expression of the Escherichia coli lacZ gene in Streptomyces. J Gen Microbiol. 1986 Jun;132(6):1739–1752. doi: 10.1099/00221287-132-6-1739. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mazodier P., Petter R., Thompson C. Intergeneric conjugation between Escherichia coli and Streptomyces species. J Bacteriol. 1989 Jun;171(6):3583–3585. doi: 10.1128/jb.171.6.3583-3585.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. A., Winstanley C., Pickup R. W., Jones J. G., Saunders J. R. Direct phenotypic and genotypic detection of a recombinant pseudomonad population released into lake water. Appl Environ Microbiol. 1989 Oct;55(10):2537–2544. doi: 10.1128/aem.55.10.2537-2544.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai C., Kagamiyama H., Nozaki M., Nakazawa T., Inouye S., Ebina Y., Nakazawa A. Complete nucleotide sequence of the metapyrocatechase gene on the TOI plasmid of Pseudomonas putida mt-2. J Biol Chem. 1983 Mar 10;258(5):2923–2928. [PubMed] [Google Scholar]
- Nakano T., Tucker H., Oka K., Brown W. V. A simple semi-dry capillary transfer of DNA. Biotechniques. 1990 Feb;8(2):173–174. [PubMed] [Google Scholar]
- Rafii F., Crawford D. L. Transfer of conjugative plasmids and mobilization of a nonconjugative plasmid between Streptomyces strains on agar and in soil. Appl Environ Microbiol. 1988 Jun;54(6):1334–1340. doi: 10.1128/aem.54.6.1334-1340.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Evans W. C. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971 Jun 11;20(3):400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Sayler G. S., Shields M. S., Tedford E. T., Breen A., Hooper S. W., Sirotkin K. M., Davis J. W. Application of DNA-DNA colony hybridization to the detection of catabolic genotypes in environmental samples. Appl Environ Microbiol. 1985 May;49(5):1295–1303. doi: 10.1128/aem.49.5.1295-1303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer A., Ranes M., Santamaria R., Guijarro J., Lawlor E., Mendez C., Chater K., Losick R. Visualizing gene expression in time and space in the filamentous bacterium Streptomyces coelicolor. Science. 1988 May 6;240(4853):768–772. doi: 10.1126/science.3363358. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffan R. J., Goksøyr J., Bej A. K., Atlas R. M. Recovery of DNA from soils and sediments. Appl Environ Microbiol. 1988 Dec;54(12):2908–2915. doi: 10.1128/aem.54.12.2908-2915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., Janssen G. R., Kieser T., Bibb M. J., Buttner M. J., Bibb M. J. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol Gen Genet. 1986 Jun;203(3):468–478. doi: 10.1007/BF00422072. [DOI] [PubMed] [Google Scholar]
- Wellington E. M., Cresswell N., Saunders V. A. Growth and survival of streptomycete inoculants and extent of plasmid transfer in sterile and nonsterile soil. Appl Environ Microbiol. 1990 May;56(5):1413–1419. doi: 10.1128/aem.56.5.1413-1419.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C., Morgan J. A., Pickup R. W., Jones J. G., Saunders J. R. Differential regulation of lambda pL and pR promoters by a cI repressor in a broad-host-range thermoregulated plasmid marker system. Appl Environ Microbiol. 1989 Apr;55(4):771–777. doi: 10.1128/aem.55.4.771-777.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukowski M. M., Gaffney D. F., Speck D., Kauffmann M., Findeli A., Wisecup A., Lecocq J. P. Chromogenic identification of genetic regulatory signals in Bacillus subtilis based on expression of a cloned Pseudomonas gene. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1101–1105. doi: 10.1073/pnas.80.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]