Abstract
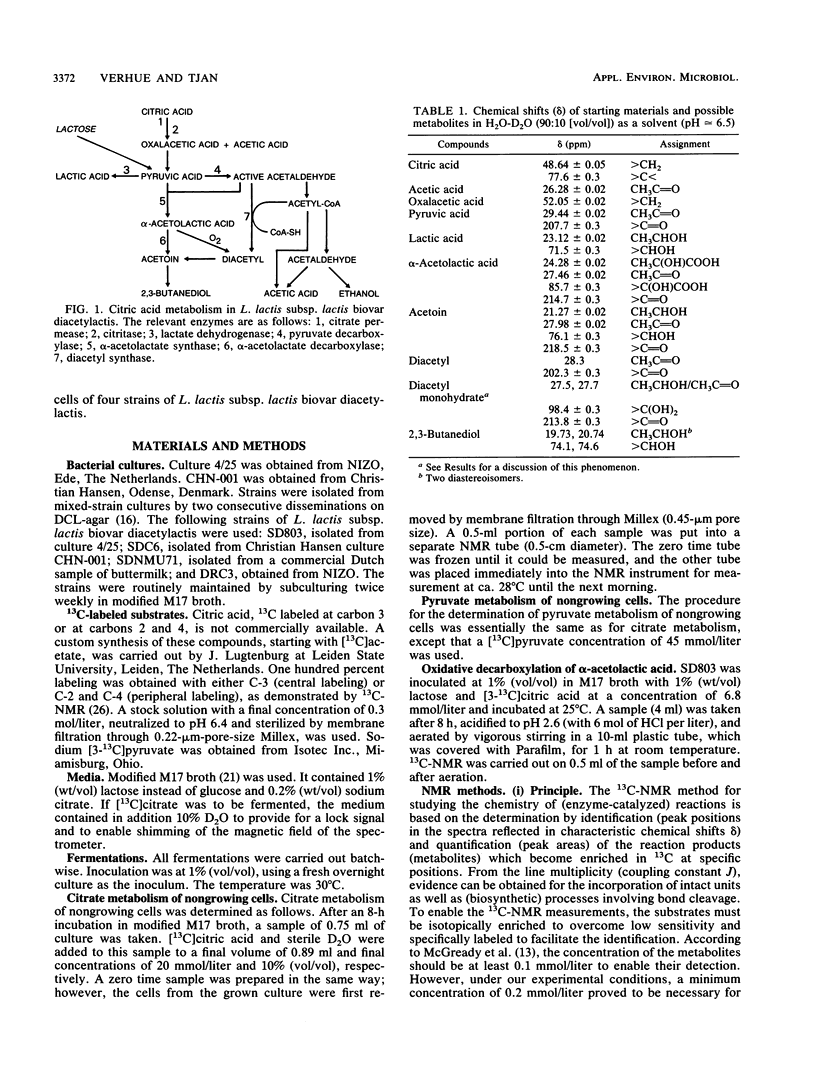
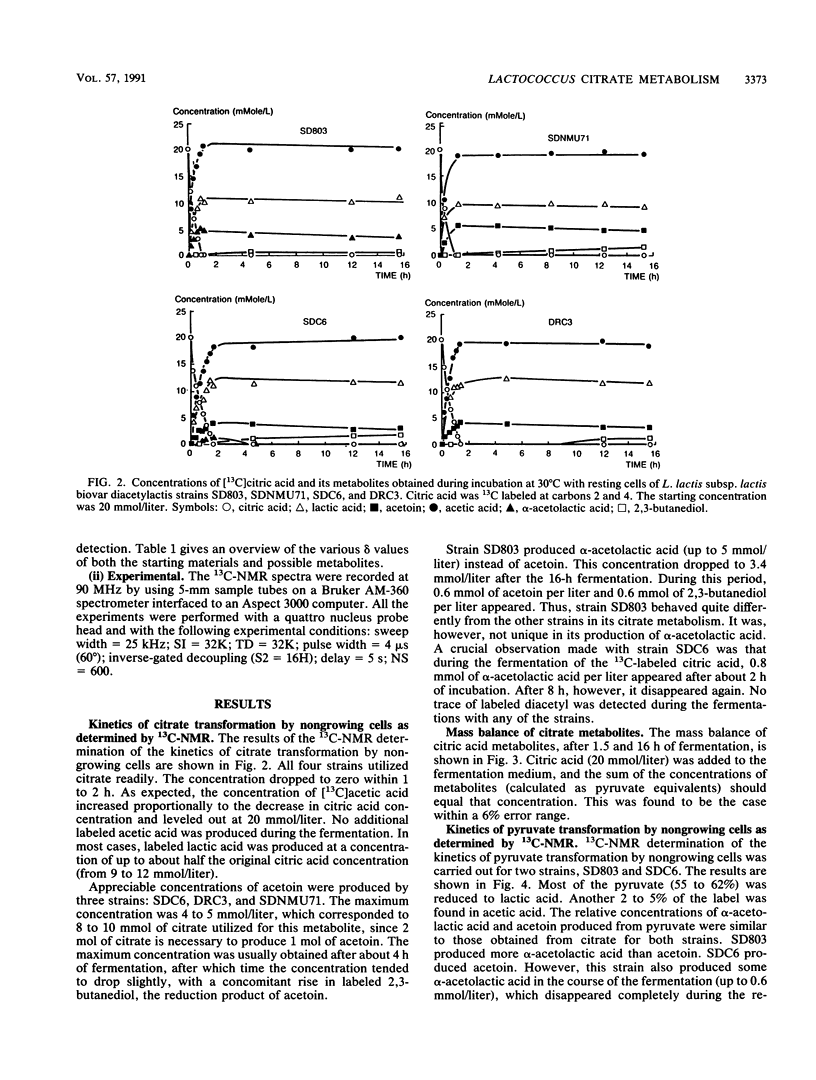
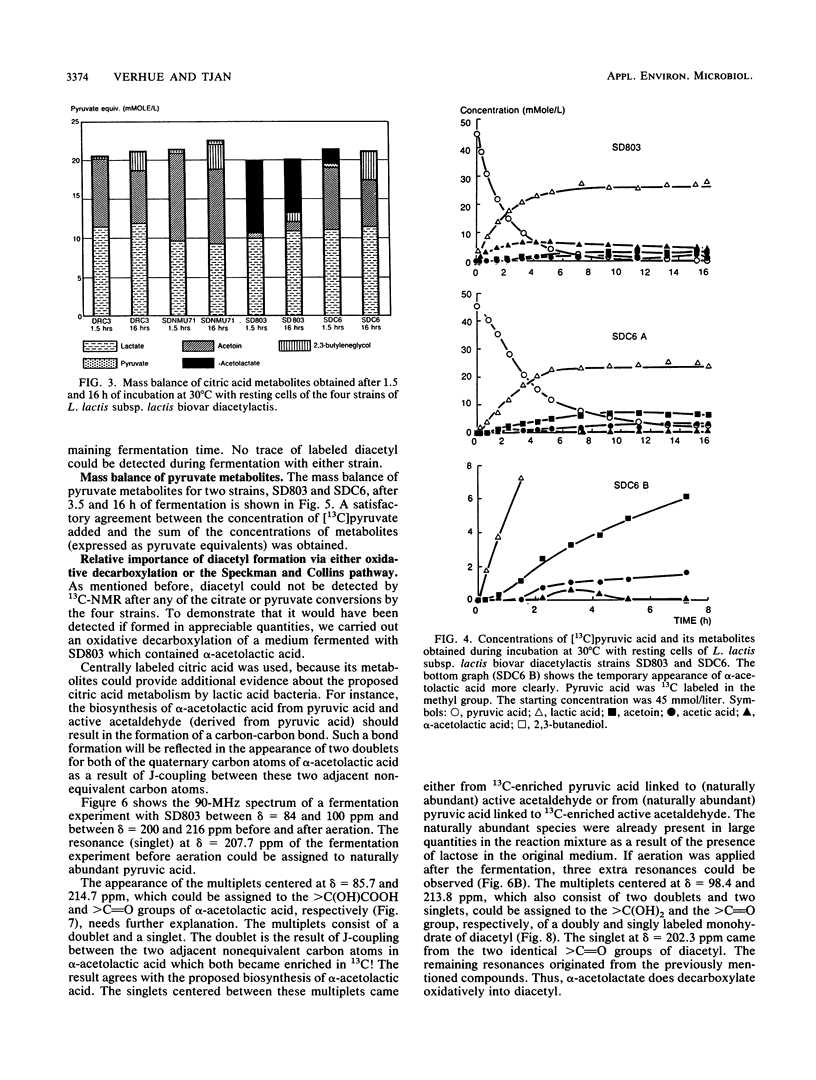
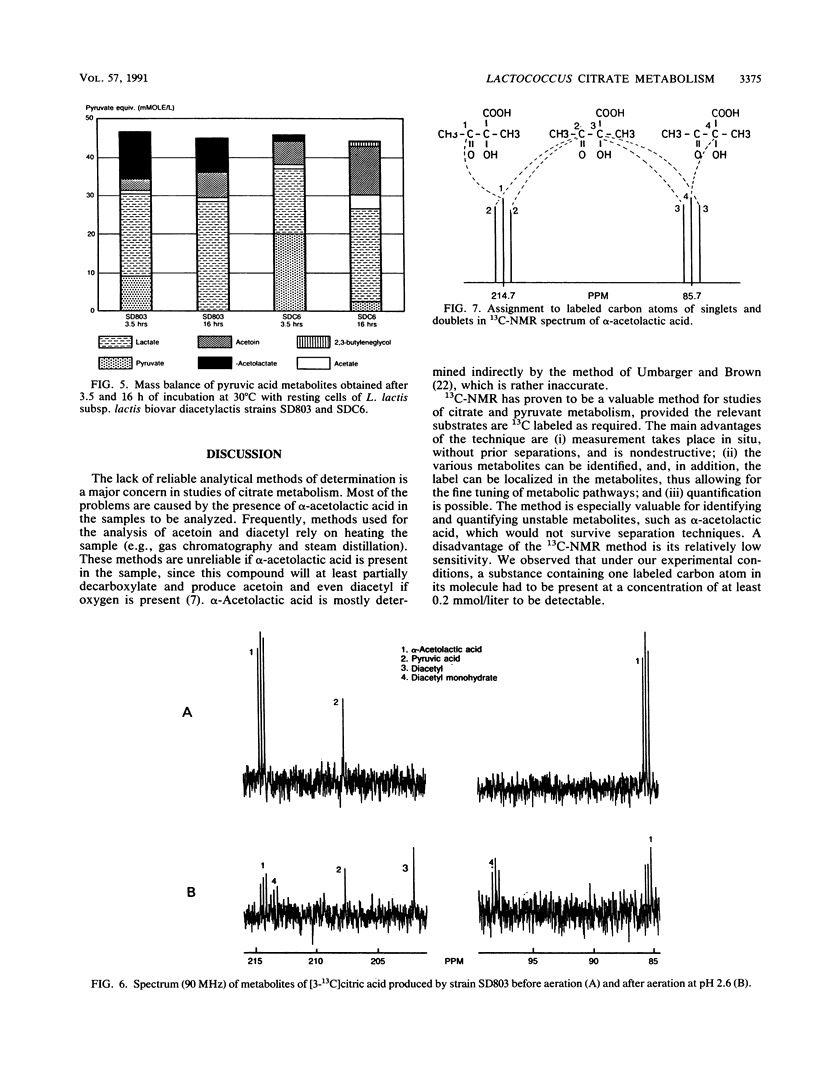
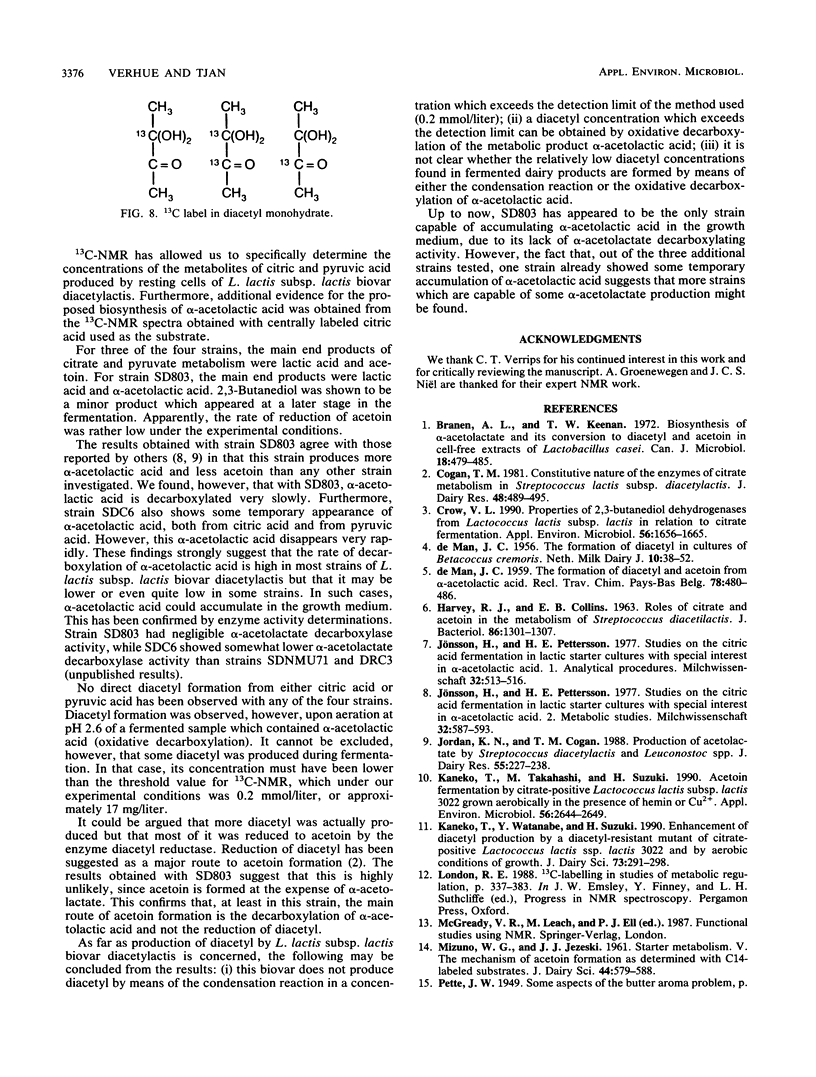
The metabolic fate of citrate and pyruvate in four strains of Lactococcus lactis subsp. lactis biovar diacetylactis has been studied by means of 13C nuclear magnetic resonance, using as a substrate either [3-13C]pyruvic acid or custom-synthesized citric acid that is 13C labeled either at carbons 2 and 4 or at carbon 3. The fermentations were carried out batchwise in modified M17 broth. For the actual conversions of the 13C-labeled substrates, cells at the end of their logarithmic growth phase were used to minimize the conversion to lactic acid. A mass balance of the main citric acid metabolites was obtained; the four strains produced from 50 to 70% (on a molar basis) lactic acid from either citrate or pyruvate. The remaining 50 to 30% was converted mainly to either α-acetolactic acid (for one strain) or acetoin (for the other three strains). One of the strains produced an exceptionally high concentration of the diacetyl precursor α-acetolactic acid. Another strain (SDC6) also produced α-acetolactic acid, but this was decarboxylated to acetoin at a high rate. The 13C nuclear magnetic resonance method confirmed that the biosynthesis of α-acetolactic acid occurs via condensation of pyruvate and “active” acetaldehyde. Diacetyl was not found as a direct metabolite of citrate or pyruvate metabolism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes A. L., Keenan T. W. Biosynthesis of alpha-acetolacetate and its converison of diacetyl and acetion in cell-free extracts of Lactobacillus casei. Can J Microbiol. 1972 Apr;18(4):479–485. doi: 10.1139/m72-074. [DOI] [PubMed] [Google Scholar]
- Crow V. L. Properties of 2,3-Butanediol Dehydrogenases from Lactococcus lactis subsp. lactis in Relation to Citrate Fermentation. Appl Environ Microbiol. 1990 Jun;56(6):1656–1665. doi: 10.1128/aem.56.6.1656-1665.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAY H. S. Planning and equipping a disease diagnostic control laboratory. Lab Anim Care. 1963 Jun;2:431–441. [PubMed] [Google Scholar]
- HARVEY R. J., COLLINS E. B. ROLES OF CITRATE AND ACETOIN IN THE METABOLISM OF STREPTOCOCCUS DIACETILACTIS. J Bacteriol. 1963 Dec;86:1301–1307. doi: 10.1128/jb.86.6.1301-1307.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Takahashi M., Suzuki H. Acetoin Fermentation by Citrate-Positive Lactococcus lactis subsp. lactis 3022 Grown Aerobically in the Presence of Hemin or Cu. Appl Environ Microbiol. 1990 Sep;56(9):2644–2649. doi: 10.1128/aem.56.9.2644-2649.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. S., Vedamuthu E. R., Washam C. J., Reinbold G. W. Agar medium for differential enumeration of lactic streptococci. Appl Microbiol. 1972 Dec;24(6):947–952. doi: 10.1128/am.24.6.947-952.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speckman R. A., Collins E. B. Diacetyl biosynthesis in Streptococcus diacetilactis and Leuconostoc citrovorum. J Bacteriol. 1968 Jan;95(1):174–180. doi: 10.1128/jb.95.1.174-180.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speckman R. A., Collins E. B. Incorporation of radioactive acetate into diacetyl by Streptococcus diacetilactis. Appl Microbiol. 1973 Nov;26(5):744–746. doi: 10.1128/am.26.5.744-746.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Isoleucine and valine metabolism in Escherichia coli. VIII. The formation of acetolactate. J Biol Chem. 1958 Nov;233(5):1156–1160. [PubMed] [Google Scholar]



